Abstract
Summary
Background and objectives
Soluble CD40 ligand (sCD40L) is a marker of platelet activation; whether platelet activation occurs in the setting of renal artery stenosis and stenting is unknown. Additionally, the effect of embolic protection devices and glycoprotein IIb/IIIa inhibitors on platelet activation during renal artery intervention is unknown.
Design, setting, participants, & measurements
Plasma levels of sCD40L were measured in healthy controls, patients with atherosclerosis without renal stenosis, and patients with renal artery stenosis before, immediately after, and 24 hours after renal artery stenting.
Results
Soluble CD40L levels were higher in renal artery stenosis patients than normal controls (347.5 ± 27.0 versus 65.2 ± 1.4 pg/ml, P < 0.001), but were similar to patients with atherosclerosis without renal artery stenosis. Platelet-rich emboli were captured in 26% (9 of 35) of embolic protection device patients, and in these patients sCD40L was elevated before the procedure. Embolic protection device use was associated with a nonsignificant increase in sCD40L, whereas sCD40L declined with abciximab after the procedure (324.9 ± 42.5 versus 188.7 ± 31.0 pg/ml, P = 0.003) and at 24 hours.
Conclusions
Atherosclerotic renal artery stenosis is associated with platelet activation, but this appears to be related to atherosclerosis, not renal artery stenosis specifically. Embolization of platelet-rich thrombi is common in renal artery stenting and is inhibited with abciximab.
Introduction
Platelet activation leading to thrombus formation is a well described complication of coronary artery disease, yet its occurrence in renal artery stenosis (RAS) is unknown (1–4). RAS is a major cause of secondary hypertension and an important cause of renal failure (1–3,5,6). Although the utility of stent revascularization in patients with RAS is uncertain, several studies suggest that at least a portion of patients develop a loss of kidney function after the procedure (1–3,6,7).
Soluble CD40 ligand (sCD40L) is expressed and secreted by platelets after activation and plays a vital role in the immune, inflammatory, and coagulative responses after injury or stress, and in the setting of transplantation has been linked to renal fibrosis (8–15). Moreover, high levels of sCD40L correlate with cardiovascular events in patients with unstable coronary syndromes (13,16–18). Glycoprotein (GP) IIb/IIIa inhibitors may lower the level of platelet activation in vitro and the level of sCD40L released from platelets upon activation (19,20). A recent report from our group has demonstrated that the use of a GPIIb/IIIa inhibitor in combination with an embolic protection device (EPD) during renal artery stenting may improve renal function after the revascularization procedure (21). However the relationship between platelet activation and patient outcome after renal artery stenting is uncertain.
On this background, the goals of the present study were to determine (1) if platelet activation is associated with atherosclerotic RAS, (2) whether platelet activation occurs during renal artery stenting, and (3) if platelet thrombus formation captured by the EPD correlates with systemic platelet activation.
Materials and Methods
The study, ClinicalTrials.gov identifier NCT00234585, was conducted with funding provided by the sponsors, but study conduct, analysis, and reporting were performed independent of the sponsors. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use good clinical practice guidelines were followed with patients providing informed consent in an institutional review board–approved protocol.
Platelet activation levels from the RAS patients were compared with 30 healthy controls and with 30 patients with atherosclerosis undergoing coronary angiography, but free of RAS. A total of 100 RAS patients were recruited from seven sites. Inclusion required a history of hypertension, renal insufficiency, heart failure, or angina with poorly controlled hypertension and also the presence of one or more stenoses, ≥50% and <100, treatable with the EPD. RAS patients were randomized to the following allocations: one-half to Angioguard (Cordis Corp.), one-half to no Angioguard; one-half to abciximab, and one-half to placebo infusion, yielding four groups: control, Angioguard only, abciximab only, and Angioguard with abciximab.
Preprocedural Care
In patients with RAS before double-blinded administration of abciximab or placebo, systolic BP was lowered to ≤160 mmHg. The target activated clotting time was 275 seconds, and if the patient was randomized to the EPD device, an activated clotting time of >300 seconds was required. A bolus of 0.25 mg/kg abciximab (or placebo) was administered 5 minutes before crossing the lesion and was followed by an infusion at 0.125 μg/kg per minute (maximum 10 μg/min) for 12 hours.
