Abstract
Summary
Background and objectives
Interferon (IFN) and pegylated-IFN treatment of hepatitis C virus (HCV) infection in hemodialysis patients result in sustained virological response (SVR) rates of 45% and 37%, respectively. Although most nonhemodialysis patients who achieve SVR remain persistently viral negative, the durability of SVR in hemodialysis patients is unknown. We analyzed the rate of long-term virological negativity in hemodialysis patients who achieved SVR after IFN or pegylated-IFN through analysis of patient-level data.
Design, setting, participants, & measurements
After performing a systematic literature review for IFN-based treatment of hemodialysis patients with chronic HCV infection, we extracted patient-level data on patients who achieved SVR. We performed life table analysis to estimate long-term virological negativity rates after SVR in patients who continued on hemodialysis or subsequently underwent kidney transplantation.
Results
Long-term HCV RNA outcomes following SVR were available for 121 hemodialysis patients (20 studies) and 45 patients who subsequently underwent transplantation (11 studies). The probability of remaining HCV RNA negative was 86% (95% confidence interval, 77% to 96%) for patients followed on hemodialysis 48 months after SVR and 95% (95% confidence interval, 89% to 100%) for kidney recipients followed 48 months after transplant.
Conclusions
Viral negativity from IFN-based HCV treatment in hemodialysis patient appears durable during extended follow-up, including after kidney transplantation. The certainty of the viral negativity estimate is limited by the small number with follow-up beyond 48 months or longer. Transplantation does not confer an increased risk of relapse. Future research should investigate whether IFN-based treatment improves clinical outcomes for hemodialysis patients.
Introduction
Hepatitis C virus (HCV) infects an estimated 170 million people worldwide (1), with prevalence rates of 13% in hemodialysis (HD) patients from European countries, the United States, and Japan (2). Compared with noninfected patients, HCV infection is associated with higher mortality in HD patients (3,4) and higher mortality and graft loss in kidney transplant recipients (5). The rate of spontaneous viral clearance is 0.5% per year for chronic HCV infection, (6) highlighting the importance of effective treatment.
The standard measure of successful HCV treatment is sustained virological response (SVR). In non-HD patients, SVR is achieved in 50% to 60% of patients treated with pegylated-IFN and ribavirin combination therapy (7,8), and over 90% of patients who achieve SVR remain persistently HCV RNA negative after 3 to 5 years of follow-up (9–13). IFN-based treatment or SVR in non-HD patients are associated with improved liver histology scores (11,14), lower incidences of hepatocellular carcinoma (15,16), and lower mortality (16,17).
Because of reduced renal clearance, higher adverse event rates with IFN and ribavirin therapy make HCV treatment of patients with chronic kidney disease (CKD) challenging (18). In kidney transplant recipients, IFN-based treatment is contraindicated because of a 6% to 20% rate of allograft loss from acute rejection (19,20), and ribavirin monotherapy is ineffective in kidney recipients (21). Treatments for HD patients have primarily focused on IFN or pegylated-IFN monotherapy because ribavirin is contraindicated in HD due to high rates of hemolytic anemia except in research settings with careful monitoring of ribavirin plasma levels. Our recent meta-analysis in chronic HCV-infected HD patients found that 45% of IFN-treated and 37% of pegylated-IFN-treated patients achieved SVR (18,22).
It is unknown whether achieving SVR in HD patients results in similar long-term persistence of viral negativity to that observed in non-HD patients. Compromised immunity associated with end-stage renal disease might theoretically increase the risk of virological relapse. Whether relapse occurs in kidney transplant recipients who previously achieved SVR while on HD is particularly important for HD patients who are transplant candidates. In IFN and ribavirin-treated liver transplant recipients, SVR was persistent in 93% (23). We summarized individual patient data from IFN or pegylated-IFN treated HD patients with SVR to determine the long-term rates of viral negativity while on HD or after subsequent transplantation.
Materials and Methods
Patient Selection for Long-Term HCV RNA Analysis
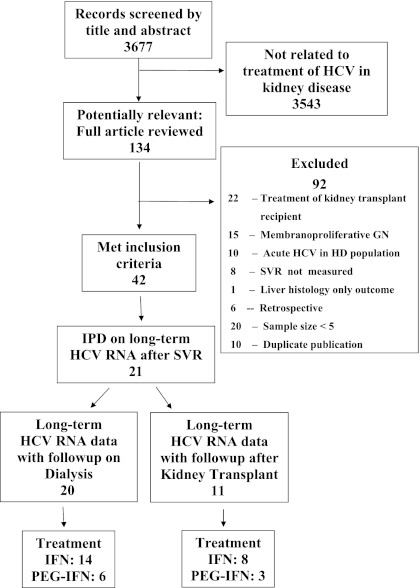
The literature search strategy and process of study selection for our systematic review have been described previously (18) and followed the protocol for meta-analysis of observational studies in epidemiology (MOOSE) (24). Briefly, we included prospective studies of HD patients with chronic HCV infection (documented by HCV RNA testing) who achieved SVR with IFN or pegylated-IFN treatment. Treatment studies of patients with CKD stages 1 to 5 not on HD and kidney transplant recipients (CKD stage 5-T) were excluded. Studies that did not assess for SVR were excluded, as were those with sample sizes less than 5. We performed a MEDLINE literature search on January 3, 2011 (Figure 1).
