Abstract
Summary
Background and objectives
Dialysis patients are at high risk for low-trauma bone fracture. Bone density measurements using dual-energy x-ray absorptiometry (DXA) do not reliably differentiate between patients with and without fractures. The aim of this study was to identify differences in bone microarchitecture between patients with and without a history of fracture using high-resolution peripheral quantitative computed tomography (HR-pQCT).
Design, setting, participants, & measurements
Seventy-four prevalent hemodialysis patients were recruited for measurements of areal bone mineral density (aBMD) by DXA and bone microarchitecture by HR-pQCT. Patients with a history of trauma-related fracture were excluded. Forty healthy volunteers served as controls. Blood levels of parathyroid hormone, vitamin D, and markers of bone turnover were determined.
Results
Dialysis patients, particularly women, had markedly impaired bone microarchitecture. Patients with fractures had significantly reduced cortical and trabecular microarchitecture compared with patients without fractures. aBMD tended to be lower in patients with fractures, but differences were statistically not significant. The strongest determinant of fracture was the HR-pQCT-measured trabecular density of the tibia, which also had the highest discriminatory power to differentiate patients according to fracture status. Radial DXA had a lower discriminatory power than trabecular density.
Conclusions
Bone microarchitecture is severely impaired in dialysis patients and even more so in patients with a history of fracture. HR-pQCT can identify dialysis patients with a history of low-trauma fracture.
Introduction
Nearly all hemodialysis patients suffer from renal osteodystrophy (1). It is part of the mineral and bone disorder complex accompanying chronic kidney disease (CKD) and leads to reduced bone mineral density (BMD), volume, and structure (2). Patients with CKD stage 5D (dialysis) have a 4.4-fold higher risk of femoral and a 2-fold higher risk of vertebral fractures than patients with normal kidney function (3,4). BMD measured by dual-energy x-ray absorptiometry (DXA) neither distinguishes among the different forms of renal osteodystrophy nor does it reliably predict fracture status in dialysis patients (5,6). Furthermore, vascular calcification, which is common in dialysis patients, may substantially contribute to BMD values measured with DXA (7). Quantification of cortical density, area, or thickness by peripheral quantitative computed tomography (pQCT) is more closely associated with bone fractures in this population (8). Despite the effective discrimination between cortical and trabecular density, pQCT is unable to depict trabecular microarchitecture. So far, trabecular structures have been assessed by two-dimensional histomorphometry or three-dimensional microcomputed tomography of an iliac crest bone biopsy. However, fractures in dialysis patients mainly occur in the appendicular skeleton and spine. Because the association of histomorphometric indices between the iliac crest and the spine or the appendicular skeleton is low (9), assessment of microarchitecture at the site of fracture will probably permit a better risk stratification.
High-resolution peripheral quantitative computed tomography (HR-pQCT) is a method that allows for in vivo assessment of bone microarchitecture at the tibia and the radius. Compared with pQCT, the resolution has been improved from 350 μm to below 100 μm (10). The higher resolution facilitates measurement of cortical and trabecular parameters.
In the study presented here, cortical and trabecular bone of the tibia and the radius were measured with HR-pQCT in hemodialysis patients and compared with values of healthy controls. To analyze the diagnostic value of HR-pQCT for the detection of patients with a history of fracture, the results were correlated with low-trauma fracture status and different markers of bone turnover.
Study Population and Methods
Dialysis Patients and Healthy Volunteers
Subjects over 18 years of age on chronic maintenance hemodialysis were recruited at the Medical University Vienna. A chart review was performed for demographic data, medications, and medical history. Dialysis vintage was defined as the time from the first day of dialysis to the measurement of HR-pQCT. In patients who had previously undergone renal transplantation, the intermittent time of a functioning graft was excluded. All kidney transplant recipients had received corticosteroids as part of their triple immunosuppressive regimen. All patients were asked about previous low-trauma fractures (fractures occurring from falls at standing height or minimal trauma). Lateral x-rays of the spine were obtained to identify vertebral fractures, and chest x-rays were reviewed for fractures of the thoracic spine as described previously (11). Patients with fractures other than low-trauma fractures were excluded from the analysis. Gender- and race-matched healthy controls were recruited by the Musculoskeletal Quantitative Imaging Research Group in the Department of Radiology at the University of California–San Francisco and measured using the same type of device (Xtreme CT, SCANCO Medical AG, Brüttisellen, Switzerland). The study was reviewed and approved by the local ethics committees according to the standards set by the Declaration of Helsinki, and all participants gave their written informed consent to participate.
HR-pQCT
Trabecular and cortical microarchitecture was determined by performing HR-pQCT at the distal radius and the tibia, as reported previously by Boutroy et al. (XtremeCT, SCANCO) (10). The data were converted into histomorphometric parameters as described by Laib et al. (12). Cortical thickness (CtTh [mm]) was defined as the mean cortical volume divided by the outer surface. Trabecular density (Dtrab, in mg hydroxyapatite [HA]/cm3), cortical density (Dcort [mg HA/cm3]), and total density (mg HA/cm3) were the mean densities in each region. The bone volume fraction (BV/TV [%]) was calculated by relating Dtrab to an arbitrary, fully mineralized bone block of 1200 mg HA/cm3 and the same size: BV/TV = 100 × (Dtrab in mg HA/cm3)/1200 mg HA/cm3.