Central Laboratory Analysis
The blinded analysis of EPD contents was performed by the CV Path core lab (Gaithersburg, MD). Platelet emboli consisted of layered platelet aggregates with varying amounts of entrapped leukocytes and fibrin as evidence on hematoxylin and eosin–stained sections (22). GFR, calculated from the modified MDRD equation (23), was used as the primary measure of renal function. Creatinine was measured by a modified Jaffe reaction using the isotope dilution mass spectrometry–traceable assay at the University of Minnesota Core Lab for all subjects.
Blood Collection
Peripheral venous blood was collected at baseline, immediately after, and 24 hours after the procedure in lithium heparin plasma separator tubes; spun at 1000 × g for 15 minutes; and frozen at −80°C until batch analysis.
Measurement of Soluble CD40 Ligand
Plasma levels of sCD40L were measured by ELISA (R&D Systems; Minneapolis, Minnesota). The ELISA kit had intra-assay and interassay coefficients of 5% and 6%, respectively. The average minimum detectible amount of sCD40L was 4.2 pg/ml.
Statistical Analysis
Study data are presented as continuous (mean ± SEM) and categorical data. Statistical analysis was performed on subjects with complete data for platelet activation measurements at the baseline, immediate after, and 24 hours after the procedure time points (n = 84). SAS one-way ANOVA was used to test for significance among groups. Paired t tests and Fisher protected least significant difference post hoc tests were used to test for significance between groups. Unpaired t tests were used to test for significance between the normal subjects, patient controls, and the RAS patients. Significance was defined as P < 0.05. All analyses were performed in SAS or JMP.
Results
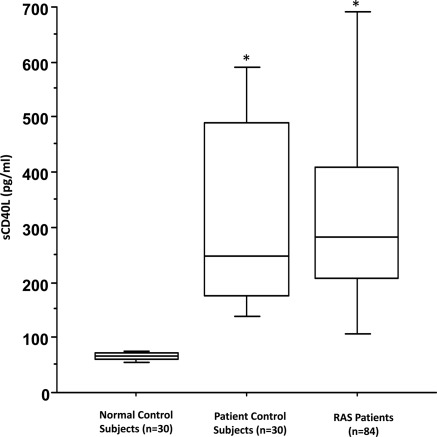
Baseline characteristics of the normal controls (n = 30), atherosclerotic controls (n = 30), and the RAS patients (n = 84) are shown in Table 1. The RAS patients had a significantly higher level of sCD40L compared with normal controls (347.5 ± 27.1 versus 65.2 ± 1.4 pg/ml, P < 0.001) (Figure 1). However, sCD40L levels were similar when compared with the patients with atherosclerosis who were free of renal artery stenosis (347.5 ± 27.1 versus 335.2 ± 38.6 pg/ml, P = 0.79) (Figure 1). Soluble CD40L, either at baseline or after the stenting, was not associated with baseline GFR or subsequent changes in kidney function.
Table 1.
Baseline characteristics of the normal controls, patient controls, and the renal artery stenosis patients
| Patients |
P-Valuea | P-Valueb | |||
|---|---|---|---|---|---|
| Normal (n = 30) | Control (n = 30) | RAS (n = 84) | |||
| Patient characteristics | |||||
| age (years) ± SD | 35 ± 12 | 63 ± 12 | 73 ± 9 | <0.001 | <0.01 |
| female, n (%) | 16 (53%) | 17 (57%) | 48 (57%) | 0.72 | 0.96 |
| white, non-Hispanic, n (%) | 23 (77%) | 26 (87%) | 77 (92%) | <0.001 | <0.01 |
| BMI | 25.9 ± 3.9 | 31.1 ± 8.7 | 28.6 ± 5.5 | 0.02 | 0.08 |
| systolic BP (mmHg) | 125 ± 2 | 126 ± 16 | 157 ± 31 | <0.001 | <0.01 |
| diastolic BP (mmHg) | 83 ± 3 | 78 ± 10 | 72 ± 16 | <0.001 | 0.13 |
| heart rate | 74 ± 3 | 65 ± 11 | 66 ± 12 | <0.001 | 0.96 |
| Laboratory values | |||||
