Abstract
Summary
Background and objectives
End-stage renal disease is linked to alterations in thyroid hormone levels and/or metabolism, resulting in a high prevalence of subclinical hypothyroidism and low triiodothyronine (T3) levels. These alterations are involved in endothelial damage, cardiac abnormalities, and inflammation, but the exact mechanisms are unclear. In this study, we investigated the relationship between serum free-T3 (fT3) and carotid artery atherosclerosis, arterial stiffness, and vascular calcification in prevalent patients on conventional hemodialysis.
Design, setting, participants, & measurements
137 patients were included. Thyroid-hormone levels were determined by chemiluminescent immunoassay, carotid artery–intima media thickness (CA-IMT) by Doppler ultrasonography, carotid-femoral pulse wave velocity (c-f PWV), and augmentation index by Sphygmocor device, and coronary artery calcification (CAC) scores by multi-slice computerized tomography.
Results
Mean fT3 level was 3.70 ± 1.23 pmol/L. Across decreasing fT3 tertiles, c-f PWV and CA-IMT values were incrementally higher, whereas CACs were not different. In adjusted ordinal logistic regression analysis, fT3 level (odds ratio, 0.81; 95% confidence interval, 0.68 to 0.97), age, and interdialytic weight gain were significantly associated with CA-IMT. fT3 level was associated with c-f PWV in nondiabetics but not in diabetics. In nondiabetics (n = 113), c-f PWV was positively associated with age and systolic BP but negatively with fT3 levels (odds ratio = 0.57, 95% confidence interval 0.39 to 0.83).
Conclusions
fT3 levels are inversely associated with carotid atherosclerosis but not with CAC in hemodialysis patients. Also, fT3 levels are inversely associated with surrogates of arterial stiffness in nondiabetics.
Introduction
End-stage renal disease is linked to alterations in thyroid hormone levels and/or metabolism, resulting in specially a high prevalence of subclinical hypothyroidism and low triiodothyronine (T3) levels (1–5). Zoccali et al. (6) initially pinpointed that uremic inflammation shared strong links with these low T3 levels, and subsequent studies have also implicated thyroid hormone alterations with endothelial damage and cardiac abnormalities (7,8). Nevertheless, the exact mechanisms for these associations are not fully clear. In experimental models, thyroid hormones via blood vessel dilation, production of vasodilator molecules, inhibition of angiotensin II receptor expression, and its signal transduction are suggested to regulate endothelial function and vascular homeostasis and have anti-atherosclerotic effects (9–12). In nonuremic populations, the presence of overt as well as subclinical hypothyroidism has been related to accelerated atherosclerosis and coronary artery disease (13,14). In contrast, some studies found that subclinical hypothyroidism was associated with an increased risk of heart failure but not with an increased risk of coronary artery disease (15).
Recently, several observational studies have suggested that low T3 levels are sensitive predictors of cardiovascular and overall mortality in hemodialysis (HD) patients (5,7,16) raising the hypothesis that these relationships may be causal. Moreover, it has been shown that serum free-T3 (fT3) level associated with impaired endothelial function as assessed by flow-mediated dilation in nondiabetic nondialyzed patients with chronic kidney disease (CKD) (17). It is presently unknown whether atherosclerotic features commonly found in dialysis stages are associated with low T3 levels. In this cross-sectional study, we investigated the relationship between serum fT3 and carotid artery atherosclerosis, arterial stiffness (AS), and vascular calcification in prevalent patients on conventional HD.
Study Population and Methods
We studied total T3, fT3, free T4 (fT4) and thyroid-stimulating hormone (TSH) levels in the stored sera samples of 172 prevalent HD patients in whom carotid artery–intima media thickness (CA-IMT), AS, and coronary artery calcification score (CACs) were measured.