To correct for partial volume effects, a thickness-independent structure extraction was used for the measurement of trabecular number (TbN [mm−1]) (13). The three-dimensional center points of the trabeculae (three-dimensional ridges) were detected on gray-level images, and TbN was taken as the inverse of the mean distance between the ridges. Trabecular thickness (TbTh) was calculated as (BV/TV)/TbN, and Tb1/NSD (SD of 1/TbN) was calculated as the SD of the intraindividual distribution of separation. Greater trabecular heterogeneity results in a higher Tb1/NSD. For measurements of the radius, the arm contralateral to the dialysis access (arteriovenous fistula) was used to exclude poor bone microarchitecture because of hemodynamic steal phenomena. In case the patient had arteriovenous fistulas on both arms, the site with the dysfunctional fistula was used for the same reasons. For measurements of the tibia, the leg of the nondominant side was used. Extremities with a history of fracture at or near the site of measurement were excluded from HR-pQCT measurements. To ensure comparability of HR-pQCT measurements between the Medical University Vienna and the University of California–San Francisco, regular quality control scans using a phantom supplied by the manufacturer were carried out. In addition, both sites were part of a preliminary international cross-site-calibration study that demonstrated an interscanner reproducibility of 2% to 3% for densities and 2% to 7% for geometrical and microarchitectural parameters except trabecular heterogeneity (13%) (14).
BMD by DXA
Areal bone mineral density (aBMD) measurements were performed with DXA on a QDR-4500 scanner (Hologic, Waltham, MA) at the posteroanterior lumbar spine at L1 to L4 and the proximal femur at the femoral neck. At the radius, the ultradistal site was measured because this location overlaps with the site of radial HR-pQCT measurements.
Serologic Parameters
Each parameter was determined by calculating the mean of all measurements performed during the 12 months before the HR-pQCT and DXA investigations. Alkaline phosphatase, osteocalcin, intact parathyroid hormone (iPTH), c-telopeptide pyridinoline crosslinks of type I collagen (CrossLaps), 25-hydroxycholecalciferol, calcitriol, calcium, and phosphate were quantified as described previously (15) according to Good Laboratory Practice standards.
Statistical Analyses
The differences between healthy controls and patients as well as between patients with and without fractures were calculated using a t test for independent samples. The number of men and women with and without fractures were compared by Pearson chi-squared test. Differences in age between men and women were assessed by a two-way ANOVA. Analyses of covariance were used to compare the age-corrected HR-pQCT measurements between patients with or without fractures. Univariate associations between HR-pQCT bone indices and all other data were analyzed by the Spearman correlation coefficient.
A logistic regression model was constructed to estimate the odds of fracture for different bone parameters. The independent variables were the HR-pQCT and the DXA parameters. The odds ratios (ORs) are given per 1 SD decrease. Receiver-operating characteristic (ROC) curves were constructed for all variables to estimate their efficacy in separating patients with from those without fractures. Data were analyzed with SPSS (version 17.0; SPSS, Chicago, IL). P values <0.05 were considered statistically significant.
Results
Characteristics of Patients and Controls
Male and female patients had similar characteristics (Table 1). Of 74 patients studied, 68 were Caucasian, three were African, and three were Indian. Thirteen patients had received one kidney transplant, five patients had received two, and two patients had received three transplants. Regarding phosphate binder use, 35% of patients were taking calcium carbonate, 54% sevelamer hydrochloride, 12% lanthanum carbonate, and 60% aluminum hydroxide. Active vitamin D (alfacalcidol, calcitriol, paricalcitol) was given intravenously or orally to 52% of patients. Twenty women and 20 men (80% Caucasian, 12.5% Hispanic, 7.5% Asian) without a history of bone or kidney disease served as a healthy control group. The mean age of dialysis patients was 59 ± 15 years, compared with 56 ± 10 years in healthy controls (P = NS). The body mass index (BMI) was similar in both groups (dialysis: 25.1 ± 5.1 versus controls: 26.1 ± 5.7; P = 0.35.). Thirty fractures had occurred in 24 dialysis patients: 21 lumbar or thoracic spine fractures, six femoral neck fractures, and three rib fractures. Patients with fractures were older, mainly women, and had a higher serum alkaline phosphatase level (Table 1). There was no statistical difference in the type of phosphate binder or active vitamin D use between patients with or without a history of fracture. Patients with a history of femoral neck fracture (two women, four men) were of similar age (mean 67 years) as patients with a history of any other fracture (mean 70 years). As for corticosteroid exposure, two of six patients with a femoral neck fracture had a history of corticosteroid therapy because of their underlying disease, but none had undergone kidney transplantation before.
Table 1.
