Abstract
Summary
Background and objectives
Travel distance to healthcare facilities affects healthcare access and utilization. Using the example of patients with kidney disease and nephrology services, we investigated the feasibility and utility of using geographic information system (GIS) techniques to identify the ideal location for new clinics to improve care for patients with kidney disease, on the basis of systematically minimizing travel time for remote dwellers.
Design, setting, participants, & measurements
Using a provincial laboratory database to identify patients with kidney disease and where they lived, we used GIS techniques of buffer and network analysis to determine ideal locations for up to four new nephrology clinics. Service-area polygons for different travel-time intervals were generated and used to determine the best locations for the four new facilities that would minimize the number of patients with kidney disease who were traveling >2 hours.
Results
We studied 31,452 adults with living in Alberta, Canada. Adding the four new facilities would increase the number of patients living <30 minutes from a clinic by 2.2% and reduce the number living >120 minutes away by 72.5%. Different two- and three-clinic scenarios reduced the number of people living >120 minutes away by as much as 65% or as little as 32%, emphasizing the importance of systematic evaluation.
Conclusions
GIS techniques are an attractive alternative to the current practice of arbitrarily locating new facilities on the basis of perceptions about patient demand. Optimal location of new clinical services to minimize travel time might facilitate better patient care.
Introduction
Residents of remote communities may experience increased morbidity and mortality because of chronic diseases such as cardiovascular disease, chronic kidney disease (CKD), and cancer (1–9). Increased travel time to receive specialty care for such illnesses is a potentially reversible determinant of adverse outcomes, and several studies have shown that increased distance between the patient's residence and the closest relevant medical specialist is associated with adverse outcomes (9–12). Even within a relatively small geographic area, shorter distances to healthcare services and availability of such services at the nearest center were strongly related to increased utilization of healthcare and better outcomes (12–20). Given that geography is a potentially reversible barrier to accessing healthcare services, careful planning to optimize the placement of future healthcare facilities with the goal of enhancing service and improving healthcare outcomes for remote dwellers is critical.
The last decade has witnessed increased use of geographic information systems (GIS) analysis for measuring access to health services and facilities, healthcare utilization, and relationships with healthcare outcomes (6,7,9,12,21–24). However, the use of GIS techniques to determine the optimal location of a healthcare facility with the goal of optimizing patient access is limited (12,18,20,25).
Given Canada's vast size and relatively sparse population, GIS techniques have the potential to inform decisions on location of healthcare facilities including planning for clinics to care for people with chronic diseases. This study utilizes GIS techniques to identify the ideal locations for up to four new nephrology clinics in Alberta, considered the maximum feasible number of new facilities given current resource constraints.
Study Population and Methods
Study Population
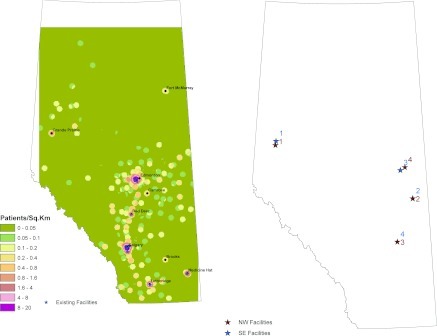
Patients with CKD were identified from the Alberta Kidney Disease Network database (26) using a cross-section of 31,452 adults who had chronic kidney disease on the basis of outpatient serum creatinine measurement between January 1, 2005, and December 31, 2005. Patients on renal replacement therapy at baseline were excluded from the analysis. The first outpatient serum creatinine measurement during this period was used to define baseline kidney function. For the purposes of this analysis and to ensure that that the identified patients had significant CKD, we defined a population of patients with estimated GFR (eGFR) of <45 ml/min per 1.73 m2 (as estimated from the Modification of Diet in Renal Disease prediction equation). Residence location of participants was on the basis of the residential postal code as recorded in the Alberta Health and Wellness (provincial health ministry) registry file during the calendar year corresponding to the first eGFR measurement. The locations of the 17 existing clinics were determined by obtaining their postal codes from the Northern and Southern Alberta Renal Programs (Figure 1A). These clinics were mapped on the basis of 2009 postal code locations.