Figure 1.
Systematic review of the literature. GN, glomerulonephritis; HCV, hepatitis C virus; HD, hemodialysis; IFN, interferon; IPD, individual patient data; PEG-IFN, pegylated-IFN; SVR, sustained virological response.
Retrieved studies often reported patient-level data on long-term virological outcomes in patients who achieved SVR. Of 21 IFN treatment studies, 16 reported patient-level data on long-term virological outcomes following SVR (25–40), and of 21 pegylated-IFN studies, six studies provided such data (41–46). For unpublished data on long-term HCV RNA outcomes, we directly contacted authors. Patient-level data on long-term clinical outcomes, including mortality and graft loss, were unavailable.
Statistical Analysis
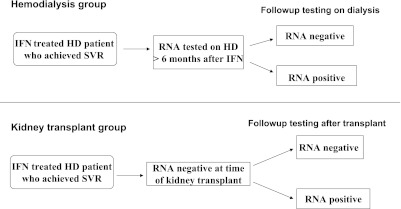
To explore the long-term persistence of HCV RNA negativity in patients who achieved SVR after IFN-based treatment while on HD, we performed life table analysis of patient-level data after accounting for censoring. Because of immunosuppressive agents used for kidney transplantation recipients, we analyzed HD patients and transplant recipients separately. The HD group comprised HD patients who had long-term HCV RNA data after SVR, with censoring at the time of kidney transplantation. The kidney transplantation group included patients achieving SVR while on HD who remained HCV RNA negative until kidney transplantation and had long-term HCV RNA values thereafter (Figure 2). In our analysis, follow-up began from the time SVR was achieved for the HD group, and from the time of transplantation for the kidney transplantation group. For inclusion, patients must have had both documented long-term HCV RNA results and specified duration of follow-up, but we did not require a minimum follow-up duration beyond SVR or transplantation for inclusion into either group.
Figure 2.
Persistence of HCV RNA negativity after SVR. HCV, hepatitis C virus; HD, hemodialysis; IFN, interferon; SVR, sustained virological response.
Based on our review of previous publications on long-term response in non-HD patients (9–13) and the follow-up duration observed in our data, we investigated the probability of remaining HCV RNA negative after 48 months of follow-up in the HD and transplant groups, with separate analyses for IFN, pegylated-IFN, and either treatment. We also examined the probability of HCV RNA negativity at last follow-up.
Results
Characteristics of Studies
We identified 16 studies of IFN and 6 of pegylated-IFN that met inclusion criteria and had long-term HCV RNA data following SVR. Twenty studies of 121 patients (25–37,40–46) and 11 studies of 45 patients (25,26,28,31,32,38–40,42,43,45) contributed patient-level data on long-term HCV RNA outcomes in patients who achieved SVR and were followed on HD or after kidney transplantation, respectively (Tables 1 and 2). Although, in most cases, long-term data were presented on all patients who achieved SVR, some studies only reported on a subset of those who achieved SVR. Long-term data were available for 32 patients from 9 studies, with both pre- and post-transplant status reported separately (25,26,28,31,32,40,42,43,45). These patients contributed data to both analyses, with the HD and transplant states examined separately. The majority of patients were treated with IFN, but pegylated-IFN was used in 25 patients followed on HD and seven patients who underwent kidney transplantation.
Table 1.