Patients
The patients enrolled were a subgroup of the participants of a prospective clinical trial (Ege Study; Clinicaltrials ID NCT00295191). Inclusion criteria for the Ege Study were to be aged between 18 and 80 years and were on thrice weekly HD; main exclusion criterion was life expectancy less than a year. Patients for this study were selected among Ege Study participants on the basis of the following inclusion and exclusion criteria. Inclusion criteria were CA-IMT, AS and CAC data measured within a period of 3 months in the context of the Ege Study and available stored sera sample for measurement of thyroid hormones obtained within the same interval. Exclusion criteria were abnormal TSH levels and using anti-thyroid medications. In total, 35 patients were excluded because of (1) abnormal TSH and/or use of anti-thyroid medications (n = 29) and (2) presence of cardiac arrhythmia (n = 6). Thus, this study analyzes the remaining 137 patients.
Demographic, clinical, and laboratory parameters were collected from patients' charts. The local ethics committee approved the study and informed consent was obtained from all patients. The study was performed according to the recommendations of the Declaration of Helsinki of 1975 (and as revised in 1983). The etiology of CKD was diabetic nephropathy in 24, chronic glomerulonephritis in 11, chronic pyelonephritis in 12, hypertensive renal disease in 29, polycystic kidney disease in 14, amyloidosis in two, and unknown in 45. At the time of enrollment, only 18 (13.1%) patients were on anti-hypertensive medication: mainly calcium channel blockers (n = 12) and angiotensin-converting enzyme inhibitors (n = 6). Thirty-three percent of the patients were on active vitamin D treatment, 27% on treatment with erythropoietin, 25% on intravenous iron therapy, and 84% on treatment with calcium-based phosphate binders. Only 8.2% percent of the patients were receiving statins. All of the patients were on thrice weekly 4-hour hemodialysis treatment with standard bicarbonate dialysis. Sixty percent of them were dialyzed by high-flux membrane (FX60/80; Fresenius Medical Care, Bad Homburg, Germany).
Laboratory Measurements
Blood samples were collected at the beginning of the HD session under fasting conditions. Sera from the blood samples were separated and kept frozen at − 70°C until use. All of the biochemical parameters including albumin, creatinine, total cholesterol, triglyceride, HDL cholesterol, phosphate, and highly-sensitive C-reactive protein (hs-CRP) were performed by standard auto analyzers (Architect C8000 and CELL-DYN 3700) in the same central laboratory registered to external quality control programs. LDL cholesterol was calculated by the Friedewald formula (18). Serum TSH (reference range, 0.27 to 4.2 mU/L), total T3 (reference range, 0.84 to 2.02 ng/ml), fT3 (reference range, 3.10 to 6.80 pmol/L), and fT4 levels (reference range, 12 to 20 pmol/L) were measured by a chemiluminescent immunoassay method (Cobas E systems; Roche Diagnostics GmbH). The coefficient of variations were 4.2% for fT3, 3.2% for T3, 3.9% for fT4, and 6.1% for TSH.
Measurement of CA-IMT Thickness
Ultrasonographic studies on common carotid arteries were carried out by gray scale high-resolution color Doppler ultrasound (ATL HDI 5000 scanner Philips, ATL ultrasound, Bothell, WA, ABD) equipped with 5 to 12 MHz linear transducer. The same operator performed all procedures on both sides of two longitudinal images of the each common carotid artery in the morning. An average of two CA-IMT values from each side were used to calculate mean CA-IMT. In addition, presence of CA plaque was recorded. The intraobserver coefficient of variation was 2.68%.
Measurement of AS
AS was evaluated using Sphygmocor device (AtCor Medical, Sydney, Australia) by the same operator. Augmentation index (AIx) was calculated from pulse waves of the radial artery that were recorded by applanation tonometry, as described previously (19). Carotid-femoral pulse wave velocity (c-f PWV) was measured by sequential recordings of the arterial pressure wave at the carotid and femoral arteries and by measurement of the distance from the carotid sampling site to the suprasternal notch and from the suprasternal notch to the femoral sampling site. With a simultaneous electrocardiography recording of the R-wave as reference, the integral software calculated the pulse wave transit time. The intraobserver variability was 4.1%.
