Abstract
Sepsis research relies heavily on animal models. One of the most frequently used models, cecal ligation and puncture (CLP), involves surgery, and animal use committees may require the use of analgesics after CLP. However, some analgesics are immunomodulatory and may affect research outcomes. In addition, both septic inflammation and responses to opioids may vary with the sex of the subject. Therefore, we investigated the effects of buprenorphine in inbred mice of both sexes undergoing CLP. We hypothesized that buprenorphine would not significantly change the outcome or patterns of inflammation in C57BL/6 mice after CLP. Male and female C57BL/6 mice underwent CLP surgery and were randomized into 2 groups to receive either buprenorphine or saline. Three-week survival studies were performed (n = 20 per group). Survival did not differ between groups of female mice, but male mice that received buprenorphine had decreased survival compared with that of controls. Reducing the dose of buprenorphine in male mice ameliorated the difference in survival. To examine inflammation, mice (n = 10 per group) were euthanized at 12, 24, or 48 h after CLP. Cell counts and cytokines were measured in the blood and peritoneal lavage fluid. In female and male C57BL/6 mice, buprenorphine treatment resulted in few differences in inflammatory parameters, although peripheral neutrophil counts were decreased transiently in male mice. The findings suggest that the effects of buprenorphine on sepsis models in C57BL/6 mice may be sex-specific. Consequently the use of analgesics must be assessed on a study-by-study basis, and investigators should define analgesic regimens when publishing sepsis studies.
Abbreviation: CLP, cecal ligation and puncture; KC, keratinocyte chemoattractant; MIP2α, macrophage inflammatory protein 2α
Sepsis is a complex, multifactorial inflammatory response to an infection4,26 that can lead to serious consequences, including multiple organ failure and death. Animal models are of vital importance to improving the understanding of this complex condition.7 Cecal ligation and puncture (CLP) is considered by some to be the ‘gold standard’ of sepsis models because it recreates the clinical progression observed in human sepsis.8,15 This model requires abdominal surgery to create a polymicrobial peritonitis, thus prompting animal welfare concerns, particularly when analgesics are not used in the perioperative period. Analgesics are often withheld from animal models of sepsis due to concerns over potential immunomodulatory effects that may interfere with the scientific goals of the study. However, administration of perioperative analgesia is considered to be the standard of care in veterinary medicine. In addition, federally funded animal research must adhere to the Public Health Service Policy on Humane Care and Use of Laboratory Animals, which states that “procedures that may cause more than momentary or slight pain or distress to animals will be performed with appropriate sedation, analgesia, or anesthesia” unless the procedure is justified for scientific reasons in writing by the investigator.30 These government and institutional policies place the onus on investigators and IACUC to determine whether withholding analgesic agents is scientifically justified in sepsis models. Unfortunately, few experimental sepsis studies directly address this controversial issue.
The scientific justifications for specific analgesic policies often are based on results extrapolated from different analgesics or models. These educated opinions may or may not be relevant to a new situation. Historically, many studies investigating the immunomodulatory effects of opioid analgesics were performed with morphine. Morphine, the prototypical μ receptor agonist, increases corticosteroid secretion and decreases natural killer cell activity.29 Overall, morphine administration leads to suppression of inflammatory cell proliferation, with an increase in proinflammatory mediators and decrease in antiinflammatory mediators.29 In addition, studies have associated morphine administration with increased endotoxin sensitivity secondary to a combination of decreased gastrointestinal motility, immunosuppression, and increased bacterial translocation.14 However, buprenorphine is a commonly used analgesic in current research settings.34 It is a strong analgesic recommended for moderate to severe pain and is often administered to ameliorate surgical pain.36 Buprenorphine is considered to be a partial μ agonist and κ antagonist, with limited immunomodulatory effects.30 The lack of immunosuppression with buprenorphine administration is hypothesized to be due to its lack of neuroendocrine effects.11,23 In fact, our laboratory previously demonstrated that buprenorphine administration to female ICR mice undergoing CLP did not have significant effects on mortality or several immune parameters when compared with those of control animals.16 These results suggest that buprenorphine could be used in surgical models of sepsis with limited adverse effects to the model.
Although buprenorphine may not affect the inflammatory response in some situations, previous studies have not assessed its effects on the CLP model. The CLP model is implemented in a variety of mouse strains, and each strain potentially could demonstrate a different response to analgesic administration. Furthermore, an outbred stock, such as ICR, could have a more variable immune response than would an inbred strain, such as C57BL/6. In addition, responses to sepsis are known to differ between male and female mice.21,37 Overall, women are believed to produce stronger humoral and T-cell responses than do men, leading to higher rates of autoimmune disease and potentially more effective resolution of infections in women.19,37 Reports also indicate that women are less likely to develop sepsis than are men, particularly in the postsurgical intensive care unit.21,22,35 The distinct, albeit variable, effects of sex on sepsis survival have been studied extensively. Clinical studies and rodent models have demonstrated better survival in female compared with male subjects.18,33,37 In contrast, female patients have demonstrated worse survival than male in other clinical studies as well as animal models.20,25 Likewise, analgesic responses to opioids differ between male and female hosts.6 Therefore, further investigation into the effect of analgesic administration is necessary for accurate refinement of sepsis models. The purpose of our study was to investigate the effects of buprenorphine administration on mortality and inflammation, assessed through cell counts and cytokine levels, in both sexes of a commonly used inbred mouse strain, C57BL/6J, undergoing CLP-induced polymicrobial sepsis.