Characteristics of studies of interferon or pegylated-interferon treatment of chronic HCV-infected hemodialysis patients with long-term HCV RNA outcomes on hemodialysis
| Study, Country, Year | Overall Sample Size (n) | Number with SVR (n) | Number with Long-Term HCV RNA Outcomes (n) | Treatment Dosea | Treatment Duration (mo)b | Long-Term Follow-Up, Median (mo) |
|---|---|---|---|---|---|---|
| Studies with long-term HCV RNA outcomes with interferon | ||||||
| Pol, France, 1995 | 19 | 6 | 6 | 3 | 6 | 17 |
| Raptopoulou-Gigi, Greece, 1995 | 19 | 13 | 13 | 3 | 6 | 8 |
| Okuda, Japan, 1995 | 15 | 8 | 1 | 6 | 6 | 18 |
| Izopet, France, 1997 | 23 | 13 | 12 | 3 | 6 (n = 5) 12 (n = 7) |
28 |
| Fernandez, Argentina, 1997c | 14 | 3 | 3 | 1.5 | 6 | 12 |
| Chan, Hong Kong, 1997 | 11 | 3 | 3 | 3 | 6 | 18 |
| Campistol, Spain, 1999c | 19 | 11 | 11 | 3 | 6 | 20 |
| Huraib, Saudi Arabia, 1999 | 17 | 12 | 2 | 3 | 12 | 10 |
| Espinosa, Spain, 2001 | 13 | 6 | 6 | 3 | 12 | 27 |
| Hanrotel, France, 2001 | 12 | 4 | 2 | 3 | 12 | 24 |
| Kamar, France, 2003 | 55 | 21 | 16 | 3 | 6 (n = 3) 12 (n = 13) |
37 |
| Ozdemir, Turkey, 2004 | 20 | 8 | 8 | 6 (n = 5) 3 (n = 3) |
6 (n = 5) 12 (n = 3) |
64 |
| Mousa, Saudi Arabia, 2004 | 20 | 11 | 10 | 3 RBV (200 TIW) |
6 (n = 6) 12 (n = 4) |
6 |
| Buargub, Libya, 2006 | 35 | 9 | 4 | 3 | 12 | 12 |
| Studies with long-term HCV RNA outcomes with pegylated-interferon | ||||||
| Bruchfeld, Sweden, 2006 | 6 | 3 | 3 | 135 (α-2a), n = 2 50 (α-2b), n = 1 200–300 daily (RBV) |
6 (n = 1) 12 (n = 2) |
23 |
| Amarapurkar, India, 2007 | 6 | 3 | 3 | 1.0/kg (α-2b) | 6 | 12 |
| Ucmaky, Turkey, 2008 | 12 | 9 | 9 | 135 (α-2a) | 12 | 30 |
| Zoppoli, Italy, 2008 | 10 | 2 | 2 | 135 (α-2a) | 12 | 22 |
| Kose, Turkey, 2009 | 26 | 9 | 2 | 135 (α-2a) | 12 | 9 |
| Dzekova, Macedonia, 2009 | 14 | 5 | 5 | 135 (α-2a) | 12 | 18 |
| All hemodialysis | 366 | 159 | 121 | Variable | 6 (n = 60) 12 (n = 61) |
18 |
α-2a, pegylated-interferon alpha-2a; α-2b, pegylated-interferon alpha-2b; RBV, ribavirin; TIW, three times weekly.
Doses presented for interferon are in million units, three times weekly, and for pegylated interferon alpha-2a or 2b are in micrograms, once weekly. When studies used more than one treatment dose, the numbers reported are based on the group with long-term data.
Intended treatment duration in months. When studies used different durations, the numbers reported in parentheses are from the group with long-term data. More recent studies selected treatment duration based on HCV genotype with 12 months of treatment for genotypes 1 or 4 and 6 months for genotypes 2 or 3.
Randomized controlled trial comparing treated patients versus untreated controls. Sample size reported is the treatment group only.
Table 2.
Characteristics of studies of interferon or pegylated-interferon treatment of chronic HCV-infected hemodialysis patients with long-term HCV RNA outcomes after transplantation
| Study, Country, Year | Overall Sample Size (n) | Number with SVR (n) | Number with Long-Term HCV RNA Outcomes (n) | Treatment Dosea | Treatment Duration (mo)b | Long-Term Follow-Up, Median (mo) |
|---|---|---|---|---|---|---|
| Studies with long-term HCV RNA outcomes after interferon | ||||||
| Izopet, France, 1997 | 23 | 13 | 2 | 3 | ND | 21 |
| Campistol, Spain, 1999c | 19 | 11 | 3 | 3 | 6 | 20 |
| Huraib, Saudi Arabia, 1999 | 17 | 12 | 2 | 3 | 12 | 23 |
| Casanovas-Taltavull, Spain, 2001 | 29 | 18 | 9 | 3 | 12 | 50 |
| Huraib, Saudi Arabia, 2001c | 11 | 4 | 4 | 3 | 12 | 12 |
| Espinosa, Spain, 2001 | 13 | 6 | 1 | 3 | 12 | 27 |
| Kamar, France, 2003 | 55 | 21 | 16 | 3 | 6 (n = 3) 12 (n = 13) |
24 |
| Buargub, Libya, 2006 | 35 | 9 | 1 | 3 | 12 | 12 |
| Studies with long-term HCV RNA outcomes after pegylated-interferon | ||||||
| Bruchfeld, Sweden, 2006 | 6 | 3 | 2 | 135 (α-2a), n = 1 50 (α-2b), n = 1 200–300 daily (RBV) |
6 (n = 1) 12 (n = 1) |
5 |
| Amarapurkar, India, 2007 | 6 | 3 | 3 | 1.0/kg (α-2b) | 6 | 6 |
| Kose, Turkey, 2009 | 26 | 9 | 2 | 135 (α-2a) | 12 | 24 |
| All transplant | 240 | 109 | 45 | Variable | 6 (n = 10) 12 (n = 33) |
20 |
α-2a, pegylated-interferon alpha-2a; α-2b, pegylated-interferon alpha-2b; ND, not documented; RBV, ribavirin; TIW, three times weekly.
Doses presented for interferon are in million units, three times weekly, and for pegylated interferon alpha-2a or 2b are in micrograms, once weekly. When studies used more than one treatment dose, the numbers reported are based on the group with long-term data.