Figure 1.
(A) The existing facilities and population density of patients with eGFR <45 ml/min per 1.73 m2. Red shading denotes higher density; green shading denotes lower density. (B) The proposed nephrology facilities and the effect of the starting the search for a new nephrologist facility in the southeast corner of Alberta instead of the northwest corner as in the primary analysis.
GIS Techniques
We used buffer analysis and network analysis to determine the ideal locations for new nephrology practices (clinics) that would provide services for diagnosing and managing patients with kidney disease. Buffer analysis involves creating a specified distance barrier around a given point or line and estimates the geographic variables (e.g., occurrence of postal codes) that occur within the barrier (27). Network analysis involves the computations of the distances of interest within a set road network (28) (such as calculating the distance or travel time between patients' residences and the closest healthcare facility). Travel times were calculated using posted speed limits. An origin-destination cost matrix was created using ESRI Network Analyst (www.esri.com) with patients' homes as origins and facilities as destinations (28).
Our specific objective was to determine the best locations for four new clinics by minimizing the number of patients traveling >120 minutes to see a nephrologist. We chose to focus on travel time because greater distance and travel time from the closest nephrologist have both been associated with worse outcomes in people with advanced CKD (29), but travel time is arguably more relevant to patients.
The patients' residence locations and the 17 existing nephrology clinics were mapped on the basis of their postal codes. Because postal codes correspond to a region rather than a discrete location, we mapped the residence location of participants using the single linkage indicator (SLI). The SLI is the geographic location within a postal code that is associated with the majority of dwellings (Figure 1A). Although not all residents of a given postal code will live at the precise coordinates of the SLI, the SLI represents the best single estimate for the residents of that postal code. To minimize misclassification resulting from use of SLI to classify residence location, we took two steps. First, we considered only valid postal codes to ensure that inaccuracies caused by outdated postal codes were not a concern. Second, we used broad categories of travel time to classify participants with respect to residence location (0 to 30, 30 to 60, 60 to 90, 90 to 120, and >120 minutes, all referring to the distance between each participant's home and the location of the closest nephrologist). These categories were selected to reduce the risk that small inaccuracies resulting from use of the SLI would lead to misclassification regarding expected travel time to the closest nephrologist.
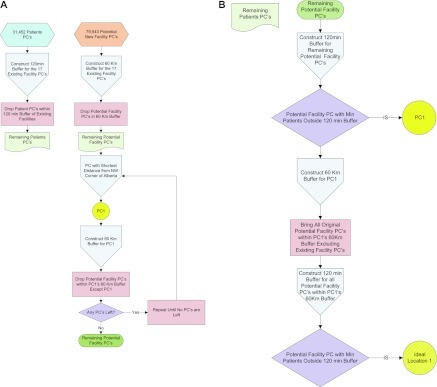
The algorithm used to determine this objective was developed specifically for this project and designed to reduce the number of calculations and thus computation time (Figure 2). It uses a two-step sampling process that is consistent with conventional GIS methodology. Starting in the northwest corner, we narrowed our search by cycling through adjacent 60-km buffers, temporarily eliminating all but one postal code in each buffer. Then after selecting the buffer containing the postal code that performed our objective best, we considered each of the previously eliminated postal codes within the selected buffer and reapplied our objective. Once a potential “best” location for a new clinic was identified, it was added to the existing list of potential clinic locations, and this procedure was repeated to determine the locations of additional clinics. The ideal locations for up to four new clinics are shown in Figure 1B. We then generated 16 scenarios (Table 1) representing the possible combinations of not adding any new clinics or adding one, two, three, or four new clinics at these four locations. In a sensitivity analysis, we tested the effect of starting in the southeast corner.