Materials and Methods
Study design.
All male and female mice underwent cecal ligation and puncture (CLP). They then were assigned randomly to receive either buprenorphine or an equal volume of saline.
Survival study.
For survival studies, buprenorphine-treated and -untreated female C57BL/6J mice (n = 20 per group) were observed for 3 wk after CLP. Each survival study was performed in 2 separate runs. Mice were removed from the study when they met institutional criteria for endstage illness. Clinical signs used to determine the presence of a moribund condition, as indicated by impaired mobility, inability to remain upright, dyspnea, dehydration or decreased food intake for greater than 48 h, muscle atrophy, unconsciousness, or chronic diarrhea or constipation.9 Any animal meeting one or more of these criteria was removed from the study. Mice were monitored at least twice daily for the first week after surgery and more often if warranted by disease state. The study design was repeated with male mice.
Immunologic study.
To examine the effect of analgesics on inflammation, buprenorphine-treated and -untreated female C57BL/6J mice (n = 10 per group) were euthanized at 12, 24, or 48 h after surgery. The experiment was performed in 2 separate runs. Blood collection and peritoneal lavage were performed at sample harvest. WBC counts and differentials and cytokine levels (IL6, IL1β, TNFα, IL10, keratinocyte chemoattractant [KC; also known as CXCL1], and macrophage inhibitory protein [MIP2α; also known as CXCL2]) were determined from these biologic samples. The study was repeated with male mice.
Experimental animals.
Male and female C57BL/6 mice (age, 12 wk) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were housed 5 per cage in static microisolation caging in an SPF barrier facility. Male mice were housed only with the same cagemates throughout the study, and no evidence of fighting was observed. Mice were SPF for viruses including mouse hepatitis virus, minute virus of mice, mouse parvovirus, enzootic diarrhea of infant mice virus, ectromelia virus, Sendai, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus. Mice had ad libitum access to food (Laboratory Rodent Diet 5001, PMI LabDiet, St Louis, MO) and water. The animal housing room was maintained on a 12:12-h light:dark cycle with constant temperature (72 ± 2 °F [22.2 ± 1.1 °C]). Mice were acclimated for at least 5 d prior to experimental use. All procedures were approved by the University of Michigan's Animal Care and Use Committee.
Surgical procedures.
All mice underwent CLP surgery under isoflurane anesthesia (Vetone, Boise, ID).31 A single ligature of 4-0 silk was used to ligate half of the cecum distal to the ileocecal junction, and 2 punctures were made with a 21-gauge needle. The cecum was expressed gently to ensure patency of the punctures. The abdominal musculature was closed with sutures, followed by closure of the skin with tissue glue. Lactated Ringer solution (1 mL) was administered subcutaneously immediately after surgery. Mice were given heat support in a holding cage and were returned to their home cage once they had recovered completely from anesthesia.
Analgesic dosing.
All analgesic treatments were provided subcutaneously after induction of anesthesia and again at 12 h after surgery for 2 total doses. The high-dose buprenorphine group received 0.1 mg/kg buprenorphine (Bedford Labs, Bedford, OH). Mice in the low-dose buprenorphine group each received 0.05 mg/kg buprenorphine, based on the low end of a published reference range.13 The control group received an equal volume of saline without analgesics.
Behavioral analysis.
All mice in the survival studies were evaluated daily by institutional criteria for endstage illness. However, a subset of male mice receiving either 0.1-mg/kg buprenorphine or saline (control; n = 10 per group) were assigned scores for specific parameters that might further characterize the cause of mortality. Animal behavioral evaluations first occurred at 24 h after surgery and continued daily for 5 d. Evaluations were performed at the same time each day, and the mice initially were observed, undisturbed, within their home cages. Two evaluators, blinded to the study condition, performed independent, behavioral scoring to quantify pain responses in each mouse. Previously published parameters used in mice provided the basis behind the scoring.24 The scoring system used behavioral and physiologic indicators, specifically through 6 recognizable factors: activity, posture, breathing, coat condition, relation to other mice, and response to external stimulation. Clinical signs associated with abnormal scores include lameness, vocalization, lethargy, hunched posture, scruffy or unkempt hair coat, dyspnea, separation or isolation for other animals, decreased or exaggerated responsiveness to handling. Scores of 0 (normal) or 1 (abnormal) were provided for each of these features for every mouse; the maximal total behavioral score was 6.
Blood collection and processing.
Mice (n = 10 per group) were anesthetized with isoflurane, and approximately 500 μL blood was collected from the retroorbital sinus into two 500-μL EDTA tubes (Becton Dickinson, Franklin Lakes, NJ) prior to euthanasia at 12, 24, or 48 h after CLP surgery. One tube of blood was centrifuged (2000 × g, 5 min) and the plasma stored at −20 °C for later cytokine analysis. The second tube was used to obtain an automated CBC analysis (Hemavet Veterinary Multispecies Hematology System, Drew Scientific, Waterbury, CT). Mice then were euthanized by cervical dislocation.
Peritoneal lavage.