| serum creatinine (mg/dl) | 0.91 ± 0.10 | 0.85 ± 0.24 | 1.14 ± 0.41 | 0.003 | <0.01 |
| sCD40L (pg/ml) | 65.2 ± 7.4 | 335.2 ± 211.6 | 347.5 ± 248.3 | <0.001 | 0.81 |
| platelet count (×1000) | 298 ± 27 | 236 ± 54 | 239 ± 73 | <0.001 | 0.84 |
| Indications for treatment, n (%) | |||||
| hypertension | NA | 24 (80%) | 84 (100%) | NA | <0.01 |
| congestive heart failure | NA | 1 (3%) | 20 (24%) | NA | 0.01 |
| renal dysfunction | NA | 0 (0%) | 18 (21%) | NA | 0.06 |
| angina | NA | 21 (70%) | 31 (37%) | NA | 0.02 |
| Risk factors, n (%) | |||||
| diabetes mellitus | NA | 7 (23%) | 21 (25%) | NA | 0.86 |
| peripheral vascular disease | NA | 1 (3%) | 43 (51%) | NA | <0.01 |
| hyperlipidemia | NA | 22 (73%) | 71 (85%) | NA | 0.17 |
| coronary artery disease | NA | 13 (43%) | 55 (65%) | NA | 0.03 |
| previous MI | NA | 5 (17%) | 26 (31%) | NA | 0.13 |
| history of smoking | NA | 17 (57%) | 53 (63%) | NA | 0.53 |
| Medications, n (%) | |||||
| antiplatelet | |||||
| aspirin | NA | 20 (67%) | 71 (85%) | NA | 0.04 |
| thienopyridines | NA | 9 (30%) | 39 (46%) | NA | 0.12 |
| warfarin | NA | 2 (7%) | 11 (13%) | NA | 0.34 |
Values are mean ± SD or number and percentage of patients. BMI, body mass index; sCD40L, soluble CD40 ligand; MI, myocardial infarction; NA, not applicable.
Normal versus RAS.
Patient control versus RAS.
Figure 1.
Soluble CD40L (sCD40L) levels in normal controls, patient controls, and renal artery stenosis (RAS) patients. Box plot represents interquartile range with the median value shown as a horizontal bar within each box. Minimum and maximum values are shown in the bars outside each box. *P < 0.001 versus normal control subjects.
EPD Content, Platelet Embolization, and sCD40L
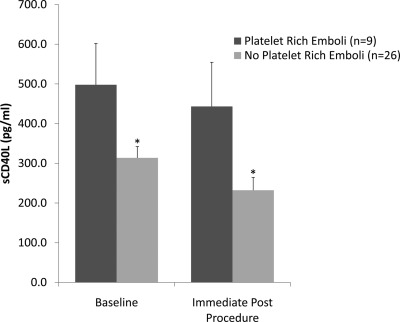
Twenty-six percent (nine of 35) of the patients who received the Angioguard had platelet-rich emboli captured within the filter. In these patients with platelet-rich emboli, sCD40L levels were higher than in patients without platelet emboli both before the procedure (497.9 ± 105.0 versus 313.7 ± 28.4 pg/ml, P = 0.02) and after the procedure (443.3 ± 111.3 versus 232.2 ± 32.4 pg/ml, P = 0.02) (Figure 2).
Figure 2.
Soluble CD40L (sCD40L) levels in patients with platelet-rich emboli captured within the filter immediately after the procedure. Analysis of Angioguard contents was performed in 35 of 39 (90%) patients randomized to Angioguard. Nine of 35 patients (26%) had platelet-rich emboli captured. Data are presented as mean ± SEM, *P = 0.02 versus platelet-rich emboli.
Effect of Distal Protection and Drug Treatment
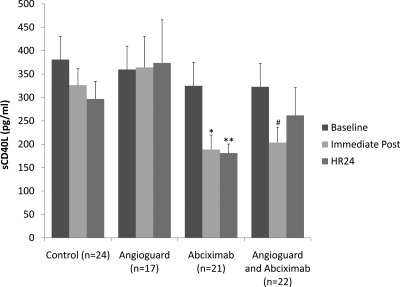
Patients with RAS randomized to abciximab had a significant decrease in sCD40L levels immediately after the procedure (324.9 ± 42.5 versus 188.7 ± 31.0 pg/ml, P = 0.003), which persisted at 24 hours (324.9 ± 42.5 versus 181.2 ± 19.3 pg/ml, P = 0.002) (Figure 3). In patients randomized to the Angioguard, sCD40L levels rose slightly immediately after the procedure and at 24 hours (P = 0.90) (Figure 3). Patients randomized to both the Angioguard device and abciximab showed a significant decrease in sCD40L immediately after the procedure (322.8 ± 35.2 versus 203.6 ± 33.1 pg/ml, P = 0.03), but this difference was no longer significant at 24 hours (Figure 3).