Figure 2.
Diagram showing the process of eliminating postal codes. PC, postal code.
Table 1.
Percentage of chronic kidney disease patients residing in different categories of travel time from the closest nephrologist for each scenario (n = 31,452)
| Scenario | New facilities included |
Travel Time from the Closest Nephrologist (min) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 0 to 30 | 30 to 60 | 60 to 90 | 90 to 120 | >120 | |
| A | Y | Y | Y | Y | 76.0 | 10.7 | 8.4 | 2.6 | 2.4 |
| B | Y | Y | Y | 75.9 | 9.9 | 8.5 | 2.7 | 3.0 | |
| C | Y | Y | Y | 76.0 | 10.3 | 7.7 | 3.0 | 3.0 | |
| D | Y | Y | Y | 75.5 | 9.5 | 8.5 | 3.4 | 3.0 | |
| E | Y | Y | Y | 74.9 | 10.3 | 8.0 | 1.6 | 5.2 | |
| F | Y | Y | 75.9 | 9.6 | 7.7 | 3.1 | 3.6 | ||
| G | Y | Y | 75.5 | 8.8 | 7.6 | 3.5 | 4.7 | ||
| H | Y | Y | 75.5 | 9.1 | 7.8 | 3.4 | 4.2 | ||
| I | Y | Y | 74.8 | 9.6 | 8.1 | 1.8 | 5.8 | ||
| J | Y | Y | 74.9 | 10.0 | 7.3 | 2.1 | 5.8 | ||
| K | Y | Y | 74.4 | 9.2 | 8.1 | 2.5 | 5.8 | ||
| L | Y | 75.5 | 8.4 | 6.9 | 3.5 | 5.8 | |||
| M | Y | 74.8 | 9.3 | 7.3 | 2.2 | 6.4 | |||
| N | Y | 74.3 | 8.5 | 7.2 | 2.6 | 7.5 | |||
| O | Y | 74.4 | 8.8 | 7.4 | 2.5 | 6.9 | |||
| P | 74.3 | 8.1 | 6.5 | 2.5 | 8.6 | ||||
Each scenario includes the possible combinations of zero, one, two, three, and four new clinics at the four selected locations. For example, scenario A includes four new clinics; scenarios B through E include three new clinics; scenarios F through K include two new clinics; scenarios L through O include one new clinic; and scenario P is the status quo (no new clinics).
Service-area polygons for each of the different travel-time intervals (0 to 30, 30 to 60, 60 to 90, 90 to 120, and >120 minutes) were generated for each of the scenarios. The number of patients residing in each of these service-area polygons was calculated and recorded as a percentage of patients for each travel-time category. This would determine the closest facility for each patient and also compute the individual patient travel time. Total travel times for all patients and by eGFR subgroups were calculated for each of the scenarios. Cartographic techniques were used to visualize the results.
Results
We studied a population-based sample of 31,452 patients with eGFR <45 ml/min per 1.73 m2, most of whom lived <30 minutes from a nephrology clinic (Table 1). Figure 1A indicates the locations of major cities and existing nephrology clinics in Alberta; 14 of the 17 existing clinics are located in Edmonton and Calgary. Figure 1A represents the density of CKD patients distributed across Alberta. Red shading is shown near Calgary, Edmonton, Lethbridge, and Medicine Hat, indicating a higher density of patients. Cities such as Grande Prairie, Red Deer, and Fort McMurray have a density of patients that is intermediate between rural areas and those of the four major cities.
We were able to identify geographic locations for all clinics and for 99.7% of patients within the existing road network. To examine the possibility that use of the SLI as the primary index of residence location would affect our results, we calculated the number of postal codes for which using an alternative (SLI = 0) location would lead to a shift of estimated residence location by >50 km (selected as a distance that was likely to lead to movement between travel-time categories). Of approximately 80,000 postal codes in Alberta, only 35 postal codes met this criterion, affecting a maximum of 531 participants (1.7%) in the worst case scenario.
