Abstract
Summary
Background and objectives
Data are needed to assess safety and efficacy of the 2009 pandemic influenza A H1N1 vaccine in renal patients.
Design, setting, participants, & measurements
We prospectively evaluated seroconversion, predictors of response, and vaccine safety in renal patients. Hemagglutination inhibition tests to detect serum antibodies against a new influenza A-H1N1 virus were performed in 79 transplant patients, 48 hemodialysis patients, and 15 healthy workers before and 1 month after vaccination. Healthy controls and 88 of 127 renal patients were vaccinated. Seroconversion was defined as at least 2 dilutions increase in titer.
Results
We excluded 19 individuals seroprotected (≥1/40) against the novel H1N1 in the initial sample. Efficacy rate in the 96 vaccinated individuals was 43.7% (42 of 96 seroconverted versus four of 27 nonvaccinated patients, P = 0.007). For vaccinated subgroups, efficacy was 41.8% in transplant patients (P = 0.039 versus nonvaccinated), 33.3% in hemodialysis patients (P = 0.450), and 81.8% in controls. Healthy controls showed better response to vaccine than transplant (P = 0.021) and dialysis (P = 0.012) patients. For the transplant subgroup, longer time after transplantation (P = 0.028) was associated with seroconversion, but no influence was found for age, gender, renal function, or immunosuppression. In the hemodialysis subgroup, younger age was associated with response (55.7 ± 20.8 versus 71.6 ± 10.1 years, P = 0.042), but other specific variables, including Kt/V or time on dialysis, were not. No serious adverse events were reported, and kidney function was stable.
Conclusion
The novel influenza A 2009 H1N1 vaccine was safe in renal patients, although administration of a single dose of adjuvanted vaccine induced a poor response in these patients.
Introduction
A pandemic infection caused by a novel influenza A (H1N1) was initiated in April 2009 (1). Infection was more common in young persons and more severe in pregnancy and, presumably, in immunosuppressed patients (2–4). Immunization was immediately thought to be an important method of controlling the pandemic (5,6). The American Society of Transplantation (AST) and the Transplantation Society (TTS) developed a guidance document to manage novel H1N1 in solid organ transplant recipients, published in November 2009 when vaccines became available (7). Recommendations for immunization of transplant recipients included administration of at least one dose of the novel H1N1 vaccine (in spite of the lack of information on efficacy of this vaccine in this population), the seasonal inactivated influenza vaccine, and an update of pneumococcal vaccine. The proposal of administering at least one dose of the novel vaccine, even as soon as 1 month post transplantation, was based on the presumed risk for this type of population, despite the absence of published data in this specific subgroup. Vajo and collaborators showed that both influenza vaccines could be administered safely (5).
Although systematic annual vaccination with seasonal influenza vaccine is performed in many transplant centers, efficacy of this vaccine in immunosuppressed patients is still controversial. Some authors have communicated acceptable antibody responses 1 month after vaccination against seasonal H1N1 in a series of renal transplant recipients, but starting from a high seroprotection rate before seasonal vaccination (8). Seroconversion for seasonal H1N1 in those patients was only 30.3%, compared with 45% in controls, probably due to a high baseline level of protection against seasonal influenza for yearly vaccination. Other studies have shown mediocre results in spite of combining seroprotection and seroconversion concepts. In a recent study on the possible interactions between seasonal influenza vaccine and immune allograft responses, fewer than 10% of renal transplant patients showed seroconversion for seasonal H1N1, compared with 58% of controls (9). Most of them increased antibody titers without reaching seroconversion. Age, gender, time after transplantation, graft function, and type of immunosuppression had no influence on seroconversion (9).
We prospectively designed a study to evaluate the efficacy of H1N1 vaccines in renal allograft recipients and chronic renal failure patients on hemodialysis. The main objective was to establish possible predictors of response to 2009 pandemic influenza virus vaccine in this population.