After euthanasia of mice, 10 mL Hanks Balanced Salt Solution (Invitrogen, Grand Island, NY) containing 1:100 heparin sodium (1000 USP U/mL; Abraxis, Schaumberg, IL) was injected into the peritoneal cavity, and 5 mL of the solution was retrieved by using a 21-gauge needle. The peritoneal lavage fluid was centrifuged (600 × g, 5 min) and the supernatant stored at −20 °C for later cytokine analysis. The cell pellet was reconstituted in 200 μL RPMI Medium 1640 (Invitrogen, Grand Island, NY) containing 0.1% heat-inactivated fetal bovine serum (Invitrogen, Grand Island, NY) for cell count and differential analyses.
Cytokine ELISA.
Proinflammatory (IL6, IL1β, TNFα), antiinflammatory (IL10), and chemotactic (KC and MIP2α) cytokines were examined. Cytokines were measured in plasma (dilution, 1:10) and peritoneal lavage fluids (dilution, 1:2) by using sandwich ELISA. Matched pairs (biotinylated and nonbiotinylated) of antimurine antibodies with their recombinant proteins (R and D Systems, Minneapolis, MN) were used as previously described by this laboratory.27 Peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA) and the color reagent tetramethylbenzidine were used as the detection system. The reaction was stopped by using 1.5 N sulfuric acid, and absorbance was read at 465 and 590 nm.
Statistical analysis.
Kaplan–Meier survival curves were calculated for each treatment group (Prism, Graphpad, La Jolla, CA), and differences between survival curves were analyzed by log rank tests. The relative risk of mortality with 95% 2-sided confidence intervals was calculated. A t test was used to compare differences in weight change during the first 24 h after surgery between control and treated mice.
Two-way ANOVA (Prism, Graphpad) was used to evaluate the effect of treatment on each immune parameter at each of the 3 time points. When an independent variable had a significant P value, a posthoc t test with Bonferroni correction for multiple comparisons was performed for that variable. In addition, for the 24-h data, the 2-way ANOVA was used for each parameter with the additional level of 0.05-mg/kg buprenorphine as a treatment variable. If the treatment or interaction variables were significant, posthoc t tests with Bonferroni correction for multiple comparisons were performed. Parameters in control mice were compared across time by using a one-way ANOVA with Bonferroni correction for multiple comparisons posthoc. For the behavioral study, individual parameters were compared in buprenorphine-treated and control mice by using the Fischer exact test for each observer. The behavioral scores also were totaled for the 24-h time point, and the total scores were compared separately for each observer by using a t test.
Results
Buprenorphine use in female C57BL/6J mice.
Survival studies.
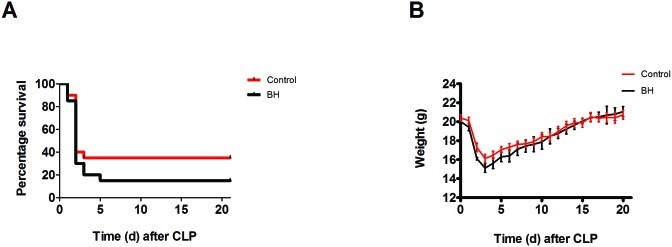
Mortality (defined as meeting moribund criteria for euthanasia) in female mice was greatest during the first 5 d after CLP (Figure 1 A). Administration of buprenorphine (0.1 mg/kg) did not significantly change survival. Mortality was 1.3 times more likely in buprenorphine-treated than control mice, but this difference was not significant at the 0.05-level (Relative rate, 1.308; 95% confidence interval, 0.9027 to 1.894). Percentage weight change during the first 24 h after CLP did not differ significantly between buprenorphine-treated and control mice (Figure 1 B).
Figure 1.
Kaplan–Meier survival curves and daily weight graph for female mice (n = 20 per group). Mice underwent CLP and received treatment with saline (control) or high-dose buprenorphine (BH). Weight was measured daily. (A) There were no significant differences in survival between groups. (B) Weight patterns were similar between groups.
Immune parameters.
Cell counts and differentials.
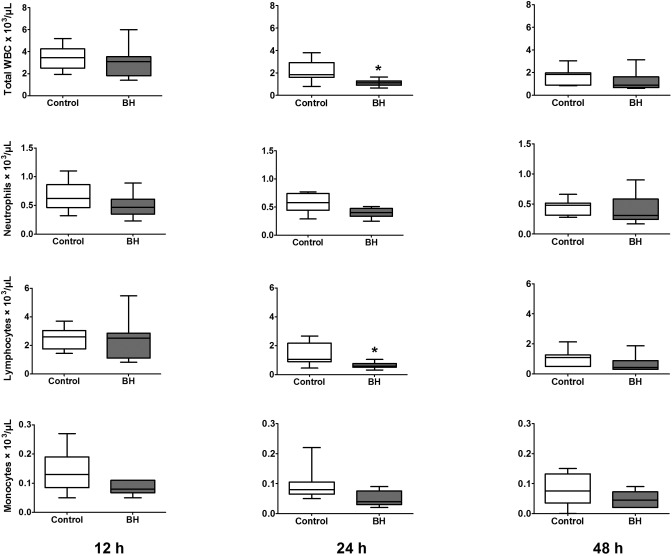
In female control mice, total plasma WBC, neutrophils, and lymphocytes demonstrated significant (P = 0.0003, 0.0480, and 0.0001, respectively) decreases from 12 to 48 h after surgery, as expected in the CLP model (Figure 2). The buprenorphine-treated group had lower mean peripheral WBC counts at all time points compared with control mice, but differences were significant only at the 24-h time point for total WBC counts (P = 0.0052) and lymphocytes (P = 0.0224).
Figure 2.