Measurement of CAC
Multi-slice computed tomography scans were performed with a 16-slice technique (Aquilion 16; Toshiba Medical Systems, Tokyo, Japan). All of the scans with slices of 3.0 mm thickness were acquired under the following conditions: 250 mA of tube current, 62 mA effective. The images were obtained during a single breath-hold of 12 to 15 seconds. The data obtained during the diastolic phase of the cardiac cycle were used for image reconstruction, with electrocardiography monitoring. Calcium scoring was performed on the reconstructed image sets with commercially available software (Terarecon 3.4.2.11). Threshold calcium determination was set using a density of at least 130 Hounsfield units. CAC score was calculated by summing the calcification score in the left main, the left anterior descending, the left circumflex, and the right coronary arteries. The calcium score was blindly evaluated by the same radiologist, according to the method described by Agatston et al. (20). The intraobserver variability for CAC was 1.7% with using volume calculations.
Statistical analyses
All of the parameters were expressed as the means ± SD. P value less than 0.05 was considered statistically significant. Comparisons between two groups were assessed by chi-squared and independent t test analysis. Differences between more than two groups were analyzed by ANOVA. Spearman analysis was used for univariate correlations between thyroid hormones (total T3 and fT3) and other variables. Ordinary logistic regression analysis was performed to study the predictive factors for CA-IMT and c-f PWV tertiles as well as the severity of CACs (dichotomized as ≥400 versus <400). For each examination, only variables found as significant in univariate analyses were included in logistic regressions. All of the statistical analyses were performed using SPSS, version 15 (Chicago, IL).
Results
Patients
Patient characteristics are given in Table 1 according to tertiles of distribution of fT3. The presence of diabetes and that of cardiovascular disease (CVD) history were 18% and 21%, respectively. Only 2.8% of the patients had hypoalbuminemia (<3.5 g/dl). Hyperphosphatemia (>5.5 mg/dl) was present in 21% of the patients. Most of the patients (88%) had acceptable ekt/V values (>1.2). Eighty-one percent of the patients had normotension without using antihypertensive medication; hypertension prevalence was only 19%. Mean fT3 level was 3.70 ± 1.23 pmol/L, total T3 was 0.87 ± 0.18 ng/ml, fT4 was 12.5 ± 2.34 pmol/L, and TSH was 1.57 ± 0.80 mIU/ml. The patients in the low fT3 tertile were older, had lower hemoglobin and albumin levels, and had higher hs-CRP levels compared with middle and high fT3 tertiles. CACs did not vary across fT3 tertiles, and a weak inverse association was found between fT3 and CACs (r = − 0.16, P = 0.05), which did not stand multivariate adjustment (not shown). However, across decreasing fT3 tertiles, AS and CA-IMT were incrementally higher. Given that these associations were of interest, we studied them in multivariate analysis.