Figure 3.
Soluble CD40L (sCD40L) in patients with atherosclerotic renal artery stenosis randomly assigned to either abciximab or Angioguard embolic protection, both, or neither. Data presented as mean ± SEM, *P = 0.003 versus baseline, **P = 0.002 versus baseline, #P = 0.03 versus baseline.
Effect of Abciximab and Clopidogrel
Clopiogrel use was not associated with lower sCD40L at baseline. For patients on clopidogrel before intervention, sCD40L levels rose slightly immediately after the procedure and decreased at 24 hours (P = 0.53). For patients randomized to Abciximab and not taking clopidogrel, sCD40L levels decreased significantly immediately after the procedure (310.5 ± 33.0 versus 195.2 ± 31.3 pg/ml, P = 0.008), which persisted at 24 hours (310.5 ± 33.0 versus 173.1 ± 18.7 pg/ml, P < 0.001. Patients on clopidogrel and randomized to Abciximab showed a significant decrease in sCD40L immediately after the procedure (346.3 ± 48.1 versus 198.3 ± 30.6 pg/ml, P = 0.02). Similar effects were seen in patients who were prescribed clopidogrel on the day of procedure.
Discussion
Platelet activation is a major cause of events and complications in coronary artery disease and with coronary revascularization (24,25). The use of platelet inhibitors during coronary stenting reduces the potentially harmful effects of platelet activation including abrupt vessel occlusion, myocardial infarction, and stent thrombosis (25). To date, the extent of platelet activation and the effect of antiplatelet therapies in the setting of renal artery stenting has not been established. Thus, in the current study we sought to determine whether atherosclerotic renal artery stenosis was associated with platelet activation and the effect(s) of embolic protection and or use of platelet inhibitors on markers of platelet activation.
Increased platelet activation is associated with a variety of vascular disorders including acute coronary syndromes, stable coronary artery disease, and restenosis after percutaneous coronary intervention (26,27). Soluble CD40L is a particularly attractive marker for platelet activation because it is shed from the surface of activated platelets, is easily measured, and meaningfully participates in a number of important biologic processes including activation of immunity and thrombosis (28). The current study found increased levels of sCD40L in the setting of RAS; however, this appears to be a nonspecific association with atherosclerosis in general as opposed to being attributable to RAS specifically. More importantly, although increased levels of sCD40L before the procedure were more likely to have embolization of platelet-rich thrombi, these patients had persistently elevated levels of sCD40L after the procedure. This finding may represent a potentially modifiable feature denoting increased risk for patients referred for renal artery revascularization.
The current study also demonstrated that abciximab effectively inhibits platelet activation, as denoted by substantial suppression of sCD40L, up to 24 hours after the procedure. Others have also observed the ability of GPIIb/IIIa inhibitors to lower levels of sCD40L in settings such as acute coronary syndromes and in ST-elevation myocardial infarction patients undergoing coronary intervention (29,30). The current finding extends the prior observation that a GPIIb/IIIa inhibitor, when combined with an embolic protection device to capture atheroembolic debris, resulted in the most favorable renal function outcome (21).