Peripheral WBC counts for female mice (n = 8 to 10 per group). Mice underwent CLP and received treatment with saline (control) or high-dose buprenorphine (BH). Whole blood was collected for automated cell counts at 12, 24, and 48 h after CLP. Box indicates interquartile range; each horizontal bar represents the median value; whiskers represent the minimal and maximal values. *, P < 0.05 compared with control value.
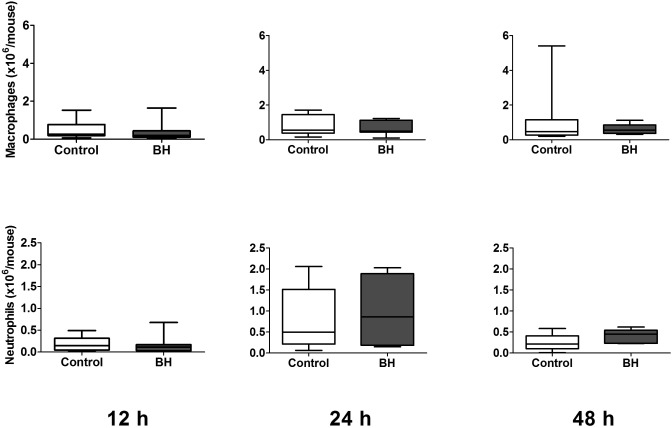
Macrophages and neutrophils comprised the predominant WBC in peritoneal lavage fluid (Figure 3). In control mice, neutrophil counts increased significantly (P = 0.0170) from 12 to 24 h after CLP. Buprenorphine treatment did not result in significant differences between treated and control mice at any time point.
Figure 3.
Peritoneal cell counts for female mice (n = 8 to 10 per group). Mice underwent CLP and received treatment with saline (control) or high-dose buprenorphine (BH). Peritoneal lavage was performed for automated cell counts and manual differential at 12, 24, and 48 h after CLP. Box indicates interquartile range; each horizontal bar represents the median value; whiskers represent the minimal and maximal values.
Cytokine levels.
Regardless of treatment, plasma levels of TNFα and IL1β in female mice were below the lower limit of detection at all time points. In control mice, IL10 and MIP2α did not vary significantly between time points (Table 1). However, IL6 increased significantly (P = 0.0461) from 24 to 48 h after CLP, and KC increased significantly (P = 0.0198) from 12 to 24 h after surgery. No other significant differences in cytokine parameters were present in buprenorphine treated animals compared with control animals for any time point.
Table 1.
Cytokine levels (ng/mL; mean ± 1 SEM) in female mice after CLP
| 12 h |
24 h |
48 h |
|||||
| Control | BH | Control | BH | Control | BH | ||
| Plasma | IL6 | 3.2 ± 0.7 | 4.6 ± 1.7 | 1.0 ± 0.6 | 0.4 ± 0.1 | 5.2 ± 1.3 | 3.7 ± 2.2 |
| IL10 | 0.8 ± 0.4 | 1.4 ± 0.8 | 0.8 ± 0.8 | 0.1 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.3 | |
| KC | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.3 | 0.3 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| MIP2α | 1.7 ± 0.3 | 1.5 ± 0.5 | 1.0 ± 0.7 | 0.2 ± 0.1 | 3.7 ± 1.4 | 1.4 ± 1.0 | |
| IL1β | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Peritoneum | IL6 | 2.3 ± 0.3 | 1.1 ± 0.3a | 0.6 ± 0.3 | 0.3 ± 0.1 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| IL10 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| KC | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | |
| MIP2α | 0.4 ± 0.0 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.0 ± 0.0 | 0.5 ± 0.2 | 0.2 ± 0.1 | |
| IL1β | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
BH, high-dose buprenorphine; KC, keratinocyte chemoattractant; MIP2α, macrophage inflammatory protein 2α
P < 0.05 compared with value for control animal at same time point.
The TNFα level in peritoneal lavage fluid was below the lower limit of detection at all 3 time points and therefore was excluded from Table 1. In control mice, IL6 was highest at 12 h after CLP and had decreased significantly (P < 0.0001) by 48 h after surgery. In contrast, IL10, MIP2α, and KC did not demonstrate a significant difference over time (Table 1). At 12 h after surgery, plasma IL6 was significantly (P = 0.0305) lower in buprenorphine-treated mice (1.1± 0.3 ng/mL) compared with control mice (2.3 ± 0.3 ng/mL). This finding was not observed at the other time points. Buprenorphine treatment did not produce significant changes in other cytokines when compared with values from control animals at any time point.
Buprenorphine use in male C57BL/6J mice.
Survival studies.