Table 1.
Demographic, clinic, and laboratory data according to serum free triiodothyronine (fT3) tertiles
| Lowest Tertile (<3.27 pg/ml) (n = 44) | Middle Tertile (3.27 to 3.85 pg/ml) (n = 50) | Highest Tertile (>3.85) (n = 43) | P | Rho (P) | |
|---|---|---|---|---|---|
| Age (years) | 62 | 62 | 52 | <0.01 | −0.32 (<0.01) |
| Men (%) | 45 | 42 | 53 | 0.53 | |
| Duration on HD (months) | 74 | 73 | 85 | 0.44 | |
| Smokers (%) | 23 | 44 | 28 | 0.06 | |
| Diabetes mellitus (%) | 23 | 18 | 12 | 0.40 | |
| Cardiovascular disease (%) | 23 | 24 | 12 | 0.28 | |
| Kt/V | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.3 | 0.48 | |
| T3 (ng/ml) | 0.73 ± 0.12 | 0.90 ± 0.11 | 1.02 ± 0.19 | <0.01 | 0.63 (<0.01) |
| fT4 (pmol/l) | 11.7 ± 2.1 | 12.4 ± 1.9 | 13.4 ± 2.8 | <0.01 | 0.28 (<0.01) |
| TSH (uIU/ml) | 1.68 ± 0.8 | 1.66 ± 0.9 | 1.36 ± 0.7 | 0.12 | |
| Hemoglobin (g/dl) | 11.2 ± 1.0 | 11.0 ± 0.8 | 11.7 ± 0.7 | <0.01 | 0.19 (0.02) |
| Albumin (g/dl) | 3.98 ± 0.3 | 4.03 ± 0.3 | 4.14 ± 0.2 | 0.02 | 0.19 (0.03) |
| CRP (mg/dl) | 1.65 ± 2.2 | 1.13 ± 1.13 | 0.9 ± 1.15 | 0.09 | |
| Total cholesterol (mg/dl) | 169 ± 48 | 172 ± 41 | 169 ± 46 | 0.91 | |
| Triglycerides (mg/dl) | 179 ± 105 | 171 ± 92 | 187 ± 79 | 0.72 | |
| High-density lipoprotein cholesterol (mg/dl) | 31 ± 7 | 32 ± 9 | 31 ± 10 | 0.45 | |
| Low-density lipoprotein cholesterol (mg/dl) | 103 ± 36 | 105 ± 34 | 101 ± 34 | 0.81 | |
| Augmentation index (%) | 30 ± 11 | 28 ± 9 | 27 ± 11 | 0.24 | −0.18 (0.04) |
| c-f PWV (m/s) | 11 ± 3 | 11 ± 4 | 8.5 ± 2.6 | <0.01 | −0.35 (<0.01) |
| CA-IMT (mm) | 0.78 ± 0.18 | 0.77 ± 0.16 | 0.70 ± 0.14 | 0.06 | −0.23 (<0.01) |
| CA plaque (%) | 65.8 | 55.7 | 42.1 | 0.10 | −0.19 (0.03) |
| CAC score | 796 ± 1672 | 830 ± 1117 | 368 ± 876 | 0.17 | −0.16 (0.05) |
| Erythropoietine use (%) | 29.5 | 22.2 | 30.7 | 0.59 | |
| Vitamin D use (%) | 27.2 | 30.0 | 39.0 | 0.42 |
fT4, free T4; TSH, thyroid-stimulating hormone; CRP, C-reactive protein; c-f PWV, carotid-femoral pulse wave velocity; CA-IMT, carotid artery–intima media thickness; CA, coronary artery; CAC, coronary artery calcification.
Free T3 Level and CA-IMT
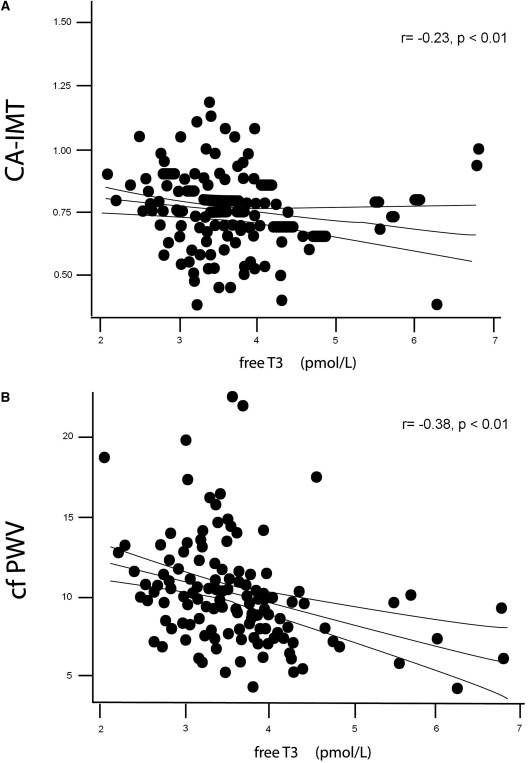
Mean CA-IMT was 0.75 ± 0.16 mm. fT3 level was inversely correlated with CA-IMT (r = − 0.23, P <0.01) (Figure 1A) in univariate analysis. The patients in the highest CA-IMT tertile (IMT >0.8 mm) were older, more likely to be diabetic, and had lower serum albumin and fT3 levels and higher systolic and diastolic BP, interdialytic weight gain, and hs-CRP compared with middle and lowest tertiles (data not shown). In ordinal logistic regression analysis adjusted with variables related to CA-IMT, fT3 level along with age and interdialytic weight gain were significantly associated with CA-IMT (model χ2: 51.18, P < 0.0001; pseudo r2 = 0.35) (Table 2). The lowest fT3 tertile was associated with higher CA-IMT values compared with high fT3 tertile (odds ratio [OR], 2.17; 95% confidence interval [CI], 1.24 to 3.29; P = 0.006).