Mean (SD) or percentage of the descriptive characteristics of dialysis patients
| Characteristic | Men (n = 40) | Women (n = 34) | Total (n = 74) | Patients with Fracture (n = 24) | Patients without Fracture (n = 50) |
|---|---|---|---|---|---|
| Men | 100% | 0% | 54% | 33%a | 64% |
| Age (years) | 59 (13) | 56 (16) | 59 (15) | 69 (13)a | 54 (13) |
| BMI (kg/m2) | 25 (4) | 25 (6) | 25 (5) | 24 (6) | 25 (5) |
| Dialysis (months) | 44 (37) | 70 (99) | 56 (73) | 76 (113) | 47 (45) |
| Smoking (%) | 26 | 12 | 20 | 10 | 24 |
| Diabetes mellitus (%) | 38 | 26 | 32 | 29 | 34 |
| Previous transplantations (%) | 28 | 26 | 27 | 21 | 30 |
| Calcium supplement (%) | 37 | 47 | 42 | 39 | 43 |
| Vitamin D supplement (%) | 60 | 44 | 53 | 50 | 54 |
| Calcimimetic (%) | 18 | 21 | 20 | 27 | 16 |
| Oral anticoagulants (%) | 0 | 6 | 3 | 4 | 2 |
| Past corticosteroid use (%)b | 21 | 21 | 22 | 12 | 26 |
| Current corticosteroid use (%) | 5 | 0 | 3 | 0 | 4 |
| Calcium (mg/dl) | 8.8 (1.2) | 9.0 (1.3) | 8.9 (1.3) | 8.9 (1.3) | 8.9 (1.3) |
| Phosphate (mg/dl) | 5.8 (1.2) | 5.0 (1.2) | 5.8 (1.2) | 5.5 (1.1) | 5.9 (1.2) |
| Alkaline phosphatase (U/L) | 96 (56) | 112 (110) | 103 (85) | 135 (125)a | 88 (51) |
| Osteocalcin (pg/ml) | 177 (139) | 221 (167) | 196 (152) | 161 (136) | 210 (157) |
| C-telopeptide (ng/ml) | 1.89 (0.77) | 2.21 (1.15) | 2.03 (0.96) | 1.77 (0.78) | 2.13 (1.02) |
| iPTH (pg/ml) | 270 (283) | 355 (397) | 309 (340) | 338 (453) | 295 (277) |
| 25-Hydroxycholecalciferol (ng/ml) | 17 (6.8) | 15 (8.0) | 16 (7.6) | 15 (8.0) | 17 (7.2) |
| Calcitriol (pg/ml) | 5.0 (2.9) | 4.2 (2.5) | 4.6 (2.7) | 5.4 (2.9) | 4.2 (2.6) |
Conversion factors for units: calcium in mg/dl to mmol/L, ×0.25; phosphate in mg/dl to mmol/L, ×0.32; 25-hydroxycholecalciferol in ng/ml to nmol/L, ×2.5; calcitrol in pg/ml to pmol/L, ×2.6. No conversion necessary for alkaline phosphatase, osteocalcin, C-telopeptide, and iPTH. BMI, body mass index; iPTH, intact parathyroid hormone.
Significant difference between patients with and without fracture (P < 0.05).
Excluding corticosteroid use because of renal transplantation.
HR-pQCT and DXA Measurements in Dialysis Patients and Healthy Controls
HR-pQCT measurements revealed impaired bone microarchitecture in dialysis patients compared with healthy controls in all parameters studied (Table 2), TbTh being the only exception. In particular, the tibias of women on dialysis showed highly significant differences in structural parameters compared with healthy controls. In dialysis patients, aBMD (T-scores) using DXA were (mean [SD]): −2.0 (1.2) at the femoral neck (women: −2.0 [1.1], men: −2.0 [1.3]), −1.3 (1.5) at the lumbar spine (women: −1.4 [1.7], men: −1.1 [1.4]), and −3.1 (1.7) at the radius (women: −2.9 [1.6], men: −3.2 [1.9]).
Table 2.
Mean (SD) of HR-pQCT and DXA parameters in hemodialysis patients and healthy controls
| HR-pQCT | n | Dtot (mg HA/cm3) | Dcort (mg HA/cm3) | CtTh (mm) | Dtrab (mg HA/cm3) | BV/TV (%) | TbN (mm−1) | TbTh (mm) | Tb1/NSD (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Tibia | |||||||||
| women | |||||||||
| controls | 20 | 287 (49) | 850 (53) | 1.11 (0.21) | 156 (30) | 13 (2.5) | 1.87 (0.34) | 0.07 (0.01) | 0.22 (0.06) |
| patients | 34 | 214 (69)b | 778 (79)b | 0.77 (0.33)b | 144 (42)b | 10.4 (3.7)b | 1.45 (0.45)b | 0.07 (0.02) | 0.46 (0.38)b |
| men | |||||||||
| controls | 20 | 294 (46) | 846 (58) | 1.28 (0.27) | 163 (32) | 13.6 (2.6) | 1.87 (0.36) | 0.07 (0.01) | 0.23 (0.08) |
| patients | 40 | 243 (65)b | 787 (79)b | 0.96 (0.34)b | 144 (42) | 12 (3.5) | 1.81 (0.43) | 0.07 (0.01) | 0.25 (0.10) |
| Radius | |||||||||
| women | |||||||||
| controls | 20 | 294 (53) | 837 (74) | 0.69 (0.18) | 150 (31) | 12.5 (2.6) | 1.95 (0.29) | 0.06 (0.01) | 0.20 (0.09) |
| patients | 34 | 255 (85) | 797 (102) | 0.61 (0.26) | 114 (55)b | 9.6 (4.6)b | 1.50 (0.45)b | 0.06 (0.01) | 0.35 (0.19)b |
| men | |||||||||
| controls | 20 | 317 (38) | 848 (45) | 0.80 (0.014) | 170 (28) | 14.2 (2.3) | 2.03 (0.33) | 0.07 (0.01) | 0.19 (0.06) |
| patients | 40 | 268 (79)b | 787 (92)b | 0.65 (0.26)b | 144 (45)a | 12.1 (3.8)a | 1.84 (0.47) | 0.06 (0.01)a | 0.26 (0.16) |
HR-pQCT, high-resolution peripheral quantitative computed tomography; DXA, dual-energy x-ray absorptiometry; Dtot, total density; HA, hydroxyapatite; Dcort, cortical density; CtTh, cortical thickness; Dtrab, trabecular thickness; BV/TV, trabecular bone volume; TbN, trabecular number; TbTh, trabecular thickness; Tb1/NSD, SD of 1/TbN.