Materials and Methods
Study Population and Vaccine
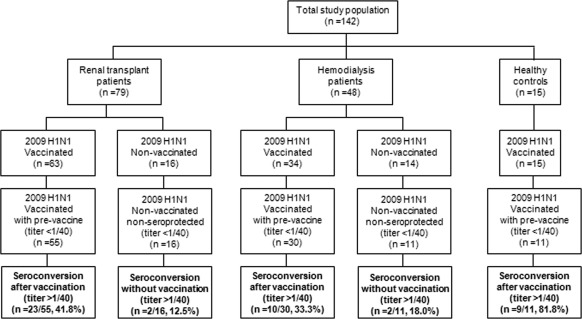
A total of 142 individuals were included in the study: renal allograft recipients (n = 79), chronic hemodialysis patients (n = 48), and health care workers with normal renal function as controls (n = 15). Patients were on hemodialysis in two units (one in-hospital unit and the other under our supervision), and renal transplant recipients attended our outpatient clinic between November and December 2009. All patients who consented were included. A total of 30 renal patients (16 renal allograft recipients and 14 patients on hemodialysis) refused to be vaccinated but accepted participation in the study as nonvaccinated patients for the new H1N1 antibody detection. Consequently, 63 renal transplant recipients and 34 hemodialysis patients were vaccinated. The 15 healthy controls were vaccinated. The study groups are summarized in Figure 1.
Figure 1.
Patient flow in the study.
All vaccinated individuals involved in the study received one dose of a split-virus inactivated vaccine with adjuvants against the H1N1 virus. All healthy controls received one dose of 3.75 μg of adjuvant-containing A/California/7/2009 (H1N1) v-like strain vaccine (Pandemrix®), as did 34 renal transplant patients and eight chronic renal failure patients on hemodialysis. The remaining 29 renal transplant patients and 26 patients on hemodialysis were vaccinated with one dose of 7.5 μg of adjuvant-containing A/California/7/2009 (H1N1) v-like strain vaccine Focetria®. In summary, 56 individuals received Pandemrix® and 56 received Focetria®. The two adjuvanted vaccines approved by the European Commission in November 2009 were used indiscriminately in our renal patients and the decision was made by primary care physicians as to which vaccine was administered. No differences were detected between the vaccines in each group of patients, so we considered all vaccinated patients as a group, without distinguishing the type of vaccine used.
The study was approved by our internal ethics review board. All patients and controls agreed to participate in the study and signed the informed consent form accepted by the internal review board at our hospital for the antibody tests. Demographics as well as laboratory data were obtained from clinical charts.
Sample Sera
Two sample sera were obtained from each individual, once before vaccination and again 8 weeks after vaccination. Samples from nonvaccinated patients were obtained contemporaneously. Sera were stored at –80 °C until tests were performed.
Antibody Test: Hemagglutination Inhibition Test
Antibodies against influenza A virus H1N1 (strain A/Brisbane/59/2007 [H1N1]) and against influenza A virus H1N1 new virus (strain A/California/7/2009 [H1N1]v), strains kindly provided by the WHO Influenza Center, MRC (UK), were detected by a hemagglutination inhibition (HI) test, performed as recommended by the WHO (10). Briefly, antigens for the HI test were prepared in our laboratory following cultivation of the virus strains in MDCK trypsin-treated cells, and the hemagglutination titer in the supernatant from cell cultures was quantified by hemagglutination using 0.75% guinea pig erythrocytes after virus ultraviolet inactivation. The HI test was performed in duplicate using 96-well plates, with sera treated with the trypsin-heat-periodate method, followed by adsorption with guinea pig erythrocytes to remove antierythrocyte agglutinins. Sera were diluted from 1:10 to 1:1280 (25 μl), and 4 HA (hemoagglutination) units of virus antigen (25 μl) added per well, followed by incubation for 45 minutes at room temperature, the addition of 0.75% guinea pig erythrocytes (50 μl), and incubation at room temperature for 60 minutes before reading of results. Seroprotection was considered when individuals showed HI titers were equal to or greater than 1/4. Seroconversion was considered when at least two dilutions increase in serum HI titer between the prevaccinated sample and the postvaccinated sample was observed, that is, four times the initial titer.
Statisticical Analyses
Comparisons of seroconversion rates were made between vaccinated and nonvaccinated patients and between the kidney transplant recipients, hemodialysis patients, and healthy controls. With a 5% type 1 error rate and a power of 90%, 16 renal patients and 16 healthy controls were required to show a difference of 50% in immunogenicity. In a more conservative approach, with a 5% type 1 error rate and a power of 80%, given that the response rate to seasonal vaccination in renal allograft recipients was 46% and the response in the general population was 72% to 96% in the different age groups, the number of transplant patients needed to show significant differences was 60. Calculated sample size for hemodialysis patients (about 35% vaccination response rate) was 40.
Univariate analyses were performed to assess the possible association between seroconversion and demographic, clinical, and analytical factors. Continuous variables are expressed as mean values ± SD or median and interquartile range, and categorical variables as numbers and percentages. The continuous data were analyzed using t test if they were normally distributed or a Wilcoxon rank-sum test if they were not. The categorical data were analyzed using contingency tables and the chi-squared or Fisher test, as appropriate. All of the analyses were two-tailed, and P values of <0.05 were considered significant.