Figure 1.
Univariate correlations between serum free-T3 level and CA-IMT (carotid artery–intima media thickness, A) and c-f PWV (carotid-femoral pulse wave velocity, B).
Table 2.
Ordinal regression showing predictors associated with carotid artery–intima media thickness in 137 prevalent hemodialysis patients
| Odds Ratio (95% CI) |
|||
|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | |
| Free T3 (per 1 pmol/L) | 0.81 (0.66 to 0.98), P = 0.03 | 0.81 (0.67 to 0.97), P = 0.02 | 0.81 (0.68 to 0.97), P = 0.02 |
| Age (per 1 year) | 1.05 (1.03 to 1.08), P < 0.01 | 1.05 (1.02 to 1.07), P < 0.01 | |
| IDWG (per 100 ml) | 1.06 (1.02 to 1.10), P < 0.01 | 1.06 (1.02 to 1.09), P < 0.01 | |
The values of carotid artery–intima media thickness were categorized according to tertiles. Model 1 was adjusted for age, gender, diabetes, cardiovascular disease history, smoking, time on hemodialysis, systolic and diastolic blood pressure, and IDWG. Model 2 was model 1 + serum albumin and C-reactive protein. (Model chi-squared: 51.18, P < 0.0001; pseudo r2 = 0.35.) CI, confidence interval; IDWG, interdialytic weight gain; T3, triiodothyronine.
The presence of carotid artery plaque was 54.9%. fT3 levels were not different between the patients with and without carotid artery plaques (3.66 ± 1.55 versus 3.79 ± 0.76 pmol/L, P = 0.55).
Free T3 Level and AS
Mean c-f PWV and AIx values were 10 ± 3.2 m/s and 28% ± 10%, respectively. fT3 level was inversely correlated with c-f PWV (r = − 0.37, P <0.01) (Figure 1B) and AIx (r = − 0.18, P = 0.04) in univariate analysis. However, fT3 was not a significant predictor for arterial stiffness parameters in the whole group in adjusted models.
Because diabetes is an important confounder in the associations with surrogates of endothelial function, we reanalyzed the association between fT3 and c-f PWV in nondiabetics separately (n = 113). Moreover, fT3 level was associated with c-f PWV in nondiabetics but not in diabetics (P <0.01 for the interaction between c-f PWV and diabetes status). Nondiabetic patients in the highest c-f PWV tertile were older, more likely to be men, had higher frequency of CVD history, and had longer time on HD, higher BP and CRP, and lower albumin and fT3 levels compared with other tertiles (not shown). Multivariate analysis showed that an increase in c-f PWV was positively associated with age and systolic BP and negatively with fT3 level (model r2 = 0.69; Table 3). Compared with the highest fT3 tertile, both the lowest and middle fT3 levels were associated with higher c-f PWV (OR, 2.88; 95% CI, 1.62 to 5.13; P <0.01 and OR, 2.53; 95% CI, 1.49 to 4.29; P <0.01, respectively).
Table 3.
Ordinal regression analysis for the risk of being in a higher pulse wave velocity tertile in 113 nondiabetic patients
| Variables | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Age (years) | 1.07 | 1.04 to 1.10 | <0.01 |
| Systolic blood pressure (mmHg) | 1.03 | 1.01 to 1.05 | 0.01 |
| Free T3 (pmol/L) | 0.57 | 0.39 to 0.83 | <0.01 |
This multivariate model includes age, gender, cardiovascular disease history, time on hemodialysis, systolic and diastolic blood pressure, serum albumin, and C-reactive protein. (Model chi-squared: 124, P <0.01; pseudo r2 = 0.65.) T3: triiodothyronine.