P < 0.05,
P < 0.01.
Highly significant correlations were found between bone indices of the tibia and the radius in dialysis patients. The lowest correlation was registered for TbTh (R = 0.46, P = 0.0001) and the highest for Dtrab (R = 0.83, P < 0.0001).
A weak albeit significant correlation was found between duration of dialysis, fracture, or previous kidney transplantation and most HR-pQCT parameters. A similar association was present for radius aBMD (Table 3). Age was mainly associated with cortical and gender with trabecular structure. The only serologic measures related to some of the HR-pQCT indices were alkaline phosphatase and, to a lesser extent, iPTH (Table 3). To study the effect of parathyroid hormone (PTH) on bone architecture more closely, patients were classified in three groups according to PTH levels (<100 pg/ml, 100 to 400 pg/ml, and >400 pg/ml). However, no differences in HR-pQCT values between PTH groups were found. No associations were found between vitamin D status (25-hydroxycholecalciferol and calcitrol), use of active vitamin D compounds, or type of phosphate binder and HR-pQCT measurements.
Table 3.
Relations between variables associated with an increased risk of fracture and HR-pQCT or DXA (Spearman correlations)
| Age | Dialysis Duration | BMI | Gender | Fracture | Previous KTX | PTH | Osteocalcin | CTX | AP | |
|---|---|---|---|---|---|---|---|---|---|---|
| HR-pQCT tibia | ||||||||||
| Dtot | −0.19a | −0.36b | 0.21 | −0.19 | −0.34b | −0.29a | −0.20 | −0.05 | 0.05 | −0.36b |
| Dcort | −0.51c | −0.14 | −0.07 | −0.03 | −0.37c | −0.11 | −0.07 | 0.18 | 0.13 | −0.34b |
| CtTh | −0.30b | −0.23 | 0.22 | −0.26a | −0.42c | −0.24a | −0.06 | 0.11 | 0.10 | −0.30a |
| Dtrab | −0.02 | −0.44c | 0.19 | −0.24a | −0.23a | −0.25a | −0.26a | −0.15 | 0.03 | −0.26a |
| BV/TV | −0.03 | −0.44c | 0.14 | −0.20 | −0.23a | −0.26a | −0.24a | −0.10 | 0.09 | −0.25a |
| TbN | −0.07 | 0.24a | 0.30b | −0.33b | −0.23a | −0.21 | −0.14 | −0.10 | 0.03 | −0.29a |
| TbTh | 0.02 | −0.33b | −0.24a | 0.15 | −0.05 | −0.16 | −0.17 | 0.01 | 0.10 | 0.01 |
| Tb1/NSD | 0.06 | 0.27a | −0.32b | 0.35b | 0.25a | 0.20 | 0.18 | 0.13 | −0.01 | 0.36b |
| HR-pQCT radius | ||||||||||
| Dtot | −0.21a | −0.49c | 0.17 | −0.07 | −0.21 | −0.41c | −0.16 | 0.04 | 0.10 | −0.27a |
| Dcort | −0.36b | −0.36b | 0.10 | 0.08 | −0.24a | −0.36b | −0.06 | 0.19 | 0.16 | −0.29a |
| CtTh | −0.25b | −0.38b | 0.30a | −0.08 | −0.30a | −0.38c | −0.05 | 0.18 | 0.19 | −0.25a |
| Dtrab | −0.11 | −0.43c | 0.02 | −0.35b | −0.22 | −0.29a | −0.27a | −0.19 | −0.08 | −0.22 |
| BV/TV | −0.11 | −0.45c | 0.01 | −0.34b | −0.21 | −0.30a | −0.26 | −0.18 | −0.04 | −0.21 |
| TbN | −0.06 | −0.22 | 0.08 | −0.37b | −0.24a | −0.28a | −0.27a | −0.16 | −0.16 | −0.22 |
| TbTh | −0.10 | −0.39b | −0.11 | −0.08 | −0.04 | −0.18 | −0.21 | −0.11 | 0.01 | −0.03 |
| Tb1/NSD | 0.06 | 0.27a | −0.15 | 0.30a | 0.20 | 0.34b | 0.33b | 0.18 | 0.12 | 0.31b |
| DXA | ||||||||||
| femoral neck aBMD | −0.21 | −0.18 | 0.39c | −0.14 | −0.32b | 0.03 | −0.23 | 0.15 | 0.09 | −0.20 |
| lumbar spine aBMD | 0.21 | −0.25a | 0.12 | −0.23 | −0.12 | −0.16 | −0.37b | −0.06 | 0.03 | −0.21 |
| distal radius aBMD | −0.19 | −0.56c | 0.35b | −0.49c | −0.49c | −0.37b | −0.03 | 0.26 | 0.36b | −0.24 |
HRpQCT, high-resolution peripheral quantitative computed tomography; DXA, dual-energy x-ray absorptiometry; BMI, body mass index; KTX, kidney transplantation; PTH, parathyroid hormone; CTX, c-telopeptide crosslinks; AP, alkaline phosphatase; Dtot, total density; Dcort, cortical density; CtTh, cortical thickness; Dtrab, trabecular thickness; BV/TV, trabecular bone volume; TbN, trabecular number; TbTh, trabecular thickness; Tb1/NSD, SD of 1/TbN; aBMD, areal bone mineral density.