Locations for the four proposed clinic locations as determined by the algorithm (Figure 2) are shown in Figure 1B. Under the status quo, 74.3%, 8.1%, 6.5%, 2.5%, and 8.6% of patients lived <30, 30 to 60, 60 to 120, and >120 minutes from the closest nephrology clinic, respectively. Adding the four proposed facilities would reduce the number of patients living >120 minutes away by 1955 (72.5%) and increase the number of patients living <30 minutes from a clinic by 520 (2.2%).
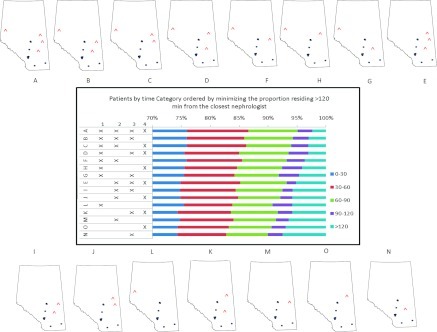
Figure 3 indicates the implications of adding new facilities for the percentage of patients in each travel time category. Adding three new clinics could reduce the number of patients living >120 minutes away by as much as 65% (scenario B) and as little as 40% (scenario E). Adding two new clinics could reduce the number of patients living >120 minutes away by as much as 57% (scenario F) and as little as 32% (scenario K). In fact, scenarios F and H (each with two new clinics) were better for reducing the number of patients living >120 minutes away than scenario E (three new clinics). Adding one new clinic could reduce the number of patients living >120 minutes away by as much as 32% (scenario L) and as little as 13% (scenario D); scenario L (one new clinic at location 1) was preferable to scenario K (two new clinics at locations 3 and 4).
Figure 3.
The implications of adding new facilities (denoted with ^) for the percentage of patients in each travel-time category. The bar graph in the center is rank ordered (from lowest to highest) by the percentage of patients who must travel more than 120 minutes to the practice location of the closest nephrologist. The locations of the proposed facilities are numbered 1 through 4. The different combinations of proposed facilities 1 through 4 are shown in the small maps labeled A through O. Scenario A (four new facilities) has the lowest percentage of patients in the >120 minutes category; scenario N (only one new facility at location 3) has the highest percent of patients in the >120 minutes category.
Figure 1B shows the effect of using the southeast corner of Alberta as a starting point rather than the northwest corner in the algorithm for location selection and indicates the alternative locations. The locations of the four clinics are similar for both starting points, although the rank ordering of location 3 and 4 is reversed when the alternative algorithm was used.
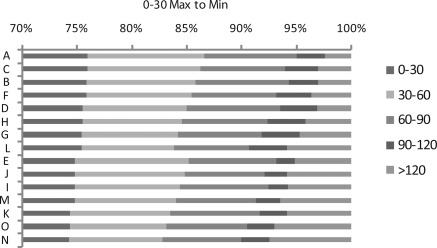
Our primary analysis ranked each scenario with the goal of minimizing the number of patients who must travel >120 minutes to reach the closest nephrology clinic. Figure 4 presents the results sorted to maximize the percentage of patients living within 30 minutes of travel time of the closest nephrologist (highest to lowest). The results were similar to those for the primary analysis: adding four new clinics was most effective; of the one- and two-clinic scenarios, scenarios L and F were again most effective. However, of the three-clinic solutions, scenario C was slightly more effective than scenario B. Thus, if funds were available for three new clinics, choosing between locations 3 and 4 would require a trade-off between alternative objectives (e.g., minimizing >120 minutes and maximizing <30 minutes). Location 3 serves fewer patients in the <30 minutes category and more patients in the >120 minutes category than location 4.
Figure 4.
Patients by time category ordered by maximizing the proportion residing <30 minutes from the closest nephrologist.