Total T3 Level and Associated Variables
Serum T3 level was directly correlated with fT3 (r = 0.63, P <0.01) and inversely correlated with age (r = − 0.33, P <0.01), presence of diabetes (r = − 0.23, P <0.01), CA-IMT (r = − 0.21, P = 0.01), and c-f PWV (r = − 0.29, P <0.01). T3 levels were not different between patients with and without carotid artery plaques. T3 was not a predictor both CA-IMT and c-f PWV after adjustment for age and diabetes (data not shown).
Discussion
Our results show that among patients on long-term chronic hemodialysis, fT3 level is associated with the degree of carotid atherosclerosis. Serum fT3 level is also associated with AS in nondiabetic subjects.
In the nonuremic population, the presence of both overt hypothyroidism and subclinical hypothyroidism is related to accelerated atherosclerosis (14,21). Hypothyroidism is associated with several traditional risk factors for atherosclerosis such as elevation of total cholesterol, LDL cholesterol level and oxidation, apolipoprotein B level, and reduction of HDL cholesterol levels. In addition, hyperhomocysteinemia, increased CRP level, and decreased fibrinolytic activity (low D-dimer and high plasminogen activator inhibitor-1 levels) have been proposed as novel risk factors in hypothyroidic patients (21–23). Although CKD patients are reported to have increased frequency of subclinical hypothyroidism (1,2) and Low-T3 syndrome (5), the clinical implications of this altered thyroid metabolism are not well defined.
Accelerated atherosclerosis is commonly observed in dialysis patients (24), and as a novel finding in our study, we report that low serum fT3 levels were an important determinant of carotid atherosclerosis in HD patients. Several mechanistic explanations may justify this association. First, the association between fT3 and both albumin and hs-CRP found in our study may reflect the role of malnutrition-inflammation in the pathogenesis of atherosclerosis in this population (5). Second, low T3 level is significantly associated with left ventricular dysfunction and hypertrophy in dialysis patients (25), an association that may in part depend on arterial rigidity. Because adjustment for inflammation markers (IL-6 and albumin) in that study abrogated these relationships, the authors suggested that the link between low fT3 and cardiomyopathy was mediated by inflammation (25). In our study, however, adjustment for CRP and albumin did minimally affect the link between fT3 and IMT. It is possible that differences in sample size, definitions, and confounders used in multivariate adjustment as well as methodology (multivariate versus logistic regression) may infer in this apparent contradiction that warrants confirmation. However, several studies have suggested anti-atherosclerotic effects for fT3 on the vascular bed via its effect on mitochondrial oxidation systems, induction of vasodilatatory molecules, and inhibition of angiotensin II receptor expression and downstream signal transduction not necessarily involving the inflammatory cascade (12,26,27).
Another novel finding in our study is that serum fT3 levels were inversely related to AS in nondiabetic HD patients. Dialysis patients have increased AS as compared with the general population. However, the underlying mechanisms are not well defined and may include chronic fluid overload, arterial calcifications, microinflammation, abnormalities of the nitric oxide system, dyslipidemia, and endothelial dysfunction (28). In nonuremic populations, overt as well as subclinical hypothyroidisms are linked to increased AS (29). Nevertheless, it has been suggested that T3 has important effects on the vascular system, by inducing relaxation of vascular smooth muscle cells through a direct or indirect effect via activation of nitric oxide synthase and inducing adrenomedullin expression in endothelial cells (9,10,30). Also, thyroid hormone enhances angiogenesis (31). The association between thyroid hormone levels and AS has never been investigated in end-stage renal disease subjects. Our results therefore agree with and expand a recent study including nondiabetic patients with stage 3 to 4 CKD that reported an association between low fT3 level and endothelial dysfunction as assessed by flow-mediated vasodilation (17).
Finally, we fail to show a multivariate association between fT3 and vascular calcification. This rationale is based on experimental studies showing that T3 stimulates osteoblast activity and vascular smooth muscle cells both directly and indirectly via complex pathways involving many growth factors and cytokines (32,33). However, there are no data available on the effects of fT3 levels on in vivo vascular calcification in humans. Additionally, it is possible that progressive vascular calcification in dialysis patients has subtle differences from other diseases (34). Also, vascular calcification is not observed in every HD patient despite the presence of a similar risk profile (35).