P < 0.05,
P < 0.01,
P < 0.001.
HR-pQCT and DXA Differences between Dialysis Patients with and without a History of Fracture
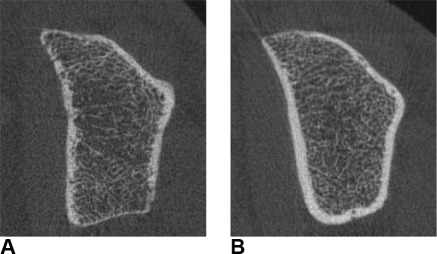
An analysis of age-corrected HR-pQCT and DXA measurements according to gender and fracture status is presented in Table 4. With the exception of TbTh, all HR-pQCT parameters were found to differ significantly between patients with and without a history of fracture at the tibia. At the radius, only TbN and trabecular heterogeneity differed significantly according to fracture status. At both anatomical sites, differences between patients with and without fracture history were more pronounced in women than in men. Differences in DXA measurements between fractured and nonfractured patients did not reach statistical significance. Among the patients with a history of fracture, the subgroup of patients with a history of femoral neck fracture had similar DXA and HR-pQCT values as patients with a history of fracture at any other anatomical site. Representative two-dimensional reconstructions of patients with and without a history of low-trauma fracture are shown in Figure 1.
Table 4.
Age-corrected HR-pQCT and DXA results in women and men with and without a history of fracture
| HR-pQCT | Women |
Men |
P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Fracture (n = 18) |
Fracture (n = 16) |
Percent Difference (95% CI) | No Fracture (n = 32) |
Fracture (n = 8) |
Percent Difference (95% CI) | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| HR-pQCT tibia | |||||||||||
| Dtot (mg HA/cm3) | 254 | 15 | 178 | 15 | −30 (−26 to −34) | 246 | 10 | 222 | 22 | −10 (−6 to −14) | <0.01 |
| Dcort (mg HA/cm3) | 812 | 19 | 739 | 20 | −10 (−7 to −11) | 792 | 13 | 769 | 28 | −3 (−1 to −5) | <0.05 |
| CtTh (mm) | 0.929 | 0.055 | 0.642 | 0.081 | −31 (−26 to −37) | 0.993 | 0.055 | 0.816 | 0.114 | −18 (−12 to −23) | <0.01 |
| Dtrab (mg HA/cm3) | 147 | 10 | 95 | 10 | −35 (−31 to −40) | 147 | 7.35 | 123 | 15 | −16 (−11 to −21) | <0.01 |
| BV/TV (%) | 12.3 | 0.9 | 8.2 | 0.9 | −33 (−28 to −38) | 12.3 | 0.6 | 10.5 | 1.3 | −15 (−10 to −20) | <0.01 |
| TbN (mm−1) | 1.67 | 0.104 | 1.24 | 0.1 | −25 (−22 to −30) | 1.84 | 0.074 | 1.67 | 0.153 | −9 (−5 to −13) | <0.05 |
| TbTh (mm) | 0.074 | 0.004 | 0.071 | 0.005 | −4 (0 to −14) | 0.067 | 0.003 | 0.063 | 0.007 | −6 (0 to −14) | 0.55 |
| Tb1/NSD (mm) | 0.286 | 0.062 | 0.641 | 0.065 | 124 (107 to 138) | 0.237 | 0.044 | 0.311 | 0.092 | 31 (13 to 50) | <0.01 |
| HR-pQCT radius | |||||||||||
| Dtot (mg HA/cm3) | 293 | 14 | 217 | 21 | −26 (−22 to −30) | 269 | 14 | 261 | 29 | −3 (2 to −8) | 0.09 |
| Dcort (mg HA/cm3) | 826 | 22 | 760 | 24 | −8 (−6 to −10) | 784 | 16 | 799 | 33 | 2 (0 to −4) | 0.35 |
| CtTh (mm) | 0.721 | 0.064 | 0.479 | 0.067 | −34 (−28 to −40) | 0.655 | 0.045 | 0.608 | 0.093 | −7 (0 to −14) | 0.06 |
| Dtrab (mg HA/cm3) | 134 | 12 | 96 | 12 | −28 (−22 to −35) | 145 | 8 | 133 | 17 | −8 (−3 to −14) | 0.08 |
| BV/TV (%) | 11.3 | 1 | 8.2 | 1.1 | −27 (−21 to −34) | 12.3 | 0.7 | 11.1 | 1.5 | −10 (−4 to −16) | 0.08 |
| TbN (mm−1) | 1.71 | 0.109 | 1.35 | 0.115 | −21 (−16 to −26) | 1.90 | 0.078 | 1.60 | 0.161 | −16 (−12 to −20) | <0.01 |
| TbTh (mm) | 0.063 | 0.004 | 0.059 | 0.004 | −6 (−4 to −17) | 0.063 | 0.003 | 0.059 | 0.005 | −6 (0 to −17) | 0.30 |
| Tb1/NSD (mm) | 0.246 | 0.044 | 0.459 | 0.046 | 87 (72 to 96) | 0.25 | 0.031 | 0.282 | 0.064 | 13 (0 to 24) | <0.05 |
| DXA | |||||||||||
| lumbar spine (g/cm2) | 1.00 | 0.06 | 0.78 | 0.059 | −22 (−18 to −26) | 0.95 | 0.046 | 0.89 | 0.079 | −6 (−2 to −11) | 0.09 |
| femoral neck (g/cm2) | 0.67 | 0.044 | 0.56 | 0.043 | −17 (−12 to −21) | 0.68 | 0.033 | 0.60 | 0.058 | −11 (−7 to −16) | 0.05 |
| radius (g/cm2) | 0.48 | 0.03 | 0.36 | 0.029 | −24 (−21 to −29) | 0.53 | 0.023 | 0.51 | 0.039 | −4 (0 to −8) | 0.06 |
P values <0.05 indicate statistically significant differences between patients with and without fractures (main effect of a two-way analysis of covariance; fracture and gender as subject factors, age as covariate). HR-pQCT, high-resolution peripheral quantitative computed tomography; DXA, dual-energy x-ray absorptiometry; CI, confidence interval; Dtot, total density; HA, hydroxyapatite; Dcort, cortical density; CtTh, cortical thickness; Dtrab, trabecular thickness; BV/TV, trabecular bone volume; TbN, trabecular number; TbTh, trabecular thickness; Tb1/NSD, SD of 1/TbN.