Results
Study Population
For the analysis, we excluded 19 individuals (eight renal transplant patients, seven on hemodialysis, and four controls) who showed seroprotection against the novel H1N1 in the initial sample (hemagglutination titers ≥1/40).
Response to Vaccination
The global vaccine efficacy rate in the 96 vaccinated individuals was 43.7% (42 of 96 seroconverted, P = 0.007, compared with four of 27 nonvaccinated renal patients). For subgroups, efficacy rate was 41.8% in renal transplant patients (23 of 55, P = 0.039, compared with two of 16 nonvaccinated transplant recipients), 33.3% in hemodialysis patients (10 of 30, P = 0.450, compared with two of 11 nonvaccinated), and 81.8% in healthy controls (Figures 1 and 2). Healthy controls showed a significantly better response to vaccine than renal transplant patients (P = 0.021) and hemodialysis patients (P = 0.012), but there was not a significant difference between transplant and hemodialysis patients (0 = 0.443).
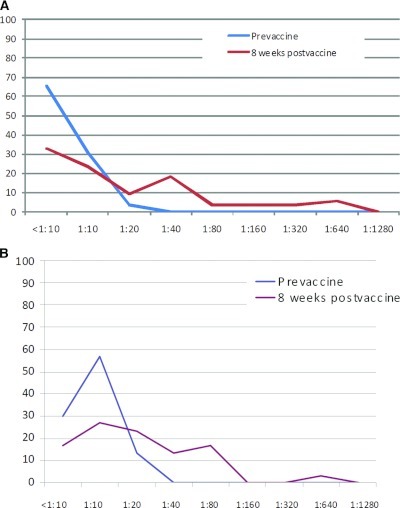
Figure 2.
(A) Distribution curves for titers of hemagglutination inhibition antibodies before and 8 weeks after the dose of 2009 pandemic influenza vaccine in vaccinated transplant patients not protected before vaccination (initial titers <1:40). (B) Distribution curves for titers of hemagglutination inhibition antibodies before and 8 weeks after the dose of 2009 pandemic influenza vaccine in vaccinated patients on hemodialysis not protected before vaccination (initial titers <1:40).
Factors Influencing Seroconversion
For the whole group, a univariate analysis showed that younger age was associated with seroconversion (53.3 ± 15.3 versus 62.1 ± 15.3 years old, P = 0.006) but not gender, weight, or the administration of the seasonal influenza vaccine. For the transplant subgroup, the univariate analysis showed that time after transplantation (92 ± 87 versus 45 ± 51 months, P = 0.028) was associated with seroconversion. Transplant patients were vaccinated a mean of 63.6 ± 68.8 months after transplantation. Only two of 79 transplant patients were vaccinated in the first 6 months after transplantation and they did not seroconvert. Additionally, 14 patients were vaccinated between 6 and 12 months after transplantation and five of 14 (35.7%) achieved serocoversion.
No influence was found for age, gender, weight, serum creatinine, Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate (MDRD-GFR), proteinuria, serum proteins, albumin, hemoglobin, leukocytes, ferritin, parathyroid hormone, vitamin D or immunosuppression (presence or absence of steroids, tacrolimus or cyclosporine, mycophenolate or whole blood trough tacrolimus, or cyclosporine levels).
In the hemodialysis subgroup, only age was associated with response (55.7 ± 20.8 versus 71.6 ± 10.1 years old, P = 0.042); other specific variables, including dose of dialysis measured as Kt/V or time on dialysis, were not. All data are shown in Table 1. The analysis of efficacy rates between both vaccines showed a trend toward a significant higher efficacy rate for Pandemrix® over Focetria® but did not reach significance in either group of patients (transplant patients, P = 0.107; hemodialysis patients, P = 0.078).
Table 1.