The suppression of sCD40L release, observed with abciximab administration in the current study, creates a plausible biologic pathway to explain the observation that abciximab use was associated with improved renal function after stenting. In the kidney the sCD40L/CD40 may be directly responsible for renal injury. Previously, others have shown that angiotensin II stimulates release of renal TGF-β that in turn increases expression of the CD40 receptor on the proximal tubule of the kidney (31). Pontrelli et al. has shown that CD40 cross-linking on proximal tubular epithelial cells is proinflammatory and induces fibrosis by stimulating the expression of plasminogen activator inhibitor-1 (PAI-1) acting through a signaling pathway that is independent of the proinflammatory signaling effects of CD40L (15). In addition, activation of the CD40 receptor results in infiltration of inflammatory cells into the interstitium of the kidney through monocyte chemoattractant protein-1 (MCP-1) and intercellular adhesion molecule-1 (ICAM-1) expression (32). IL-8 amplifies CD40/CD154-mediated ICAM-1 production via the CXCR-1 receptor and p38-MAPK pathway in human renal proximal tubule cells (32). Furthermore, inhibition of the CD40/CD40L significantly decreased the severity of renal injury in an animal model of chronic proteinuric renal disease (33). Thus, it is conceivable that in patients with renal ischemia (1) the CD40 receptor is overexpressed due to angiotensin II stimulation, (2) sCD40L shed by locally activated platelets may activate the receptor and stimulate peritubular fibrosis in a manner independent of renal blood flow or ischemia, and (3) this process may be accelerated at the time of a stent procedure. In this regard the association between the GPIIb/IIIa inhibitor abciximab and improved renal function outcomes observed in the RESIST study (A prospective Randomized Multicenter Study Comparing the Safety and Efficacy of Renal Artery Stenting With/Without a Distal Embolic Protection Device (AngioGuard) and With/Without the Use of a Platelet Inhibitor (Abciximab-Reopro) (21) may be attributable to the drug's effects in suppressing sCD40L as opposed to an effect on thrombosis per se.
An observation from the RESIST study was that the EPD, when used without abciximab, did not appear to improve renal function despite capturing debris. In the current study we saw a slight increase in platelet activation with the use of the EPD occurring immediately after the procedure, although this increase was not statistically significant. Conceivably, the EPD may slow blood flow in the vessel, provide a surface upon which platelets can aggregate, and increase local platelet activation an effect inhibited by the GPIIb/IIIa inhibitor. Admittedly the observed increase in circulating levels of sCD40L with the use of the EPD was not statistically significant; however, it may be unrealistic to expect that effects occurring on the surface of an EPD would be detected systemically.
Several studies suggest a benefit of reducing platelet activation with loading doses of 300 to 600 mg of clopidogrel before coronary interventions (34–36). However, in the current study pretreatment with clopidogrel or clopidogrel administration on the day of procedure did not significantly effect sCD40L levels. This may result from confounding because patients were not randomized to clopidogrel treatment and had a significantly higher prevalence of coronary artery and peripheral vascular disease, which may account for the lack of difference observed in sCD40L levels. Work by Azar et al. reported a reduction in sCD40L at a clopidogrel dose of 75 mg/d when preceded by a loading dose of 300 mg in patients with stable CAD (37). Others, however, have failed to demonstrate an effect of clopidogrel on levels of sCD40L (38).
Increased levels of circulating sCD40L and the impact on renal function in the setting of RAS remain speculative. Future clinical trials should address the effect of sCD40L inhibition on distal embolization and renal function with long-term follow-up. The current study provides a foundation for exploring the role of CD40/CD40L signaling and the generation of renal fibrosis during ischemic renal injury.
The following limitations of our study warrant mentioning: The current study used sCD40L as the key measure of platelet activation. We did not measure sCD40L at 1 month, and we do not have longer-term follow-up of renal function beyond 1 month. Thus, it remains uncertain whether other indices of platelet activation would provide additional insights or whether longer-term follow-up would have yielded similar results for kidney function.
Atherosclerotic RAS is associated with increased platelet activation, but this increase appears to be attributable to atherosclerosis in general, not RAS specifically. However, in patients with higher levels of platelet activation before the procedure, embolization of platelet-rich thrombi is more common. Abciximab effectively inhibits platelet activation and sCD40L release, a mechanism that may explain the beneficial effect on renal function 1 month after the procedure that has been previously observed.
Disclosures
Dr. Cooper and P. Brewster have received research grants from the RESIST and CORAL studies. Dr. Colyer has received research support from Astra-Zeneca and Sanofi-Aventis and has served on the speakers' bureau for Boehringer-Ingleheim, Pfizer, and Radi. Dr. Burket has received a research grant from the RESIST study and has served on the speakers' bureau for Cordis and BMS/Sanofi.