Figure 1.
Two-dimensional reconstructions of high-resolution peripheral quantitative computed tomography measurements at the radius in two male dialysis patients (A) with and (B) without a history of low-impact fracture. Note the differences in cortical thickness and trabecular connectivity.
Table 5 shows the results from the age-corrected, multivariable logistic regression analysis and the ROC curves. A decrease of 1 SD was associated with a significant increased prevalence of fractures in most obtained parameters. Only the derived TbTh, the Dcort of the radius, and the aBMD of the lumbar spine were not associated with fracture status. The areas under the ROC curves (ROC-AUC) were significant for all DXA and HR-pQCT parameters. The highest individual ROC-AUC was obtained for the Dtrab at the tibia. Because of the positive association between fracture status and heterogeneity of the trabecular structure, the OR of the Tb1/NSD is <1.
Table 5.
Age-adjusted OR per 1 SD decrease and the area under the ROC curve for imaging parameters in patients with fractures
| OR (95% CI) | P | Area under ROC Curve (95% CI) | P | |
|---|---|---|---|---|
| HR-pQCT tibia | ||||
| Dtot | 2.86 (1.29 to 5.80) | 0.01 | 0.87 (0.77 to 0.98) | <0.01 |
| Dcort | 1.89 (1.02 to 3.57) | 0.04 | 0.84 (0.69 to 0.96) | <0.01 |
| CtTh | 2.56 (1.30 to 5.00) | 0.01 | 0.86 (0.76 to 0.97) | <0.01 |
| Dtrab | 3.70 (1.59 to 8.33) | 0.01 | 0.90 (0.80 to 0.99) | <0.01 |
| BV/TV | 3.45 (1.52 to 8.33) | 0.01 | 0.89 (0.79 to 0.99) | <0.01 |
| TbN | 2.86 (1.39 to 5.88) | 0.01 | 0.88 (0.77 to 0.98) | <0.01 |
| TbTh | 1.04 (0.63 to 1.61) | 0.95 | 0.83 (0.69 to 0.95) | <0.01 |
| Tb1/NSD | 0.28 (0.11 to 0.72) | 0.01 | 0.89 (0.79 to 0.99) | <0.01 |
| HR-pQCT radius | ||||
| Dtot | 1.82 (0.99 to 3.33) | 0.06 | 0.84 (0.71 to 0.96) | <0.01 |
| Dcort | 1.27 (0.74 to 2.13) | 0.39 | 0.83 (0.69 to 0.96) | <0.01 |
| CtTh | 1.85 (1.01 to 3.33) | 0.05 | 0.83 (0.70 to 0.96) | <0.01 |
| Dtrab | 2.17 (1.12 to 4.17) | 0.02 | 0.84 (0.72 to 0.96) | <0.01 |
| BV/TV | 2.17 (1.12 to 4.17) | 0.02 | 0.83 (0.70 to 0.96) | <0.01 |
| TbN | 2.86 (1.37 to 5.56) | 0.01 | 0.85 (0.72 to 0.98) | <0.01 |
| TbTh | 1.33 (0.76 to 2.33) | 0.31 | 0.84 (0.72 to 0.96) | <0.01 |
| Tb1/NSD | 0.49 (0.29 to 0.83) | 0.01 | 0.87 (0.76 to 0.98) | <0.01 |
| DXA | ||||
| femoral neck aBMD | 2.04 (1.04 to 3.85) | 0.04 | 0.84 (0.71 to 0.97) | <0.01 |
| lumbar spine aBMD | 1.96 (0.99 to 3.85) | 0.06 | 0.84 (0.71 to 0.97) | <0.01 |
| radius aBMD | 3.33 (1.35 to 8.33) | 0.01 | 0.85 (0.73 to 0.97) | <0.01 |
OR, odds ratio; ROC, receiver-operating characteristic; CI, confidence interval; HR-pQCT, high-resolution peripheral quantitative computed tomography; Dtot, total density; HA, hydroxyapatite; Dcort, cortical density; CtTh, cortical thickness; Dtrab, trabecular thickness; BV/TV, trabecular bone volume; TbN, trabecular number; TbTh, trabecular thickness; Tb1/NSD, SD of 1/TbN; DXA, dual-energy x-ray absorptiometry; aBMD, areal bone mineral density.