Intended treatment duration in months. When studies used different durations, the numbers reported in parentheses are from the group with long-term data. More recent studies selected treatment duration based on HCV genotype with 12 months treatment for genotypes 1 or 4 and 6 months for genotypes 2 or 3.
Randomized controlled trial comparing treated patients versus untreated controls. Sample size reported is the treatment group only.
The HD group had a mean age of 43 years and 51% were male. Before treatment, the mean duration of HD was 74 months and the mean duration of HCV infection was 56 months. Mean pretreatment HCV RNA was 172,300 IU/ml. The prevalence of HCV genotype 1 was 66%. IFN dose was 3 million units, three times weekly, in 91% of patients, with higher doses in 3% and lower doses in 6%. For studies of pegylated-IFN, 74% of patients received the alfa-2a formulation, with alfa-2b used in the remainder. Half of the patients were prescribed treatment duration of 6 months, with 12 months intended for the remainder.
In the kidney transplant group, mean age was 40 years and 58% were male. The mean pretreatment duration of HD and HCV were 45 and 65 months, respectively. Mean pretreatment HCV RNA was 342,400 IU/ml. The proportion with HCV genotype 1 was 65%. Treatment dose was 3 million units of standard IFN, three times weekly, or 135 μg of pegylated-IFN alfa-2a once weekly. The intended treatment duration was 6 months in 29%, with the remainder intended for 12 months.
Long-Term Persistence of HCV RNA Negativity
Hemodialysis Patients.
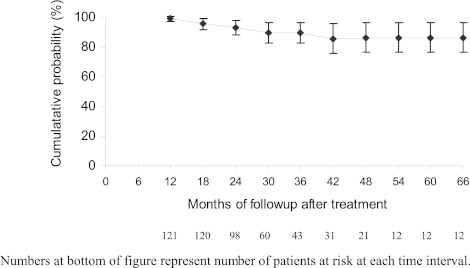
Long-term HCV RNA results were available for 121 HD patients from 20 studies (25–37,40–46) with median follow-up of 18 months (range 1 to 78 months) after achieving SVR (Table 1). Relapse to HCV RNA positivity during follow-up occurred in 10 of 121 (8%) including 6 of 97 (6%) receiving IFN and 4 of 24 (17%) receiving pegylated-IFN. By life table analysis, the probability of remaining HCV RNA negative 48 months after achieving SVR on HD in the overall group was 86% (95% confidence interval [CI], 77% to 96%; Figure 2), was 89% (95% CI, 78% to 98%) for IFN-treated patients, and was 79% (95% CI, 61% to 98%) for patients treated with pegylated-IFN. Those who were HCV RNA negative 48 months after SVR (n = 10) remained negative through last follow-up (62 to 78 months after SVR). An additional 22 patients from two studies (38,39) achieved SVR after IFN and remained HCV RNA negative on HD before undergoing kidney transplantation but were not analyzed because follow-up time on HD was not specified.
Kidney Transplantation Recipients.
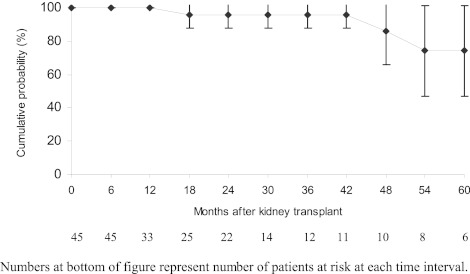
From 11 studies, 45 HD patients achieved SVR and remained HCV RNA negative until transplantation (Table 2) (25,26,28,31,32,38–40,42,43,45). Median follow-up after transplantation was 20 months (range 2 to 88 months). No patient underwent combined liver and kidney transplant. Out of 38 IFN-treated patients who were negative at the time of transplantation, three (8%) relapsed following transplantation but none of seven pegylated-IFN treated patients experienced a relapse with a 6-month median follow-up (range, 3 to 24 months). Transplantation patients had a 95% (95% CI, 89% to 100%) probability of remaining HCV RNA negative 48 months after transplantation but the probability decreased to 74% (95% CI, 47% to 100%) at 60 months (Figure 3). However, only 10 post transplant patients had HCV RNA data beyond 48 months, and two of these patients relapsed at 50 and 55 months after transplant, respectively.
Figure 3.
Probability of long-term viral negativity in patients followed on hemodialysis.
Discussion
The decision to treat HCV infection in HD patients is complex and requires weighing the potential benefits and risks of treatment. We previously demonstrated that IFN treatment in HD patients results in SVR rates of 45% (22), higher than the 10% to 20% SVR reported for IFN monotherapy in non-HD patients (47–49). However, IFN-treated HD patients have higher rates of treatment discontinuation due to adverse events compared with non-HD patients (18,47–49). Other factors considered in the decision of which HD patients to treat include an individual's comorbidities, life expectancy, transplantation candidacy, as well as the persistence of viral negativity in patients who achieve SVR.