Acknowledgments
David Kennedy is supported by the Lerner Research Institute's Morgenthaler Fellowship and American Heart Association Postdoctoral Fellowship 0825685D. The renal artery stenosis patient samples were from the RESIST clinical trial sponsored by The University of Toledo, Health Science Campus, Toledo, Ohio, and were funded by Centocor Inc. and Cordis Corp., both Johnson & Johnson companies. Part of this work was presented in abstract format at the American College of Cardiology Annual Scientific Sessions, March, 2008 Chicago, IL.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Levin A, Linas S, Luft FC, Chapman AB, Textor S: Controversies in renal artery stenosis: A review by the American Society of Nephrology Advisory Group on Hypertension. Am J Nephrol 27: 212–220, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Balk E, Raman G, Chung M, Ip S, Tatsioni A, Alonso A, Chew P, Gilbert SJ, Lau J: Effectiveness of management strategies for renal artery stenosis: A systematic review. Ann Intern Med 145: 901–912, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Olin JW: Survival in atherosclerotic renal artery stenosis: Its all about renal function, or is it? Catheter Cardiovasc Interv 69: 1048–1049, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Arthurs Z, Starnes B, Cuadrado D, Sohn V, Cushner H, Andersen C: Renal artery stenting slows the rate of renal function decline. J Vasc Surg 45: 726–731, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Safian RD, Textor SC: Renal-artery stenosis. N Engl J Med 344: 431–442, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr., White CJ, White J, White RA, Antman EM, Smith SC, Jr., Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B: ACC/AHA 2005 Practice Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113: e463–e654, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cooper CJ, Murphy TP, Matsumoto A, Steffes M, Cohen DJ, Jaff M, Kuntz R, Jamerson K, Reid D, Rosenfield K, Rundback J, D'Agostino R, Henrich W, Dworkin L: Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: Rationale and design of the CORAL trial. Am Heart J 152: 59–66, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ: CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res 92: 1041–1048, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Chakrabarti S, Varghese S, Vitseva O, Tanriverdi K, Freedman JE: CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler Thromb Vasc Biol 25: 2428–2434, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA: CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391: 591–594, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Freedman JE: CD40-CD40L and platelet function: beyond hemostasis. Circ Res 92: 944–946, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD: CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med 8: 247–252, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Mason PJ, Chakrabarti S, Albers AA, Rex S, Vitseva O, Varghese S, Freedman JE: Plasma, serum, and platelet expression of CD40 ligand in adults with cardiovascular disease. Am J Cardiol 96: 1365–1369, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Santilli F, Davi G, Consoli A, Cipollone F, Mezzetti A, Falco A, Taraborelli T, Devangelio E, Ciabattoni G, Basili S, Patrono C: Thromboxane-dependent CD40 ligand release in type 2 diabetes mellitus. J Am Coll Cardiol 47: 391–397, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Pontrelli P, Ursi M, Ranieri E, Capobianco C, Schena FP, Gesualdo L, Grandaliano G: CD40L proinflammatory and profibrotic effects on proximal tubular epithelial cells: Role of NF-kappaB and lyn. J Am Soc Nephrol 17: 627–636, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Nannizzi-Alaimo L, Rubenstein MH, Alves VL, Leong GY, Phillips DR, Gold HK: Cardiopulmonary bypass induces release of soluble CD40 ligand. Circulation 105: 2849–2854, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Kritharides L, Lau GT, Freedman B: Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 348: 2575–2577, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Burdon KP, Langefeld CD, Beck SR, Wagenknecht LE, Carr JJ, Rich SS, Freedman BI, Herrington D, Bowden DW: Variants of the CD40 gene but not of the CD40L gene are associated with coronary artery calcification in the Diabetes Heart Study (DHS). Am Heart J 151: 706–711, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Welt FG, Rogers SD, Zhang X, Ehlers R, Chen Z, Nannizzi-Alaimo L, Phillips DR, Simon DI: GP IIb/IIIa inhibition with eptifibatide lowers levels of soluble CD40L and RANTES after percutaneous coronary intervention. Catheter Cardiovasc Interv 61: 185–189, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Nannizzi-Alaimo L, Alves VL, Phillips DR: Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation 107: 1123–1128, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, Safian R, Reddy B, Brewster P, Ankenbrandt MA, Virmani R, Dippel E, Rocha-Singh K, Murphy TP, Kennedy DJ, Shapiro JI, D'Agostino RD, Pencina MJ, Khuder S: Embolic protection and platelet inhibition during renal artery stenting. Circulation 117: 2752–2760, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Burches B, Karnicki K, Wysokinski W, McBane RD, 2nd: Immunohistochemistry of thrombi following iliac venous stenting: a novel model of venous thrombosis. Thromb Haemost 96: 618–622 2006 [PubMed] [Google Scholar]