Discussion
This study is the first to investigate the efficacy of HR-pQCT in dialysis patients and to elucidate its discriminatory capacity between patients with and without fractures in comparison to DXA.
pQCT, which does not permit assessment of trabecular microarchitecture, has been used previously in hemodialysis patients (8,16,17). The authors describe a general reduction of cortical volumetric bone mineral density (vBMD), stable trabecular vBMD, and a significant relationship between bone loss and dialysis vintage. With pQCT, a significant association with fracture was found for lower Dcort and CtTh, but not for Dtrab or DXA measurements at the lumbar spine and femur (8). An important finding reported here is that poor trabecular microarchitecture is associated with bone fractures in dialysis patients. This suggests that the contribution of the trabecular network to the mechanical properties of bone becomes more important as the quality of the bone cortex deteriorates. Because HR-pQCT achieves a better resolution of trabecular structure than conventional pQCT, this association between fracture status and trabecular microarchitecture was probably missed in previous studies because of a lack of sensitivity.
A recent study investigated fracture status in patients with predialysis CKD with DXA and HR-pQCT (18). The authors described a distinct difference in HR-pQCT and DXA parameters between patients with a history of fracture and those without fracture, but the discriminatory power was low. Overall, the ROC-AUC were below 0.75 for all parameters, but increased from 0.50 to 0.60 for patients with a disease duration <2.7 years to 0.60 to 0.80 for a CKD duration >6.9 years (18). Because all patients in the study presented here had end-stage renal disease, the duration of CKD was considerably longer and the discriminatory power of HR-pQCT and DXA therefore accordingly better. A second study reported on HR-pQCT measurements in patients with stage 2 to 4 kidney disease in comparison to controls with normal kidney function (19). When patients with a history of fracture were excluded, bone microarchitecture of CKD stages 2 to 4 patients was found to resemble the values of controls with normal and osteopenic aBMD. Compared with these data, the bone microarchitecture of the cohort of dialysis patients reported here resembles values reported from HR-pQCT measurements in osteoporotic patients (19). Duration of dialysis, age, previous kidney transplantations, and the presence of a fracture exerted the strongest influence on bone loss.
In contrast, iPTH and serologic bone turnover markers were only weakly associated with bone microarchitecture. Similar low associations were described between serologic bone markers and HR-pQCT measurements in patients with normal kidney function (20). One explanation could be that these serologic markers reflect the current bone turnover status but give no information on previous bone turnover or influences detrimental to bone health. On the other hand, bone microarchitecture may be the sum of positive and negative influences on bone that occurred in the past, such as uremia, secondary hyperparathyroidism, adynamic bone disease, and medications. Therefore, associations between serologic markers of bone turnover and bone microarchitecture could be expected to be rather weak in the dialysis population. Nevertheless, particularly the lack of associations between iPTH and bone microarchitecture seem to be surprising. This could in part be a consequence of improved treatment and alleviation of the influence of secondary hyperparathyroidism on renal osteodystrophy because most patients had iPTH levels within or below the recommended range for dialysis patients (21). Interestingly, patients with fractures had similar clinical features, as did patients with osteoporosis. They were older and tended to have a lower BMI. Thus, bone loss and fractures in these patients may be due to a combination of multifactorial osteoporosis and renal osteodystrophy (22).
Only a few studies have compared the diagnostic utility of DXA and HR-pQCT to assess fracture status in patients with normal kidney function. Significantly lower HR-pQCT parameters at the radius but not the tibia were reported in postmenopausal women with fractures (10). In a matched case-control study, significantly lower volumetric BMD values were found in patients with fractures (23). In a recent study on forearm fracture risk and a second study on fracture prediction in postmenopausal women, the discriminatory power of HR-pQCT was similar as for patients with preterminal renal failure (24,25). A remarkable difference between these studies and the data presented here is that the heterogeneity of trabecular structures was markedly higher in dialysis patients with fracture history compared with dialysis patients without a history of fracture, especially in women. Overall, Tb1/NSD had a high discriminatory power to differentiate between fracture status in dialysis patients. Because Tb1/NSD can be the consequence of long-term renal osteodystrophy, it might prove to be a reliable parameter for the prediction of fracture risk in prospective studies.
When female and male dialysis patients were analyzed separately, DXA measurements showed a tendency for lower aBMD in patients with a history of fracture, but differences did not reach statistical significance, probably because of low sample size. In contrast, the differences in many HR-pQCT values between patients with and without fracture were statistically significant, especially at the tibia. HR-pQCT measurements at the tibia might therefore prove to be superior to DXA measurements in estimating fracture risk in dialysis patients.
This study has some limitations. Because the investigated data were cross-sectional, the results cannot be used to identify patients at a higher risk of future fractures. The time period between the occurrence of asymptomatic fractures and their diagnosis was unknown in many patients. The bone structure could have been better, or different, at the time the fracture occurred. Physical activity was not assessed. Low muscle strength and poor coordination can predispose to bone fracture or can be the consequence of a previous fracture leading to immobilization, which in turn contributes to bone loss. We did not perform bone biopsy studies, and the association between bone indices measured by HR-pQCT and microcomputed tomography or two-dimensional histomorphometry in chronic renal failure remains to be determined.