This patient-level meta-analysis extends our understanding of the persistence of HCV RNA negativity in HD patients who achieve SVR. Among HD patients who achieved SVR, 86% remained HCV RNA negative after 48 months of follow-up on HD. Of those who achieved SVR and were HCV negative at the time of kidney transplantation, 95% remained HCV RNA negative 48 months after transplantation. These findings are similar to reports in non-HD patients, where over 90% of patients who achieve SVR remain persistently HCV RNA negative after approximately 4 years of follow-up (Table 3) (10,12,13). However, most studies of non-HD patients did not perform life table analysis and instead analyzed the raw proportion of patients who remained viral negative in long-term follow-up after achieving SVR. We used the more conservative life table analysis, which is the preferable manner to perform time-to-event analysis.
Table 3.
Comparison of previous studies of long-term viral response following interferon-based treatment
| Author, Year | Study Design | Treatment | Number with SVR | Follow-Up (mo)a | Long-Term Response Methodology | Long-Term Response (%) |
|---|---|---|---|---|---|---|
| Studies in nonhemodialysis population | ||||||
| Shindo, 1995 | Single center | IFN | 21 | 6 | Probability | 95 |
| Marcellin, 1997 | Single center | IFN | 75 | 42 (6–85) | Probability | 96 |
| Larghi, 1998 | Single center | IFN | 25 | 33 (15–74) | Probability | 92 |
| Lau, 1998 | Single center | IFN | 5 | NR (72–156) | Probability | 100 |
| Reichard, 1999 | 3 RCT | IFN | 26 | 59 (36–100) | Probability | 92 |
| Scvarcz, 1999 | Single center | IFN + RBV | 12 | 18 | Probability | 92 |
| Bruno, 2001 | 2 RCT | IFN | 36 | 78 | Probability | 100 |
| McHutchison, 2001b | 3 RCT | IFN + RBV (24) | 112 | 42 | Kaplan–Meier | 97 |
| IFN + RBV (48) | 151 | 42 | Kaplan–Meier | 99 | ||
| IFN | 73 | 42 | Probability | 96 | ||
| Veldt, 2004 | Meta-analysisc | IFN | 286 | 59 (12–120) | Kaplan–Meier | 95 |
| Tsuda, 2004 | Single center | IFN | 38 | 82 (53–144) | Probability | 100 |
| Dalgard, 2005 | 1 RCT | IFN +/− RBV | 27 | 59 (7–77) | Probability | 98 |
| Formann, 2006 | Single center | PEG-IFN | 187 | 29 (12–172) | Probability | 100 |
| Camma, 1999 | Meta-analysis | IFN | 453 | NR (18–93) | Pooled probability | 91 |
| Studies in transplant recipients | ||||||
| Bizollon, 2002 | Single center study of liver transplant recipients | IFN + RBV | 14 | 30 | Probability | 93 |
| Current study | ||||||
| Gordon | Hemodialysis | IFN or PEG-IFN | 121 | 18 (1–78) | Probability | 92 |
| Life table analysis | 86 (77–96) | |||||
| Gordon | Hemodialysis with subsequent kidney transplant | IFN or PEG-IFN | 45d | 20 (2–88) | Probability | 93 |
| Life table analysis | 95 (89–100) | |||||
IFN, interferon, NR, not reported; PEG-IFN, pegylated-interferon; RBV, ribavirin; RCT, randomized controlled trial.
Follow-up time reported is median, with range in parentheses, unless otherwise specified. Follow-up times reported are after sustained virologic response for consistency with our analysis. Original studies largely reported follow-up time from end of treatment.
Published as abstract in 2001 American Association for the Study of Liver Diseases meeting.
Meta-analysis of individual patient data from eight randomized controlled trials or prospective studies of interferon.
Thirty two patients had long-term HCV RNA data for both the HD and kidney transplant states. We analyzed these patients in both groups.
To compare our results to studies of non-HD patients, we determined the raw probability of long-term virological negativity for two groups, which was 92% and 93% for HD patients and kidney transplant recipients with median 18 and 20 months of follow-up, respectively. Adding the virological outcomes of the 22 excluded patients with long-term virological negativity before transplantation, but without specified follow-up time on HD (38,39), increased the probability of HCV RNA negativity in the HD group to 93%. These results in HD patients are comparable to those observed in non-HD patients (Table 3). After kidney transplantation, our results are similar to one study of IFN and ribavirin-treated liver transplant recipients receiving immunosuppressive agents, where the probability of long-term viral negativity was 93% at 30 months of follow-up (23).
Thus, the long-term virological response appears to be high both for patients who remain on HD and for those who undergo kidney transplantation. The late decrease in the probability of remaining HCV RNA negative observed beyond 60 months following transplantation may be attributable to having two relapses in the only 10 patients with this duration of follow-up, as supported by the wide confidence limits around the 60-month estimate. We would expect relapses after transplantation to be more common early after transplantation when immunosuppression is typically greatest.