Overall, if resources were available only for one new facility, then location 1 (close to Grande Prairie) would be the best choice (assuming that the goal is to minimize the number of patients living >120 km from the closest nephrology clinic). Locations 2, 3, and 4 are far from the major cities but are close to major highways, ensuring that patients could access them easily.
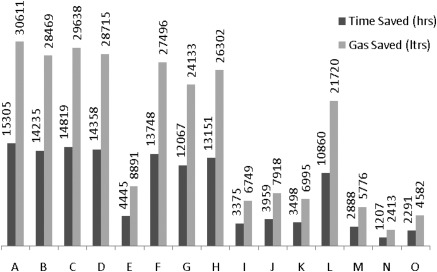
Figure 5 shows the total projected annual travel time for all CKD remote dwellers combined under each scenario, assuming that the patients with eGFR 30 to 45, 15 to 30, and 0 to 15 ml/min per 1.73 m2 visit a nephrologist twice, thrice, and four times a year, respectively. Scenarios A and C represent a substantial savings in travel time for the patients.
Figure 5.
Total time and gas saved for 2699 remotely dwelling patients by scenario.
Assuming gasoline consumption of 2 L/h, scenarios A and C would save approximately 15,305 and 14,819 L of gasoline/year for patients residing in remote areas of Alberta, respectively. Table 2 represents the total number of hours and liters of gas saved for each scenario.
Table 2.
Mean number of hours and liters of gas saved for 2699 remotely dwelling patients by scenario
| Scenario | Time Saved (hours/year) per Remote Dweller | Gasoline Saved (liters/year) per Remote Dweller | Time Saved (hours/year) by Clinic Added per Remote Dweller | Time Traveled (hours/year) per Remote Dweller |
|---|---|---|---|---|
| A | 5.7 | 11.3 | 1.4 | 1.5 |
| B | 5.3 | 10.5 | 1.8 | 1.7 |
| C | 5.5 | 11.0 | 1.8 | 1.6 |
| D | 5.3 | 10.6 | 1.8 | 1.7 |
| E | 1.6 | 3.3 | 0.5 | 3.3 |
| F | 5.1 | 10.2 | 2.5 | 1.8 |
| G | 4.5 | 8.9 | 2.2 | 2.0 |
| H | 4.9 | 9.7 | 2.4 | 1.9 |
| I | 1.3 | 2.5 | 0.6 | 3.5 |
| J | 1.5 | 2.9 | 0.7 | 3.4 |
| K | 1.3 | 2.6 | 0.6 | 3.5 |
| L | 4.0 | 8.0 | 4.0 | 2.3 |
| M | 1.1 | 2.1 | 1.1 | 3.6 |
| N | 0.4 | 0.9 | 0.4 | 3.9 |
| O | 0.8 | 1.7 | 0.8 | 3.7 |
Each scenario includes the possible combinations of zero, one, two, three, and four new clinics at the four selected locations. For example, scenario A includes four new clinics; scenarios B through E include three new clinics; scenarios F through K include two new clinics; scenarios L through O include one new clinic; and scenario P is the status quo (no new clinics).
Discussion
Our data show that it is feasible to use GIS techniques together with population-based clinical data to determine the optimal locations for new nephrology clinics. Our method was used to calculate the effect of selecting up to four new locations for conventional nephrology clinics, staffed by a full-time nephrologist and associated staff. However, similar methods could be used to select locations for a larger number of clinics or to choose the optimal locations served by mobile units that would serve multiple sites. Despite the established associations between limited access to healthcare facility locations and adverse outcomes from chronic diseases (14,18–20,25), there are surprisingly few data examining where to place new healthcare facilities to improve access and healthcare delivery. Previous studies have focused on locating facilities to serve the maximum number of patients rather than minimizing travel time for remote dwellers (17,30).