Demographics and laboratory data of patients vaccinated against 2009 pandemic influenza virus
| Non-Seroprotected Vaccinated Renal Patients (n = 85) |
Non-Seroprotected Vaccinated Transplant Patients (n = 55) |
Non-Seroprotected Vaccinated Hemodialysis Patients (n = 30) |
||||
|---|---|---|---|---|---|---|
| SCV (n = 33) | Non-SCV (n = 52) | SCV (n = 23) | Non-SCV (n = 32) | SCV (n = 10) | Non-SCV (n = 20) | |
| Age (years) | 54.67 ± 15.08 | 63 ± 15.9 | 54.22 ± 12.35 | 57.59 ± 15.36 | 55.7 ± 20.84 | 71.65 ± 10.09 |
| Gender (male/female) | 20/13 | 34/18 | 12/11 | 21/11 | 8/2 | 13/7 |
| Weight (kg) | 70.04 ± 14.21 | 71.23 ± 14.56 | 72.52 ± 12.85 | 74.83 ± 15.42 | ||
| Seasonal vaccine (yes/no) | 25/8 | 9/43 | 17/6 | 27/5 | 8/2 | 16/4 |
| Time after transplant (months) | 62.12 (12.97; 159.37) | 31.34 (8.93; 60.197) | ||||
| Creatinine pre vaccine (mg/dl) | 1.95 ± 0.62 | 1.85 ± 0.53 | ||||
| MDRD-GFR pre vaccine (ml/min/1.73 m2) | 43.55 ± 19.15 | 44.19 ± 14.70 | ||||
| Serum proteins (g/L) | 7.4 (7.12; 8) | 7.4 (7.1; 7.8) | 7.4 (7.2; 8) | 7.4 (7.1; 7.67) | 7.5 (6.35; 8.15) | 7.6 (6.4; 8.2) |
| Serum albumin (g/L) | 4.24 ± 0.36 | 4.21 ± 0.63 | 4.33 ± 0.27 | 4.36 ± 0,40 | 4.03 ± 0.47 | 3.92 ± 0.88 |
| Proteinuria pre vaccine (mg/day) | 186 (106; 514) | 159 (117; 235) | ||||
| Hemoglobin (g/dl) | 12.8 (11.45; 14.05) | 13.1 (12.1; 13.8) | 12.9 (11.5; 14.1) | 13.5 (12.32; 13.9) | 12.55 (10.2; 14) | 12.4 (11.1; 13.5) |
| Leukocytes 103/mm3 | 7.521 ± 2.798 | 7.328 ± 2.331 | 8.235 ± 3.011 | 7.618 ± 2.408 | 5.880 ± 1.179 | 6.839 ± 2.170 |
| Ferritine (mg/dl) | 238 (59.5; 341.5) | 255 (136; 470) | 149 (39; 264) | 211 (47; 321) | 272.5 (119; 366.25) | 320.5 (194.5; 503.5) |
| PTH (pg/ml) | 152.5 (66.75; 203) | 112 (62.75; 207.25) | 125 (66.75; 191.5) | 83 (59; 138) | 172 (82; 497) | 200 (107; 391) |
| Creatinine post vaccine (mg/dl) | 2.00 ± 1.15 | 1.75 ± 0.76 | ||||
| Proteinuria post vaccine (mg/day) | 158 (124.5; 727.75) | 192 (122; 270) | ||||
| Steroid treatment (yes/no) | 14/9 | 20/12 | ||||
| Tacrolimus treatment (yes/no) | 20/2 | 28/4 | ||||
| Tacrolimus dose (mg/day) | 4.45 ± 2.86 | 4.16 ± 2.38 | ||||
| Tacrolimus trough levels (ng/ml) | 9.23 ± 2.41 | 9.12 ± 2.59 | ||||
| Treatment with MPA (yes/no) | 16/6 | 27/5 | ||||
| MPA trough levels (mcg/ml) | 1.85 (1.7; 2.35) | 2.15 (1.6; 2.67) | ||||
| Kt/V single pool (dose of dialysis) | 1.63 ± 0.32 | 1.81 ± 0.29 | ||||
| Time on dialysis (months) | 25.35 ± 23.13 | 43.98 ± 42.3 | ||||
All data are pre vaccine, except for those marked as post vaccine. Normal variables are expressed as mean ± standard deviation; not normal variables are expressed as median (75th percentile; 25th percentile range). SCV, seroconversion; MDRD GFR, modification of diet in renal disease study equation for estimating glomerular filtration rate; PTH, parathyroid hormone; MPA, mycophenolate.
Given that univariate analyses gave very few significant clinical and analytical associations with seroconversion, a multivariate model was not explored.
Safety
Adverse events were recorded: mild ones after asking the patients if they noted any sign or symptom related to vaccination, and severe ones after opening a registry. No severe adverse events were detected or reported throughout the follow-up. Renal function (serum creatinine, GFR, and proteinuria) remained stable throughout the study period in transplant recipients. After 6 months of follow-up, serum creatinine was stable and no episodes of acute rejection were recorded among the vaccinated transplant recipients. No patients included in the study (either vaccinated or not) developed influenza in the year following vaccination.