One possible explanation for the observed late “relapses” involves primary reinfection with HCV, which has been described in HD patients due to breaches in infection control practices but can occur in any setting if patients use injection drugs or perform other risky behaviors. Molecular epidemiology techniques have confirmed reinfection with different HCV genotypes in successfully treated HD patients who appear to have a late virologic relapse (50). Future studies of long-term virological outcomes should determine HCV genotype and use molecular epidemiology techniques to ensure that “relapses” are not actually newly acquired infections.
This study has several limitations. Publication bias is always a concern with systematic reviews. We addressed this by searching for unpublished abstracts from recent nephrology and hepatology meetings, but we did not identify additional studies. Even among the retrieved studies, reporting bias may have occurred such that only studies with favorable results reported long-term outcomes, leading to a systematic overestimate of long-term virological outcomes following SVR. Additionally, selection bias may have occurred, particularly in the kidney transplantation group, such that only patients who remained viral negative were selected to undergo transplantation. Each of these limitations may have led us to overestimate the true long-term of viral negativity.
Missing data are a frequent problem for meta-analysis of patient-level data and may have affected our results; for example, we excluded 22 patients who had long-term HCV RNA negativity but did not have a specified follow-up time. In addition, few patients had follow-up durations beyond 48 months, thereby limiting the accuracy and precision of estimates at longer-term follow-up. However, this study's follow-up duration is similar to that reported in non-HD patients (10–12), and our choice of duration for analysis balanced the problem of overestimating the persistence of long-term viral negativity from using a shorter follow-up with the imprecision from using a smaller sample with longer follow-up. Ideally, we would have performed survival analysis with transplantation as a time-dependent covariate but were unable to because of missing data and the imprecision of the available data.
In the reviewed studies, clinical outcomes, such as mortality, were inconsistently reported, so we were unable to link the surrogate outcome, SVR, to clinical outcomes. In non-HD patients, IFN treatment and achieving SVR are associated with lower incidences of hepatocellular carcinoma (15,16) and mortality (16,17). However, the multiple competing risks for mortality among HD patients would diminish any mortality benefit of eradicating HCV infection. Future studies should investigate the clinical benefits of HCV treatment in HD patients.
Despite these limitations, this is the first systematic review to provide data on the long-term persistence of viral negativity in IFN and pegylated-IFN-treated HD patients who achieved SVR. More than 80% of HD patients who achieve SVR remain HCV RNA negative after 48 months of follow-up on dialysis. Long-term viral negativity was also observed in 95% of treated HD patients 48 months after kidney transplantation, an important finding for clinicians considering whether to treat HCV infection before transplantation. Future research should assess the clinical benefits of achieving SVR in HD patients and HCV RNA outcomes beyond 48 months after SVR to allow more precise long-term estimates of viral negativity. IFN-based treatment should be considered for HD patients who are good treatment candidates, particularly kidney transplant candidates, because SVR rates can be achieved in one third to one half of patients and, in most, viral negativity persists long term.
Figure 4.
Probability of long-term viral negativity for treated hemodialysis patients followed after kidney transplantation.
Disclosures
None.
Acknowledgments
We are indebted to the following individuals who provided individual patient data for this analysis that were not available in the original publication: Ali Akcay, M.D., Baskent University Faculty of Medicine, Ankara, Turkey; Mahdia Buargub, M.D., Tripoli Central Hospital, Tripoli, Libya; T M Chan, M.D., University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong; Jacques Chanard, M.D., Centre-Hospitalier et Universitaire, Reims, France; Jacques Izopet, M.D., and Florence Nicot, M.D., Hopital Purpan, Toulouse, France; Dujanaj Mousa, M.D. and Abdullah Al Waili, M.D., Riyadh Armed Forces Hospital, Riyadh Kingdom of Saudi Arabia; Maria Raptopolou-Gigi, M.D., University of Thessaloniki, Thessaloniki, Greece.