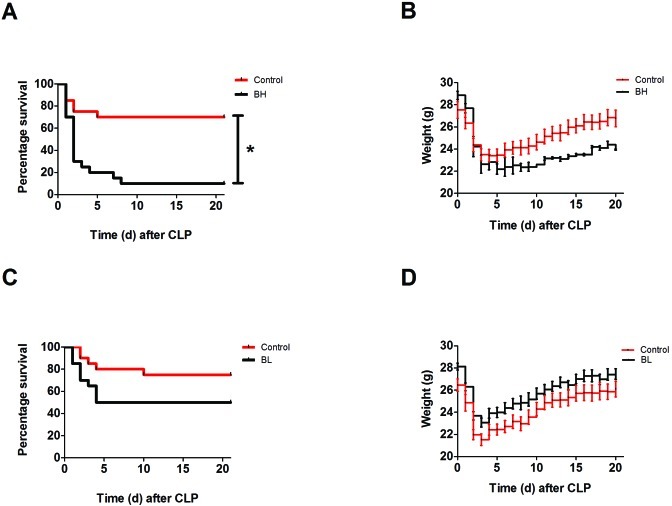
For the CLP model used in male mice, the greatest number of mortalities occurred during the first 5 d after surgery. Survival was significantly (P = 0.0002; log-rank test) lower in the the high-dose (0.1 mg/kg) buprenorphine group compared with controls (Figure 4 A). Compared with that in control mice, death was 3 times as likely to occur in mice that received high-dose buprenorphine (relative rate, 3.000; 95% confidence interval, 1.512 to 5.954). Although there was little difference in overall weight loss, mice in the high-dose group appeared to regain weight more slowly than did control mice (Figure 4 B).The percentage change in weight during the first 24 h after surgery did not differ significantly between groups (data not shown). Given the difference in survival curves, we repeated the experiment by using a lower dose of buprenorphine (0.05 mg/kg) and another group of untreated control animals (Figure 4 C). The log-rank test did not demonstrate a significant difference in survival between the low-dose buprenorphine and control groups. Compared with control mice, the low-dose group had a 2 times increased chance of mortality (relative rate, 2.000; 95% confidence interval, 0.8323 to 4.806). The percentage change in weight during the first 24 h after CLP did not differ between low-dose and control mice (Figure 4 D).
Figure 4.
Kaplan–Meier survival curves and daily weight graphs for male mice (n = 20 per group). Mice underwent CLP and received treatment with saline (control), high-dose buprenorphine (BH), or low-dose buprenorphine (BL). Weight was measured daily for all animals. (A) BH mice had a significant (*, P = 0.0002; log-rank analysis) difference in survival compared with control mice. (B) BH mice appeared to regain weight slowly compared with control mice. (C) BL mice did not have a significant difference in survival compared with controls. (D) Daily weight of BL mice compared with control mice.
Behavior analysis.
Behavior was evaluated on a subgroup of male mice to determine whether specific signs were associated with mortality. Control mice experienced the highest pain score 24 h after surgery, and the score declined over the course of 5 d. At 24 h after surgery, the total behavioral score was not significantly different between buprenorphine and control mice for either the first (4.4 ± 0.7 and 5.2 ± 0.3, respectively; P = 0.26) or second (4.4 ± 0.6 and 4.8 ± 0.5, respectively; P = 0.57). The percentages of animals with abnormal activity were not significantly different between the buprenorphine and control groups when assigned by the first (50% and 89%, respectively) or second (38% and 44%, respectively) observer. Likewise, the percentages of mice demonstrating isolation from other mice did not differ between the buprenorphine group and controls, as determined by either the first (50% and 89%, respectively) or second (75% and 100%, respectively) observer.
Immune parameters.
Cell counts and differentials.
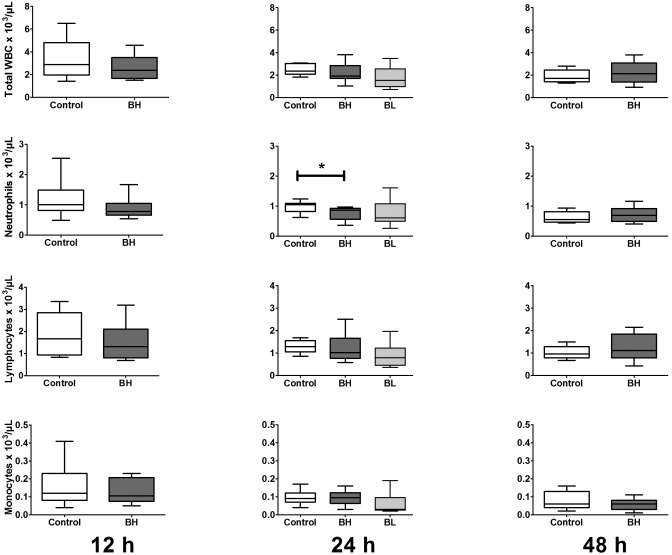
Among male mice, control animals had significant declines in total plasma WBC, neutrophils, and lymphocyte counts from 12 to 48 h after CLP (P = 0.0098, 0.0065, 0.0087, respectively). Administration of 0.1 mg/kg buprenorphine led to significant intergroup differences in specific CBC parameters (Figure 5). At 12 and 24 h after surgery, mice in the high-dose group demonstrated lower mean counts for most WBC parameters than did control mice. These decreases were significantly (P = 0.0020) different between high-dose (0.04 ± 0.01 × 103/µL) and control (0.12 ± 0.03 × 103/µL) mice for eosinophil counts at 12 h. In addition, neutrophil counts at 24 h were significantly (P = 0.0022) different between high-dose (0.80 ± 0.07 × 103/µL) and control (0.96 ± 0.06 × 103/µL) mice. None of the differences persisted beyond a single time point. Administration of 0.05 mg/kg buprenorphine (low dose) at 24 h significantly (P < 0.01) decreased eosinophil counts (0.028 ± 0.008 × 103/µL) compared with those in control mice (0.098 ± 0.017 × 103/µL). None of the other peripheral WBC parameters differed significantly between control and low-dose groups.
Figure 5.