In conclusion, patients on chronic hemodialysis show damaged bone microarchitecture, low vBMD, and low aBMD. Alterations are more pronounced in patients with a history of fracture.
Acknowledgments
We sincerely thank Professor Sharmila Majumdar and Andy Burghardt from the Musculoskeletal Quantitative Imaging Research Group in the Department of Radiology at the University of California–San Francisco for providing the HR-pQCT data of the control group.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Barreto FC, Barreto DV, Moyses RMA, Neves KR, Canziani MEF, Draibe SA, Jorgetti V, Carvalho AB: K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int 73: 771–777, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C: Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Mares J, Ohlidalova K, Opatrna S, Ferda J: Determinants of prevalent vertebral fractures and progressive bone loss in long-term hemodialysis patients. J Bone Miner Metab 27: 217–223, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Jamal SA, Hayden JA, Beyene J: Low bone mineral density and fractures in long-term hemodialysis patients: A meta-analysis. Am J Kidney Dis 49: 674–681, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Ersoy FF, Passadakis SP, Tam P, Memmos ED, Katopodis PK, Ozener C, Akcicek F, Camsari T, Ates K, Ataman R, Vlachojannis JG, Dombros AN, Utas C, Akpolat T, Bozfakioglu S, Wu G, Karayaylali I, Arinsoy T, Stathakis PC, Yavuz M, Tsakiris JD, Dimitriades CA, Yilmaz ME, Gultekin M, Karayalcin B, Yardimsever M, Oreopoulos DG: Bone mineral density and its correlation with clinical and laboratory factors in chronic peritoneal dialysis patients. J Bone Miner Metab 24: 79–86, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Smith JA, Vento JA, Spencer RP, Tendler BE: Aortic calcification contributing to bone densitometry measurement. J Clin Densitom 2: 181–183, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Jamal SA, Gilbert J, Gordon C, Bauer DC: Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res 21: 543–548, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Amling M, Herden S, Posl M, Hahn M, Ritzel H, Delling G: Heterogeneity of the skeleton: Comparison of the trabecular microarchitecture of the spine, the iliac crest, the femur, and the calcaneus. J Bone Miner Res 11: 36–45, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Boutroy S, Bouxsein ML, Munoz F, Delmas PD: In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90: 6508–6515, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Genant HK, Wu CY, van KC, Nevitt MC: Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8: 1137–1148, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Laib A, Hauselmann HJ, Ruegsegger P: In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6: 329–337, 1998 [PubMed] [Google Scholar]
- 13. Laib A, Hildebrand T, Hauselmann HJ, Ruegsegger P: Ridge number density: A new parameter for in vivo bone structure analysis. Bone 21: 541–546, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Burghardt AJ, Hermannsson B, Pialat J, Boutroy S, Burrows M, Liu D, Patsch J, Valentinitsch A, Manoharan R, McKay H, Boyd S, Boxsein M, Majumdar S: Cross-site reproducibility of cortical and trabecular bone density and micro-architecture measurements by HR-pQCT. Osteoporos Int Suppl 1: 45–46, 2010 [Google Scholar]
- 15. Cejka D, Benesch T, Krestan C, Roschger P, Klaushofer K, Pietschmann P, Haas M: Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant 8: 1864–1870, 2008 [DOI] [PubMed] [Google Scholar]
- 16. MacNeil JA, Boyd SK: Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 29: 1096–1105, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Bacchetta J, Boutroy S, Juillard L, Vilayphiou N, Guebre-Egziabher F, Pelletier S, Delmas PD, Fouque D: Bone imaging and chronic kidney disease: Will high-resolution peripheral tomography improve bone evaluation and therapeutic management? J Ren Nutr 19: 44–49, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Nickolas TL, Stein E, Cohen A, Thomas V, Staron RB, McMahon DJ, Leonard MB, Shane E: Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol 21: 1371–1380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bacchetta J, Boutroy S, Vilayphiou N, Juillard L, Guebre-Egziabher F, Rognant N, Sornay-Rendu E, Szulc P, Laville M, Delmas PD, Fouque D, Chapurlat R: Early impairment of trabecular microarchitecture assessed with HR-pQCT in patients with stage II-IV chronic kidney disease. J Bone Miner Res 25: 849–857, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Chaitou A, Boutroy S, Vilayphiou N, Munoz F, Delmas PD, Chapurlat R, Szulc P: Association between bone turnover rate and bone microarchitecture in men—The STRAMBO study. J Bone Miner Res 25: 2313–2323, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Moe S, Drüeke T, Block G, Cannata-Andia J, Elder G, Fukagawa M, Jorgetti V, Ketteler M, Langman C, Levin A, MacLeod A, McCann L, McCullough P, Ott S, Wang A, Weisinger J, Wheeler D, Persson R, Earley A, Moorthi R, Uhlig K: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int 76: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Gal-Moscovici A, Sprague SM: Osteoporosis and chronic kidney disease. Semin Dial 20: 423–430, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD: Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: The OFELY study. J Bone Miner Res 22: 425–433, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Melton LJ, III, Christen D, Riggs BL, Achenbach SJ, Muller R, van Lenthe GH, Amin S, Atkinson EJ, Khosla S: Assessing forearm fracture risk in postmenopausal women. Osteoporos Int 21: 1161–1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stein EM, Liu XS, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Zhang C, Yin PT, Cosman F, Nieves J, Guo XE, Shane E: Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res 25: 2572–2581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]