Although T3 levels were closely related with fT3 levels, T3 levels were not a predictor for carotid atherosclerosis or AS. This may be expected, because fT3 represents the active hormone form with purportedly causal links in these processes. In a cohort of euthyroid incident dialysis patients, Carrero et al. (7) reported that T3 captured a bigger all-cause and CVD mortality prediction than fT3 levels. These results are not in contradiction because it is likely that T3, including binding to several transporters such as albumin, may additionally represent the mortality risk associated to the overall inflammatory status. Our study has, nevertheless, certain limitations that merit discussion. First is the existence of only one time point; it would be imperative to study determinants and implications of fT3 changes over time in dialysis patients. Second, the fact that our cohort excluded a priori patients with serious comorbid situations or with a life expectancy of less than 1 year may underestimate the effects observed.
To conclude, fT3 levels are inversely associated with carotid atherosclerosis but not with CAC in hemodialysis patients. Also, fT3 levels are inversely associated with surrogates of AS in nondiabetics. These results add to the growing body of evidence involving thyroid alterations in the cardiovascular risk of CKD patients. Specific mechanistic and intervention studies are warranted to clarify the nature of these cross-sectional associations.
Disclosures
Ercan Ok is a member of scientific advisory board of Fresenius Medical Care (Turkey). Juan Jesus Carrero acknowledges support from the Loo and Hans Ostermans Foundation and the Swedish research council. The others declare no conflict of interest.
Acknowledgments
We thank Aynur Sener, Gulhan Ozturk, and Arzu Elmas from DIALAB for their technical support. We thank the dialysis doctors and nurses in Fresenius Medical Care hemodialysis centers. We also acknowledge Necati Sezgin for his expert technical assistance for the study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Lo JC, Chertow GM, Go AS, Hsu CY: Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int 67: 1047–1052, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Chonchol M, Lippi G, Salvagno G, Zoppini G, Muggeo M, Targher G: Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 1296–1300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim VS: Thyroid function in patients with chronic renal failure. Am J Kidney Dis 38: 80–84, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Song SH, Kwak IS, Lee DW, Kang YH, Seong EY, Park JS: The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol Dial Transplant 24: 1534–1538, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Ozen KP, Asci G, Gungor O, Carrero JJ, Kircelli F, Tatar E, Ok Sevinc E, Ozkahya M, Toz H, Cirit M, Basci A, Ok E: Nutritional state alters the association between free triiodothyronine levels and mortality in hemodialysis patients. Am J Nephrol 33: 305–312, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Zoccali C, Tripepi G, Cutrupi S, Pizzini P, Mallamaci F: Low triiodothyronine: A new facet of inflammation in end-stage renal disease. J Am Soc Nephrol 16: 2789–2795, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Carrero JJ, Qureshi AR, Axelsson J, Yilmaz MI, Rehnmark S, Witt MR, Bárány P, Heimbürger O, Suliman ME, Alvestrand A, Lindholm B, Stenvinkel P: Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med 262: 690–701, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Disthabanchong S, Treeruttanawanich A: Oral sodium bicarbonate improves thyroid function in predialysis chronic kidney disease. Am J Nephrol 32: 549–556, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Ojamaa K, Klemperer JD, Klein I: Acute effects of thyroid hormone on vascular smooth muscle. Thyroid 6: 505–512, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Napoli R, Biondi B, Guardasole V, Matarazzo M, Pardo F, Angelini V, Fazio S, Sacca L: Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation 104: 3076–3080, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, Salvetti A, Ferrannini E, Monzani F: Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: Beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 88: 3731–3737, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Fukuyama K, Ichiki T, Takeda K, Tokunou T, Iino N, Masuda S, Ishibashi M, Egashira K, Shimokawa H, Hirano K, Kanaide H, Takeshita A: Downregulation of vascular angiotensin II type 1 receptor by thyroid hormone. Hypertension 41: 598–603, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Vanhaelst L, Neve P, Chailly P, Bastenie PA: Coronary-artery disease in hypothyroidism: Observations in clinical myxoedema. Lancet 2: 800–802, 1967 [DOI] [PubMed] [Google Scholar]