Peripheral cell counts for male mice (n = 9 to 12 per group). Mice underwent CLP and received treatment with saline (control), high-dose buprenorphine (BH), or low-dose buprenorphine (BL). Whole blood was collected for automated cell counts at various time points after CLP. Box indicates interquartile range; each horizontal bar represents the median value; whiskers represent the minimal and maximal values. *, P < 0.05 compared with value for controls.
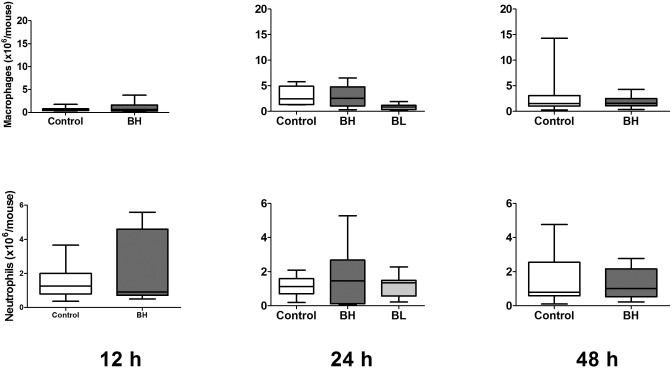
Cell counts in peritoneal lavage fluid were dominated by macrophages and neutrophils (Figure 6). Macrophage counts were significantly (P < 0.05) lower in mice in the low-dose group (0.87 ± 0. 56 × 106/mouse) compared with control animals (2.98 ± 0.78 × 106/mouse). Otherwise, buprenorphine treatment did not lead to significant differences in peritoneal lavage fluid cell counts at any time point.
Figure 6.
Peritoneal cell counts for male mice after CLP (n = 9 to 12 per group). Mice underwent CLP and received treatment with saline (control), high-dose buprenorphine (BH), or low-dose buprenorphine (BL). Peritoneal lavage was performed for automated cell counts and manual differential at 12, 24, and 48 h after CLP. Box indicates interquartile range; each horizontal bar represents the median value; whiskers represent the minimal and maximal values.
Cytokine levels.
Regardless of treatment, plasma TNFα and ILβ levels in male mice were below the lower limit of detection at all 3 time points. In control mice, IL6 was highest at the 12-h time point and fell significantly (P = 0.0209) by 24 h (Table 2). IL10 and MIP2α did not vary significantly at any time point. KC significantly (P < 0.0001) increased from 12 to 24 h after CLP and significantly (P < 0.0001) decreased between 24 and 48 h. Buprenorphine treatment did not significantly change cytokines levels as compared with control mice at any time point.
Table 2.
Cytokine levels (ng/mL; mean ± 1 SEM) in male mice after CLP
| 12 h |
24 h |
48 h |
||||||
| Control | BH | Control | BH | BL | Control | BH | ||
| Plasma | IL6 | 1.0 ± 0.2 | 0.9 ± 0.3 | 0.1 ± 0.1 | 1.3 ± 0.7 | 0.1 ± 0.0 | 0.5 ± 0.2 | 0.7 ± 0.4 |
| IL10 | 0.4 ± 0.3 | 0.4 ± 0.2 | 0.4 ± 0.3 | 0.7 ± 0.3 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.4 | |
| KC | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| MIP2α | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.3 | 1.5 ± 0.9 | 0.1 ± 0.1 | 0.9 ± 0.6 | 0.6 ± 0.4 | |
| IL1β | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Peritoneum | IL6 | 1.1 ± 0.3 | 1.2 ± 0.2 | 1.0 ± 0.3 | 1.5 ± 0.4 | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.2 ± 0.1 |
| IL10 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| KC | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.4 | |
| MIP2α | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.3 | |
| IL1β | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
BH, high-dose buprenorphine; BL, low-dose buprenorphine; KC, keratinocyte chemoattractant; MIP2α, macrophage inflammatory protein 2α
TNFα in the peritoneal lavage fluid of male mice was below the lower limit of detection at all 3 time points. In male control mice, IL6, IL1β, KC, and MIP2α did not differ significantly between time points (Table 2). IL10 was highest at 24 h after surgery and had decreased significantly (P = 0.0208) by 48 h. Buprenorphine treatment did not produce significant changes in cytokines levels when compared with those of control mice at any time point.
Discussion
Sepsis studies often evaluate the efficacy of novel treatments through the analysis of survival data.26 Previous studies in our laboratory demonstrated that buprenorphine (0.1 mg/kg SC every 12 h) did not significantly alter mortality in female ICR (outbred stock) mice after CLP. Consistent with those results, female C57BL/6J inbred mice treated with buprenorphine did not demonstrate much variation in mortality from that of control animals. However, similar buprenorphine use in male C57BL/6J mice elicited significant differences in mortality. Male mice treated with 0.1 mg/kg buprenorphine showed higher mortality within the first 5 d after CLP and demonstrated more late deaths than did untreated animals. Although the significant difference in survival rate was not apparent when male mice were given a lower buprenorphine dose (0.05 mg/kg), the current study suggests that sepsis outcome may be altered by buprenorphine use in male C57BL/6J mice.