Discussion
Although guidelines were not yet available at the peak of the novel influenza A H1N1 2009 pandemic, we administered one dose of the new vaccine to our renal patient population as soon as vaccines were available in November 2009. Our protocol was similar to that finally advised by the Transplantation Societies, which recommended vaccination of dialysis and transplant patients with at least one dose of inactivated split virus 2009 H1N1 vaccine or H1N1 vaccine containing adjuvants (7). However, administration of a single dose of adjuvanted vaccine induced a poor response in these patients. Among those renal patients showing a prevaccination titer <1/40, only 41.8% of transplant recipients and 33% of hemodialysis patients showed a titer >1/40 over 4 weeks after receiving the vaccine. Despite being a poor response when compared with healthy controls, seroconversion rate in our vaccinated transplant patients was significantly higher than in those who refused vaccination. Unfortunately, the results in vaccinated and nonvaccinated hemodialysis patients were not statistically different.
These results are not very different from those achieved in the past with seasonal influenza vaccine in immunosuppressed patients. Some authors have communicated 92.7% seroprotection against seasonal H1N1 in a series of 165 renal transplant recipients, but starting from a rate of 78.2% seroprotection before seasonal vaccination, compared with 25% baseline seroprotection and 70.7% 1 month after vaccination in 41 healthy volunteers (8). Seroconversion (defined as a fourfold rise in hemagglutination titer after vaccination) for seasonal H1N1 in those 165 renal transplant patients was only 30.3%, compared with 45% in controls, probably due to a high baseline level of protection against seasonal influenza for yearly vaccination. Other studies have shown mediocre results despite combining seroprotection and seroconversion concepts. In a recent study on the possible interactions between seasonal influenza vaccine and immune allograft responses, Candon and collaborators found that only 9.5% of 66 renal transplant patients showed seroconversion for seasonal H1N1, compared with 57.9% of controls (9). Most of them had increased antibody titers without reaching seroconversion; age, gender, time after transplantation, graft function, and type of immunosuppression had no influence on seroconversion (9).
In our experience, only length of time after transplantation was positively associated with seroconversion in transplant recipients. Seroconversion was observed in patients with a functioning graft after a mean of 8 years, whereas those without adequate vaccine response had a graft functioning for a mean of 4 years. A recent report showed that shorter time after transplantation decreased the immune response to seasonal influenza vaccine (11). In this report, use of MMF was also associated with decreased response; as most of our transplant recipients were on MMF, we could not test for this difference. However, heavy immunosuppression, mostly based on tacrolimus and MMF, may explain our low seroconversion rate. Younger age at vaccination was the only factor associated with seroconversion among hemodialysis patients.
Our control group showed a seroconversion rate of 81.8% after only one dose of vaccine. This is in agreement with the results reported in the general population. A single dose of an inactivated split influenza A (H1N1) 2009 vaccine induced an HI assay titer of 1:40 or more in nearly all 18- to 64-year-old volunteers (12,13). Similarly, a multicentered, double-blind, randomized trial in subjects receiving a single dose of a split virion A (H1N1) 2009 vaccine in China showed that potentially protective serologic titers were detected on Day 21 in 89.5% of 18- to 60-year-old adults and in 80.3% of adults older than 60 years (6). The fact that it is possible to induce potentially protective antibody levels against A (H1N1) infection in adults within 2 weeks of administration of a single dose of vaccine has now been confirmed with every pandemic H1N1 vaccine tested (5,14,15).
Most of the global pandemic influenza strategies are usually based on previous work showing that at least two doses of a pandemic vaccine would be needed to elicit a protective immune response in populations who are immunologically naive to a new strain (5). Nevertheless, publications on the immunogenicity of 2009 pandemic influenza A H1N1 vaccines showed that only children under 12 years of age needed two doses of any of the new vaccines for H1N1 2009 influenza A for adequate protection (14). Such an assessment has not been performed for other population subgroups. In our experience reported in this study, recently transplanted patients and older patients on hemodialysis are two special populations that probably need at least two doses of vaccine to seroconvert. Nevertheless, when such an approach has been used in hemodialysis, no improvement was observed in seroconversion rates over seasonal influenza vaccine (16).
In agreement with other experience in dialysis patients (17), minor side effects were reported by most vaccinated individuals, but there were no serious adverse events. Although the augmentation of cellular alloimmunity in transplant recipients after influenza vaccination is well described (18), we did not detect any effect on graft function after vaccination in our patients.