We are particularly grateful for the contributions of Dr. Joseph Lau to early portions of the analysis and writing of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. World Health Organization: Weekly Epidemiological Record. 49: December 10, 1999 [Google Scholar]
- 2. Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, Rayner HC, Greenwood RN, Akiba T, Young EW: Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int 65: 2335–2342, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjertson DW, Kopple JD, Greenland S: Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 18: 1584–1593, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G: Meta-analysis: Effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther 20: 1271–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G: Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant 5: 1452–1461, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Watanabe H, Saito T, Shinzawa H, Okumoto K, Hattori E, Adachi T, Takeda T, Sugahara K, Ito JI, Saito K, Togashi H, Suzuki R, Hayashi M, Miyamura T, Matsuura Y, Kawata S: Spontaneous elimination of serum hepatitis C virus (HCV) RNA in chronic HCV carriers: A population-based cohort study. J Med Virol 71: 56–61, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 358: 958–965, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347: 975–982, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Camma C, Giunta M, Pinzello G, Morabito A, Verderio P, Pagliaro L: Chronic hepatitis C and interferon alpha: Conventional and cumulative meta-analyses of randomized controlled trials. Am J Gastroenterol 94: 581–595, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Dalgard O: Follow-up studies of treatment for hepatitis C virus infection among injection drug users. Clin Infect Dis 40 [Suppl 5]: S336–S338, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, Kilani A, Areias J, Auperin A, Benhamou JP, Degott C, Erlinger S: Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med 127: 875–881, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Formann E, Steindl-Munda P, Hofer H, Jessner W, Bergholz U, Gurguta C, Ferenci P: Long-term follow-up of chronic hepatitis C patients with sustained virological response to various forms of interferon-based anti-viral therapy. Aliment Pharmacol Ther 23: 507–511, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Veldt BJ, Saracco G, Boyer N, Camma C, Bellobuono A, Hopf U, Castillo I, Weiland O, Nevens F, Hansen BE, Schalm SW: Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut 53: 1504–1508, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reichard O, Glaumann H, Fryden A, Norkrans G, Wejstal R, Weiland O: Long-term follow-up of chronic hepatitis C patients with sustained virological response to alpha-interferon. J Hepatol 30: 783–787, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, Nishiguchi S, Kuroki T, Imazeki F, Yokosuka O, Kinoyama S, Yamada G, Omata M: Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med 131: 174–181, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Bruno S, Battezzati PM, Bellati G, Manzin A, Maggioni M, Crosignani A, Borzio M, Solforosi L, Morabito A, Ideo G, Podda M: Long-term beneficial effects in sustained responders to interferon-alfa therapy for chronic hepatitis C. J Hepatol 34: 748–755, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Yoshida H, Arakawa Y, Sata M, Nishiguchi S, Yano M, Fujiyama S, Yamada G, Yokosuka O, Shiratori Y, Omata M: Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 123: 483–491, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB: Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: A systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis 51: 263–277, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Rostaing L, Modesto A, Baron E, Cisterne JM, Chabannier MH, Durand D: Acute renal failure in kidney transplant patients treated with interferon alpha 2b for chronic hepatitis C. Nephron 74: 512–516, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D: Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation 59: 1426–1431, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Kamar N, Sandres-Saune K, Selves J, Ribes D, Cointault O, Durand D, Izopet J, Rostaing L: Long-term ribavirin therapy in hepatitis C virus-positive renal transplant patients: effects on renal function and liver histology. Am J Kidney Dis 42: 184–192, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Gordon CE, Uhlig K, Lau J, Schmid CH, Levey AS, Wong JB: Interferon for hepatitis C virus in hemodialysis–an individual patient meta-analysis of factors associated with sustained virological response. Clin J Am Soc Nephrol 4: 1449–1458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bizollon T, Ahmed SN, Radenne S, Chevallier M, Chevallier P, Parvaz P, Guichard S, Ducerf C, Baulieux J, Zoulim F, Trepo C: Long term histological improvement and clearance of intrahepatic hepatitis C virus RNA following sustained response to interferon-ribavirin combination therapy in liver transplanted patients with hepatitis C virus recurrence. Gut 52: 283–287, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Buargub M, El Huni S, Tagdi M: Tolerance and efficacy of interferon-alpha in hemodialysis patients in Tripoli. Saudi J Kidney Dis Transpl 17: 338–343, 2006 [PubMed] [Google Scholar]
- 26. Campistol JM, Esforzado N, Martinez J, Rosello L, Veciana L, Modol J, Casellas J, Pons M, de Las Cuevas X, Piera J, Oliva JA, Costa J, Barrera JM, Bruguera M: Efficacy and tolerance of interferon-alpha(2b) in the treatment of chronic hepatitis C virus infection in haemodialysis patients. Pre- and post-renal transplantation assessment. Nephrol Dial Transplant 14: 2704–2709, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Chan TM, Wu PC, Lau JY, Lok AS, Lai CL, Cheng IK: Interferon treatment for hepatitis C virus infection in patients on haemodialysis. Nephrol Dial Transplant 12: 1414–1419, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Espinosa M, Rodriguez M, Martin-Malo A, Alvarez de Lara MA, Gonzalez R, Lopez-Rubio F, de la Mata M, Aljama P: Interferon therapy in hemodialysis patients with chronic hepatitis C virus infection induces a high rate of long-term sustained virological and biochemical response. Clin Nephrol 55: 220–226, 2001 [PubMed] [Google Scholar]