The province of Alberta, Canada, has a geographic area of 660,000 km2 but only 17 nephrology clinics, which are chiefly located in major cities. Although most CKD patients with eGFR <45 ml/min per 1.73 m2 live within 30 minutes (by road) of the closest clinic, a substantial proportion live >2 hours away, which may affect their ability to access healthcare. This is important because recent studies have documented higher mortality among patients with stage 3 to 5 CKD living farther from the closest nephrologist (5). We offer these GIS techniques as an alternative to the current practice of arbitrarily locating new facilities on the basis of perceptions about patient demand. These findings may be applicable when planning the locations for future healthcare services for other chronic diseases requiring regular medical follow-up such as diabetes, cardiovascular disease, cancer, and chronic respiratory illnesses (13–18,20,25).
In our example of the province of Alberta, the next nephrology clinic should ideally be located in Grande Prairie (approximate population 109,000) (31), which is located approximately 400 km from the nearest existing clinic. As expected, adding additional clinics continued to reduce the number of people living >120 minutes from the nearest clinic, but the incremental absolute reduction in this number declined as the number of new clinics increased. For example, the best one-clinic scenario reduced the number of people living >120 minutes by 876, whereas the best two-, three-, and four-clinic scenarios reduced this number by 1557, 1759, and 1955, respectively. In addition to Grande Prairie, our findings identify three other locations where future clinics could be purposefully located to reduce the number of people living >120 minutes from the nearest clinic. Options for staffing the new clinics would include an on-site nephrologist, off-site nephrologists with the aid of Telehealth, or nurse practitioners with telephone support from off-site nephrologists. Of these, the first would likely be the most acceptable to patients, although the latter two could be explored if difficulties were encountered in recruiting a nephrologist or nephrologists to these areas.
In addition to these Alberta-specific findings, our results have three important lessons for others to consider. First, this method is computationally intensive (the results presented herein required approximately 240 hours of processing time on a modern desktop computer). Our algorithm ultimately selected four locations from the 80,000 potential locations across Alberta. Although processing time was reduced by initially creating 60-km buffers (and excluding postal codes within these buffers from analyses) and excluding patients already served within 120-minute service-area polygons, this requirement should be borne in mind when considering GIS methods. Second, selecting the ideal locations for new clinics requires a criterion standard for “adequate” access to care. In our primary analysis, we attempted to minimize the number of patients who lived >120 minutes from the closest clinic. The results were generally similar when maximizing the number who lived <30 minutes from the closest clinic. However, the optimal three-clinic scenario was slightly different for the <30-minute objective than for the >120-minute objective. Future work should attempt to identify a more objective basis for which criterion should be used, perhaps on the basis of the preferences of patients, healthcare planners, or society in general. Third, our method assumed that new facilities would be located in the geographic area with the majority of dwellings of each postal code. Although minimally affecting our estimates of travel time, this assumption means that additional work will be required to determine the optimal location of new facilities within each postal code. Address geocoding and use of a local “points of interest” (such as shopping malls or hospitals) database are important potential tools that could be used to identify specific locations for future clinics within each postal code.
Our study also has some limitations that should be considered. Some patient residences could not be located on the road network while calculating the travel times and thus were excluded from analysis. However, because this only affected a small percentage of the study population (<0.3%), this should not have had a major effect on our results. Similarly, the road network does not include dirt or unpaved roads. This might have affected our estimates of travel time (especially for remote-dwelling patients), but the effect of this omission on our results should be very small, because approximately 88% of the Alberta roads are paved (32). As mentioned, we used the SLI to estimate the residence location of participants. However, our use of broad distance categories and the small number of postal codes for which misclassification might have occurred suggests that this is unlikely to have affected our results. Another potential limitation is that our method requires selection of an alternative starting point for computation (our primary analysis used the northwest corner of Alberta). However, results were similar in a sensitivity analysis using an alternative starting point, suggesting that this arbitrary decision did not affect our findings. We did not consider the potential effect of public transport on our findings, but public transport is not widely available outside urban areas of Alberta, making it unlikely that more detailed analyses incorporating this consideration would have affected our conclusions. Finally, we assumed that clinics would be fixed (stationary). However, our data would also permit consideration of mobile clinics that might serve several areas. Future studies should use GIS techniques to identify potential areas for mobile clinics that will minimize the travel time for remote-dwelling patients.