To further examine the effects on inflammation, this study examined numerous markers including WBC, proinflammatory and antiinflammatory cytokines, and chemokines. Male mice showed some differences in inflammatory markers between buprenorphine treatment and control groups. As in our previous studies,16 none of the parameters that demonstrated significant differences were consistently different at multiple time points. This finding is consistent with other preclinical studies in rodents that showed buprenorphine had minimal effects on immune parameters including lymphoproliferation, T-cell or macrophage function, and splenic cytokine production.23,32 The male mice with the highest mortality in our study had significantly lower peripheral neutrophil counts at 24 h after treatment compared with those of control mice. However, buprenorphine may affect additional mediators of inflammation that were not examined in the current study and that may explain the high mortality more effectively. Some studies in rodents have shown that buprenorphine can decrease lymphocyte proliferation and IFNγ release,5 but whether this effect is more prominent in male than female rodents is unknown.5 Therefore, investigators may need to evaluate the effects of buprenorphine on parameters of interest in their specific CLP models.
Other effects of buprenorphine may contribute to the increased mortality rate. Previous studies evaluating buprenorphine use in mice have demonstrated decreased activity during the first 24 h and a drop in food intake.1,34 Because most of the deaths in male mice occurred between 24 and 48 h after CLP, we compared each behavioral parameter used to evaluate pain in a subset of animals at 24 h after surgery. The percentage of mice displaying altered activity was not significantly different in the buprenorphine and control groups. Likewise, there were no differences in the percentages of animals displaying abnormal relationships to other mice, which could affect thermoregulation. However, cause and effect may be difficult to interpret in this CLP model, given that many of the behavioral parameters used to establish pain are similar to the signs of the disease being modeled. Physical parameters used to assess pain include hunched or scruffy appearance, lethargy, segregation from other mice, irregular breathing patterns, and decreased responsiveness to stimulation.24 Some of these clinical signs also occur in animals with severe infection. In addition, TNFα and IL1β, which are produced during sepsis, activate the hypothalamo–pituitary–adrenal axis, leading to production of adrenal glucocorticoids.3,12 Therefore, evaluation of parameters such as fecal cortisol may not accurately reflect the pain status in sepsis models. The severity of the model can contribute to the difficulty in determining the true effects of analgesics on behavior.
The recommended dosage of buprenorphine for postsurgical analgesia in mice is variable and controversial in the literature. It is generally accepted that male rodents are more sensitive to the antinociceptive effects of opioids.6 However, those studies were performed primarily by using morphine, and some studies in rats report no sex-associated differences in buprenorphine analgesia.2 A commonly reported dosage in clinical references is 0.05 to 0.1 mg/kg SC every 6 to 12 h.13 We used 0.1 mg/kg buprenorphine at a frequency of every 12 h, based on current clinical laboratory animal practices and consistent with our university postsurgical analgesic guidelines. However, doses as high as 2.5 mg/kg have been recommended.13,17 An evaluation of the duration of analgesic effect of buprenorphine based on hot-plate and tail-flick tests in mice recommended a dose of 2 mg/kg at a frequency of 3 to 5 h.10 Another study found decreased food consumption compared with a presurgical baseline and higher pain scores compared with nonsurgical animals at buprenorphine doses of 0.2 mg/kg.1 Therefore, although buprenorphine at 0.05 mg/kg every 12 h caused no differences in survival among our mice, this dose may not provide sufficient analgesia. This finding suggests that some sepsis studies may be better refined with other analgesics that provide reliable pain relief without immune effects. Conversely, our current study showed no differences in mortality in septic female C57BL/6J mice at buprenorphine doses of 0.1 mg/kg, a result that warrant investigating higher doses or more frequent administration with the benefit of increased analgesia. Because the effects of buprenorphine may be dependent on specific study conditions, we cannot make global recommendations at this time.
Current trends in federal regulations, local IACUC concerns, and general public opinion suggest that analgesic use may become a requirement for all surgical animal models.28 Sepsis studies often evaluate the efficacy of novel treatments through the analysis of survival data.26 If analgesic administration worsens the effects of sepsis or skews the results of mortality studies, this complication can confound the ability to evaluate the efficacy of therapeutics. This effect also could increase the numbers of animals used in studies by increasing increased mortality. Our studies suggest that the effects of buprenorphine on CLP models vary with the sex of C57BL/6J mice. Therefore, recommendations for analgesics cannot be extrapolated from unrelated models, and comprehensive recommendations cannot be given at this time. In addition, accurate documentation of analgesic use is imperative, so that studies can be compared appropriately. These recommendations and further investigation will support the refinement of surgical models of sepsis and preserve the scientific integrity of the studies.
Acknowledgment
The authors thank the American College of Laboratory Animal Medicine for supporting this project.