- 23. Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Anand SX, Kim MC, Kamran M, Sharma SK, Kini AS, Fareed J, Hoppensteadt DA, Carbon F, Cavusoglu E, Varon D, Viles-Gonzalez JF, Badimon JJ, Marmur JD: Comparison of platelet function and morphology in patients undergoing percutaneous coronary intervention receiving bivalirudin versus unfractionated heparin versus clopidogrel pretreatment and bivalirudin. Am J Cardiol 100: 417–424, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Garg R, Uretsky BF, Lev EI: Anti-platelet and anti-thrombotic approaches in patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 70: 388–406, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Santilli F, Basili S, Ferroni P, Davi G: CD40/CD40L system and vascular disease. Intern Emerg Med 2: 256–268, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Turker S, Guneri S, Akdeniz B, Ozcan MA, Baris N, Badak O, Kirimli O, Yuksel F: Usefulness of preprocedural soluble CD40 ligand for predicting restenosis after percutaneous coronary intervention in patients with stable coronary artery disease. Am J Cardiol 97: 198–202, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C: The CD40/CD40 ligand system: Linking inflammation with atherothrombosis. J Am Coll Cardiol 54: 669–677, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Furman MI, Krueger LA, Linden MD, Fox ML, Ball SP, Barnard MR, Frelinger AL, 3rd, Michelson AD: GPIIb-IIIa antagonists reduce thromboinflammatory processes in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Thromb Haemost 3: 312–320, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Dominguez-Rodriguez A, Abreu-Gonzalez P, Avanzas P, Bosa-Ojeda F, Samimi-Fard S, Marrero-Rodriguez F, Kaski JC: Intracoronary versus intravenous abciximab administration in patients with ST-elevation myocardial infarction undergoing thrombus aspiration during primary percutaneous coronary intervention—effects on soluble CD40 ligand concentrations. Atherosclerosis 206: 523–527, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Starke A, Wuthrich RP, Waeckerle-Men Y: TGF-beta treatment modulates PD-L1 and CD40 expression in proximal renal tubular epithelial cells and enhances CD8 cytotoxic T-cell responses. Nephron Exp Nephrol 107: e22–29, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Li H, Nord EP: IL-8 amplifies CD40/CD154-mediated ICAM-1 production via the CXCR-1 receptor and p38-MAPK pathway in human renal proximal tubule cells. Am J Physiol Renal Physiol 296: F438–F445, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Kairaitis L, Wang Y, Zheng L, Tay YC, Harris DC: Blockade of CD40-CD40 ligand protects against renal injury in chronic proteinuric renal disease. Kidney Int 64: 1265–1272, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Nguyen TA, Lordkipanidze M, Diodati JG, Palisaitis DA, Schampaert E, Turgeon J, Pharand C: Week-long high-maintenance dose clopidogrel regimen achieves better platelet aggregation inhibition than a standard loading dose before percutaneous coronary intervention: results of a double-blind, randomized clinical trial. J Interv Cardiol 22: 368–377, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Fefer P, Hod H, Hammerman H, Segev A, Beinart R, Boyko V, Behar S, Matetzky S: Usefulness of pretreatment with high-dose clopidogrel in patients undergoing primary angioplasty for ST-elevation myocardial infarction. Am J Cardiol 104: 514–518, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Gladding P, Webster M, Zeng I, Farrell H, Stewart J, Ruygrok P, Ormiston J, El-Jack S, Armstrong G, Kay P, Scott D, Gunes A, Dahl ML: The antiplatelet effect of higher loading and maintenance dose regimens of clopidogrel: The PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc Interv 1: 612–619, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Azar RR, Kassab R, Zoghbi A, Aboujaoude S, El-Osta H, Ghorra P, Germanos M, Salame E: Effects of clopidogrel on soluble CD40 ligand and on high-sensitivity C-reactive protein in patients with stable coronary artery disease. Am Heart J 151: 521.e1–521.e4 2006 [DOI] [PubMed] [Google Scholar]
- 38. Saw J, Madsen EH, Chan S, Maurer-Spurej E: The ELAPSE (Evaluation of Long-Term Clopidogrel Antiplatelet and Systemic Anti-Inflammatory Effects) study. J Am Coll Cardiol 52: 1826–1833, 2008 [DOI] [PubMed] [Google Scholar]