Using GIS techniques in conjunction with population-based clinical data to systematically identify potential locations for new CKD clinics (with the objective of minimizing travel time for patients) represents a significant advance over existing methods. Although we did not compare our technique against educated guesses made by knowledgeable observers, the use of GIS techniques is reproducible and objective, potential advantages compared with arbitrary clinic selection (no matter how well informed), which leaves decision-makers potentially vulnerable to criticism from jurisdictions who were not selected to receive a new clinic. Our systematic approach would save considerable travel time for patients, facilitating their access to renal services and (because distance from the closest nephrologist is associated with reduced likelihood of receiving good quality care and with excess mortality), perhaps improving their clinical outcomes. Because prolonged travel time is a major barrier to adequate healthcare, constructing new clinics with the explicit aim of minimizing travel time could not only improve patient outcomes but also might save substantial quantities of fossil fuels, thus reducing environmental pollution. These techniques could be used to improve healthcare planning for other chronic diseases.
Disclosures
None.
Acknowledgments
This work was funded by an operating grant from the Heart and Stroke Foundation of Canada and by an interdisciplinary team grant from the Alberta Heritage Foundation for Medical Research.
Drs. Hemmelgarn, Klarenbach, Manns, and Tonelli were supported by career salary awards from the Alberta Heritage Foundation for Medical Research. Dr. Tonelli was also supported by a Government of Canada Research Chair. Drs. Hemmelgarn, Klarenbach, Manns, and Tonelli were all supported by a joint initiative between Alberta Health and Wellness and the Universities of Alberta and Calgary.
The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. None of the authors have any relevant competing interests to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Call KT, Casey MM, Radcliff T: Rural beneficiaries with chronic conditions: Does prevalence pose a risk to Medicare managed care? Manag Care Q 8: 48–57, 2000 [PubMed] [Google Scholar]
- 2. Casey MM, Thiede Call K, Klingner JM: Are rural residents less likely to obtain recommended preventive healthcare services? Am J Prev Med 21: 182–188, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Dansky KH, Dirani R: The use of health care services by people with diabetes in rural areas. J Rural Health 14: 129–137, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Keppel KG, Pearcy JN, Klein RJ: Measuring progress in healthy people 2010. Healthy People 2010 Stat Notes 1–16, 2004 [PubMed] [Google Scholar]
- 5. Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, James MT, Bello A, Gordon D, Jindal KK, Tonelli M: Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int. 2011. January; 79 (2): 210–217 [DOI] [PubMed] [Google Scholar]
- 6. Tonelli M, Hemmelgarn B, Kim AK, Bertazzon S, Klarenbach S, Manns B, Wiebe N, Culleton B, Gill JS: Association between residence location and likelihood of kidney transplantation in Aboriginal patients treated with dialysis in Canada. Kidney Int 70: 924–930, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Virnig BA, Moscovice IS, Durham SB, Casey MM: Do rural elders have limited access to Medicare hospice services? J Am Geriatr Soc 52: 731–735, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bringing cancer care closer to home. Available at: http://www.albertahealthservices.ca/2835.asp Accessed November 9, 2010
- 9. Pong RW: Rural health research in Canada: At the crossroads. Aust J Rural Health 8: 261–265, 2000 [PubMed] [Google Scholar]