References
- 1.Adamson TW, Kendall L, Goss S, Grayson K, Touma C, Palme R, Chen J, Borowsky A. 2010. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci 49:610–616 [PMC free article] [PubMed] [Google Scholar]
- 2.Bartok RE, Craft RM. 1997. Sex differences in opioid antinociception. J Pharmacol Exp Ther 282:769–778 [PubMed] [Google Scholar]
- 3.Beishuizen A, Thijs LG. 2003. Endotoxin and the hypothalamo–pituitary–adrenal (HPA) axis. J Endotoxin Res 9:3–24 [DOI] [PubMed] [Google Scholar]
- 4.Buras JA, Holzmann B, Sitkovsky M. 2005. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4:854–865 [DOI] [PubMed] [Google Scholar]
- 5.Carrigan KA, Saurer TB, Ijames SG, Lysle DT. 2004. Buprenorphine produces naltrexone reversible alterations of immune status. Int Immunopharmacol 4:419–428 [DOI] [PubMed] [Google Scholar]
- 6.Dahan A, Kest B, Waxman AR, Sarton E. 2008. Sex-specific responses to opiates: animal and human studies. Anesth Analg 107:83–95 [DOI] [PubMed] [Google Scholar]
- 7.Deitch EA. 2005. Rodent models of intra-abdominal infection. Shock 24:19–23 [DOI] [PubMed] [Google Scholar]
- 8.Dejager L, Pinheiro I, Dejonckheere E, Libert C. 2011. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19:198–208 [DOI] [PubMed] [Google Scholar]
- 9.Foltz CJ, Ullman-Cullere M. 1999. Guidelines for assessing the health and condition of mice. Lab Anim 28:28–32 [PubMed] [Google Scholar]
- 10.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13 [PubMed] [Google Scholar]
- 11.Gomez-Flores R, Weber RJ. 2000. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology 48:145–156 [DOI] [PubMed] [Google Scholar]
- 12.Hadid R, Spinedi E, Chautard T, Giacomini M, Gaillard RC. 1999. Role of several mediators of inflammation on the mouse hypothalamo-pituitary-adrenal axis response during acute endotoxemia. Neuroimmunomodulation 6:336–343 [DOI] [PubMed] [Google Scholar]
- 13.Hawk CT, Leary SL, Morris TH; American College of Laboratory Animal Medicine; European College of Laboratory Animal Medicine 2005. Formulary for laboratory animals. Ames (IA): Blackwell Publishing [Google Scholar]
- 14.Hilburger ME, Adler MW, Truant AL, Meissler JJ, Satishchandran V, Rogers TJ, Eisenstein TK. 1997. Morphine induces sepsis in mice. J Infect Dis 176:183–188 [DOI] [PubMed] [Google Scholar]
- 15.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, Bland KI, Chaudry IH. 2005. Cecal ligation and puncture. Shock 24:52–57 [DOI] [PubMed] [Google Scholar]
- 16.Hugunin KMS, Fry C, Shuster K, Nemzek J. 2010. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34:250–260 [DOI] [PubMed] [Google Scholar]
- 17.Jenkins WL. 1987. Pharmacologic aspects of analgesic drugs in animals: an overview. J Am Vet Med Assoc 191:1231–1240 [PubMed] [Google Scholar]
- 18.Kahlke V, Dohm C, Mees T, Brotzmann K, Schreiber S, Schroder J. 2002. Early interleukin-10 treatment improves survival and enhances immune function only in males after hemorrhage and subsequent sepsis. Shock 18:24–28 [DOI] [PubMed] [Google Scholar]
- 19.Klein SL. 2000. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev 24:627–638 [DOI] [PubMed] [Google Scholar]
- 20.Leon LR. 1998. Role of IL6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol 275:R269–R277 [DOI] [PubMed] [Google Scholar]
- 21.Marriott I, Huet-Hudson YM. 2006. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res 34:177–192 [DOI] [PubMed] [Google Scholar]
- 22.Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 23.Martucci C, Panerai AE, Sacerdote P. 2004. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain 110:385–392 [DOI] [PubMed] [Google Scholar]
- 24.Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress, and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 116:431–436 [DOI] [PubMed] [Google Scholar]
- 25.Nachtigall I. 2011. Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care 15:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemzek JA, Hugunin KM, Opp MR. 2008. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med 58:120–128 [PMC free article] [PubMed] [Google Scholar]
- 27.Nemzek JA, Siddiqui J, Remick DG. 2001. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods 255:149–157 [DOI] [PubMed] [Google Scholar]
- 28.Nemzek JA, Xiao HY, Minard A, Bolgos G, Remick D. 2004. Humane endpoints in shock research. Shock 21:17–25 [DOI] [PubMed] [Google Scholar]
- 29.Odunayo A, Dodam JR, Kerl ME, DeClue AE. 2010. Immunomodulatory effects of opioids. J Vet Emerg Crit Care (San Antonio) 20:376–385 [DOI] [PubMed] [Google Scholar]
- 30.Office of Laboratory Animal Welfare 2002. Public Health Service policy on humane care and use of laboratory animals. Bethesda (MD): Department of Health and Human Services [Google Scholar]
- 31.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. 2002. Six at six: Interleukin-6 measured 6 H after the initiation of sepsis predicts mortality over 3 days. Shock 17:463–467 [DOI] [PubMed] [Google Scholar]
- 32.Sacerdote P. 2006. Opioids and the immune system. Palliat Med 20:S9–S15 [PubMed] [Google Scholar]
- 33.Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. 1998. Gender differences in human sepsis. Arch Surg 133:1200–1205 [DOI] [PubMed] [Google Scholar]
- 34.Tubbs JT, Kissling G, Travlos G, Goulding D, Clark J, King Herbert A, Blankenship Paris T. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191 [PMC free article] [PubMed] [Google Scholar]
- 35.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. 2000. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med 26:167–172 [DOI] [PubMed] [Google Scholar]
- 36.Yu S, Zhang X, Sun Y, Peng Y, Johnson J, Mandrell T, Shukla AJ, Laizure SC. 2006. Pharmacokinetics of buprenorphine after intravenous administration in the mouse. J Am Assoc Lab Anim Sci 45:12–16 [PubMed] [Google Scholar]
- 37.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. 1997. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med 25:106–110 [DOI] [PubMed] [Google Scholar]