- 29. Fernandez JL, Rendo P, del Pino N, Viola L: A double-blind controlled trial of recombinant interferon-alpha 2b in haemodialysis patients with chronic hepatitis C virus infection and abnormal aminotransferase levels. Nephrologists' Group for the Study of HCV infection. Journal Viral Hepat 4: 113–119, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Hanrotel C, Toupance O, Lavaud S, Thiefin G, Brodard V, Ingrand D, Diebold MD, Wynckel A, Chanard J: Virological and histological responses to one year alpha-interferon-2a in hemodialyzed patients with chronic hepatitis C. Nephron 88: 120–126, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Huraib S, Tanimu D, Romeh SA, Quadri K, Al Ghamdi G, Iqbal A, Abdulla A: Interferon-alpha in chronic hepatitis C infection in dialysis patients. Am J Kidney Dis 34: 55–60, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Izopet J, Rostaing L, Moussion F, Alric L, Dubois M, That HT, Payen JL, Duffaut M, Durand D, Suc JM, Puel J: High rate of hepatitis C virus clearance in hemodialysis patients after interferon-alpha therapy. Journal Infec Dis 176: 1614–1617, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Mousa DH, Abdalla AH, Al-Shoail G, Al-Sulaiman MH, Al-Hawas FA, Al-Khader AA: Alpha-interferon with ribavirin in the treatment of hemodialysis patients with hepatitis C. Transplantation Proc 36: 1831–1834, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Okuda K, Hayashi H, Yokozeki K, Kondo T, Kashima T, Irie Y: Interferon treatment for chronic hepatitis C in haemodialysis patients: Suggestions based on a small series. J Gastroenterol Hepatol 10: 616–620, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Ozdemir FN, Akcay A, Sezer S, Boyacioglu S, Ozdemir BH, Arat Z, Haberal M: A six-year follow-up after interferon-alpha monotherapy for chronic hepatitis C infection in hemodialysis patients. Ren Fail 26: 583–588, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Pol S, Thiers V, Carnot F, Zins B, Romeo R, Berthelot P, Brechot C: Efficacy and tolerance of alpha-2b interferon therapy on HCV infection of hemodialyzed patients. Kidney Int 47: 1412–1418, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Raptopoulou-Gigi M, Spaia S, Garifallos A, Xenou P, Orphanou H, Zarafidou E, Petridou P, Vrettou H, Vagionas G, Galaktidou G, et al. : Interferon-alpha 2b treatment of chronic hepatitis C in haemodialysis patients. Nephrol Dial Transplant 10: 1834–1837, 1995 [PubMed] [Google Scholar]
- 38. Casanovas-Taltavull T, Baliellas C, Benasco C, Serrano TT, Casanova A, Perez JL, Guerrero L, Gonzalez MT, Andres E, Gil-Vernet S, Casais LA: Efficacy of interferon for chronic hepatitis C virus-related hepatitis in kidney transplant candidates on hemodialysis: results after transplantation. Am J Gastroenterol 96: 1170–1177, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Huraib S, Iqbal A, Tanimu D, Abdullah A: Sustained virological and histological response with pretransplant interferon therapy in renal transplant patients with chronic viral hepatitis C. Am J Nephrol 21: 435–440, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Kamar N, Toupance O, Buchler M, Sandres-Saune K, Izopet J, Durand D, Rostaing L: Evidence that clearance of hepatitis C virus RNA after alpha-interferon therapy in dialysis patients is sustained after renal transplantation. J Am Soc Nephrol 14: 2092–2098, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Ucmak H, Kokoglu OF, Hosoglu S, Dogan E, Sayarlioglu H, Kuzhan N, Isik IO: Long-term efficacy of pegylated interferon alpha-2a in HCV-positive hemodialysis patients. Ren Fail 30: 227–232, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Amarapurkar DN, Patel ND, Kirpalani AL: Monotherapy with peginterferon alpha-2b {12 kDa} for chronic hepatitis C infection in patients undergoing haemodialysis. Trop Gastroenterol 28: 16–18, 2007 [PubMed] [Google Scholar]
- 43. Bruchfeld A, Lindahl K, Reichard O, Carlsson T, Schvarcz R: Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat 13: 316–321, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Dzekova P, Asani A, Selim G, Gelev S, Trajceska L, Amitov V, Selja N, Zabzun M, Mena S, Gaseva M, Sikole A: Long-term follow up of sustained viral response after treatment of hepatitis C with pegylated interferon alpha-2a in hemodialysis patients. Int J Artif Organs 32: 180–184, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Kose S, Gurkan A, Akman F, Kelesoglu M, Uner U: Treatment of hepatitis C in hemodialysis patients using pegylated interferon alpha-2a in Turkey. J Gastroenterol 44: 353–358, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Zoppoli G, DiMaio G, Artioli S, Robaudo C, Basso M, Torre F, Cannella G, Picciotto A: Monotherapy with pegylated interferon alpha-2a in hemodialyzed patients with chronic hepatitis C. Dialysis and Transplantation 37: 204–208, 2008 [Google Scholar]
- 47. McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK: Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 339: 1485–1492, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J: Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352: 1426–1432, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Zeuzem S, Teuber G, Naumann U, Berg T, Raedle J, Hartmann S, Hopf U: Randomized, double-blind, placebo-controlled trial of interferon alfa2a with and without amantadine as initial treatment for chronic hepatitis C. Hepatology 32: 835–841, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arrais TC, Van Dooren S, Vandamme AM, Brechot C, Rimlinger F, Silva AE, Perez RM, Ferraz ML, Thiers V: Change in hepatitis C virus genotype in hemodialysis patients after end-of-treatment response to interferon monotherapy–relapse or re-infection? J Med Virol 80: 80–86, 2008 [DOI] [PubMed] [Google Scholar]