- 10. Higgs G: Integrating multi-criteria techniques with geographical information systems in waste facility location to enhance public participation. Waste Manag Res 24: 105–117, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Tempest V, Higgs G, McDonald K, Iredale R, Bater T, Gray J: A pilot study of spatial patterns in referrals to a multicentre cancer genetics service. Community Genet 8: 73–79, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Higgs G, Richards W: The use of geographical information systems in examining variations in sociodemographic profiles of dental practice catchments: A case study of a Swansea practice. Prim Dent Care 9: 63–69, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Yantzi N, Rosenberg MW, Burke SO, Harrison MB: The impacts of distance to hospital on families with a child with a chronic condition. Soc Sci Med 52: 1777–1791, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Wardman D, Clement K, Quantz D: Access and utilization of health services by British Columbia's rural Aboriginal population. Int J Health Care Qual Assur Inc Leadersh Health Serv 18: xxvi–xxxi, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Thomas DP, Anderson IP, Kelaher MA: Accessibility and quality of care received in emergency departments by Aboriginal and Torres Strait Islander people. Aust Health Rev 32: 648–654, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Prakash S, Austin PC, Oliver MJ, Garg AX, Blake PG, Hux JE: Regional effects of satellite haemodialysis units on renal replacement therapy in non-urban Ontario, Canada. Nephrol Dial Transplant 22: 2297–2303, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Patel AB, Waters NM, Ghali WA: Determining geographic areas and populations with timely access to cardiac catheterization facilities for acute myocardial infarction care in Alberta, Canada. Int J Health Geogr 6: 47, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guagliardo MF: Spatial accessibility of primary care: Concepts, methods and challenges. Int J Health Geogr 3: 3, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodridge D, Lawson J, Rennie D, Marciniuk D: Rural/urban differences in health care utilization and place of death for persons with respiratory illness in the last year of life. Rural Remote Health 10: 1349, 2010 [PubMed] [Google Scholar]
- 20. Goodridge D, Hutchinson S, Wilson D, Ross C: Living in a rural area with advanced chronic respiratory illness: A qualitative study. Prim Care Respir J 2011. March; 20 (1): 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jong KE, Smith DP, Yu XQ, O'Connell DL, Goldstein D, Armstrong BK: Remoteness of residence and survival from cancer in New South Wales. Med J Aust 180: 618–622, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jiwa M, Halkett G, Aoun S, Arnet H, Smith M, Pilkington M, McMullen C: Factors influencing the speed of cancer diagnosis in rural Western Australia: A general practice perspective. BMC Fam Pract 8: 27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jong KE, Vale PJ, Armstrong BK: Rural inequalities in cancer care and outcome. Med J Aust 182: 13–14, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Tompkins JW, Luginaah IN, Booth GL, Harris SB: The geography of diabetes in London, Canada: The need for local level policy for prevention and management. Int J Environ Res Public Health 7: 2407–2422, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gregory PM, Malka ES, Kostis JB, Wilson AC, Arora JK, Rhoads GG: Impact of geographic proximity to cardiac revascularization services on service utilization. Med Care 38: 45–57, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones C, McCoy J: Geoprocessing in ArcGIS tutorial. Available at: http://webhelp.esri.com/arcgisdesktop/9.3/pdf/Geoprocessing_in_ArcGIS_Tutorial.pdf Accessed January 6, 2011
- 28. Sandhu J, Chandrasekhar T: ArcGIS network analyst tutorial. Available at: http://proteus.brown.edu/EarthlabGIS/admin/download.html?attachid=5802507 Accessed January 6, 2011
- 29. Tonelli M, Manns B, Culleton B, Klarenbach S, Hemmelgarn B, Wiebe N, Gill JS: Association between proximity to the attending nephrologist and mortality among patients receiving hemodialysis. CMAJ 177: 1039–1044, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horner MW, Mascarenhas AK: Analyzing location-based accessibility to dental services: An Ohio case study. J Public Health Dent 67: 113–118, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Government of Alberta: Alberta population projections. Available at: http://www.finance.alberta.ca/aboutalberta/population_reports/2010–2050-alberta-population-projections.pdf Accessed January 6, 2011
- 32. Government of Alberta: Transportation. Available at: http://www.transportation.alberta.ca/Content/docType437/Production/StatsandFacts.pdf Accessed January 14, 2011