- 14. Nagasaki T, Inaba M, Henmi Y, Kumeda Y, Ueda M, Tahara H, Sugiguchi S, Fujiwara S, Emoto M, Ishimura E, Onoda N, Ishikawa T, Nishizawa Y: Decrease in carotid intima-media thickness in hypothyroid patients after normalization of thyroid function. Clin Endocrinol 59: 607–612, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC: Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 165: 2460–2466, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P: Low triiodothyronine and survival in end-stage renal disease. Kidney Int 70: 523–528, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Yilmaz MI, Sonmez A, Karaman M, Ay SA, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C: Low triiodothyronine alters flow-mediated vasodilatation in advanced nondiabetic kidney disease. Am J Nephrol 33: 25–32, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 19. Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ: Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 16: 2079–2084, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Morris MS, Bostom AG, Jacques PF, Selhub J, Rosenberg IH: Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis 155: 195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Christ-Crain M, Meier C, Guglielmetti M, Huber PR, Riesen W, Staub JJ, Müller B: Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis 166: 379–386, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Chadarevian R, Bruckert E, Leenhardt L, Giral P, Ankri A, Turpin G: Components of the fibrinolytic system are differently altered in moderate and severe hypothyroidism. J Clin Endocrinol Metab 86: 732–737, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Papagianni A, Kalovoulos M, Kirmizis D, Vainas A, Belechri AM, Alexopoulos E, Memmos D: Carotid atherosclerosis is associated with inflammation and endothelial cell adhesion molecules in chronic haemodialysis patients. Nephrol Dial Transplant 18: 113–119, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Zoccali C, Benedetto F, Mallamaci F, Tripepi G, Cutrupi S, Pizzini P, Malatino LS, Bonanno G, Seminara G: Low triiodothyronine and cardiomyopathy in patients with end-stage renal disease. J Hypertension 24: 2039–2046, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Costantini F, Pierdomenico SD, De Cesare D, De Remigis P, Bucciarelli T, Bittolo-Bon G, Cazzolato G, Nubile G, Guagnano MT, Sensi S, Cuccurullo F, Mezzetti A: Effect of thyroid function on LDL oxidation. Arterioscler Thromb Vasc Biol 18: 732–737, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Fukuyama K, Ichiki T, Imayama I, Ohtsubo H, Ono H, Hashiguchi Y, Takeshita A, Sunagawa K: Thyroid hormone inhibits vascular remodeling through suppression of cAMP response element binding protein activity. Arterioscler Thromb Vasc Biol 26: 2049–2055, 2006 [DOI] [PubMed] [Google Scholar]
- 28. London GM, Marchais SJ, Guerin AP, Metivier F, Adda H: Arterial structure and function in end-stage renal disease. Nephrol Dial Transplant 17: 1713–1724, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Nagasaki T, Inaba M, Kumeda Y, Hiura Y, Shirakawa K, Yamada S, Henmi Y, Ishimura E, Nishizawa Y: Increased pulse wave velocity in subclinical hypothyroidism. J Clin Endocrinol Metab 91: 154–158, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Isumi Y, Shoji H, Sugo S, Tochimoto T, Yoshioka M, Kangawa K, Matsuo H, Minamino N: Regulation of adrenomedullin production in rat endothelial cells. Endocrinology 139: 838–846, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ: Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res 94: 1500–1506, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Gogakos AI, Duncan Bassett JH, Williams GR: Thyroid and bone. Arch Biochem Biophys 503: 129–136, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Sato Y, Nakamura R, Satoh M, Fujishita K, Mori S, Ishida S, Yamaguchi T, Inoue K, Nagao T, Ohno Y: Thyroid hormone targets matrix Gla protein gene associated with vascular smooth muscle calcification. Circ Res 97: 550–557, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Floege J, Ketteler M: Vascular calcification in patients with end-stage renal disease. Nephrol Dial Transplant 19: 59–66, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Moe SM, O'Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA: Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 19: 2387–2393, 2004 [DOI] [PubMed] [Google Scholar]