Our study has important limitations. The number of patients and controls is low; nonetheless, the results are quite consistent with those reported for seasonal influenza vaccination in previous studies. We used two different vaccines in our patient population. Since we did not see a clear significant difference in antibody response between either vaccine in each group of patients and the existing literature shows very similar efficacy and behavior, we decided to combine the results of both vaccination populations to strengthen the analysis (19). Interestingly, Pandemrix® showed a trend toward a significantly higher efficacy rate in transplant and dialysis patients compared with Focetria®, suggesting that studies including a greater number of renal patients may indicate that this specific vaccine may be more successful in our population. In addition, we decided to exclude individuals who showed seroprotection in the initial sample (antibodies against the H1N1 new virus >1:40), as the level of baseline seroprotection has been thought to impact response to seasonal influenza vaccine in previous studies (9). The 2009 H1N1 vaccine was marketed in Spain once the pandemic, in mid-winter, had already spread across the country. Individuals were vaccinated while not knowing their serologic status. Initial seroprotection can therefore be attributed to previous or contemporaneous asymptomatic new influenza A disease, which may make the interpretation of the level of the postvaccine antibody and consequently vaccine efficacy quite difficult. Only five of our 19 initially seroprotected patients showed increase in titers, four of them 1 month after vaccination and only one fulfilling seroconversion criteria. As this is the first published study on the efficacy of the influenza A H1N1 2009 vaccine in these patients, we have decided to analyze it in the “purest way.”
In conclusion, the novel influenza A 2009 H1N1 vaccine proved to be safe in renal patients, even though the administration of a single dose of adjuvanted vaccine induced a poor response. Although the WHO declared an end to the 2009 influenza A (H1N1) pandemic, influenza A H1N1 was recommended for inclusion in the 2010 trivalent influenza vaccine. Another wave of infection occurred during the 2010 to 2011 influenza season, and severity in transplant population appears to be similar to the 2009 pandemic (3,4). The response to the vaccine is suboptimal in renal patients, a population that remains uniquely susceptible to the disease in annual outbreaks.
Large-scale vaccination among renal patients is needed each year, including new virus strains and seasonal ones, probably using at least two doses and routinely assessing seroprotection.
Contributors
MC and JP designed the study, analyzed and interpreted data, and wrote the report. SC analyzed and interpreted data. MM, SH, HC, FB, and JMP managed the study, served as principal investigators for different patient populations, and collected data. CS selected data from healthy controls and analyzed and interpreted data. CH and AF collected data and assessed adverse events. JGL undertook the hemagglutination assays. MM and JPH interpreted data. All authors critically reviewed the report.
Disclosures
None.
Acknowledgments
We are indebted to Rosa Causadias, Merce Borderia, Josefina Pi-Sunyer, Ernestina Junyent, and Nuria Pujolar for their technical assistance. Data contained in this manuscript were presented at the American Transplant Congress 2011, Philadelphia, Pennsylvania. This study was performed, in part, with funding from the project ISCIII PI10/01370.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Vaccinations in Kidney Transplant Patients: Searching for Optimal Protection,” on pages 2099–2101.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) in humans. N Engl J Med 360:2602–2615, 2009 [DOI] [PubMed] [Google Scholar]
- 2. New influenza A/H1N1 virus: Global epidemiological situation. Wkly Epidemiol Rec 84: 249–257, 2009 [PubMed] [Google Scholar]
- 3. Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, Danziger-Isakov L, Stosor V, Estabrook M, Gantt S, Marr KA, Martin S, Silveira FP, Razonable RR, Allen UD, Levi ME, Lyon GM, Bell LE, Huprikar S, Patel G, Gregg KS, Pursell K, Helmersen D, Julian KG, Shiley K, Bono B, Dharnidharka VR, Alavi G, Kalpoe JS, Shoham S, Reid GE, Humar A, American Society of Transplantation H1N1 Collaborative Study Group: Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: A multicentre cohort study. Lancet Infect Dis 10: 521–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ridao-Cano N, Sanchez-Fructuoso AI, Rodriguez-Moreno A, Barrientos A: H1N1 2009 Influenza in kidney transplant patients. Transplantation 90: 224–225, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Vajo Z, Tamas F, Sinka L, Jankovics I: Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009–10 influenza season: A multicentre, randomised controlled trial. Lancet 375: 49–55, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, Li RC, Xia SL, Zhao YL, Li FJ, Yan SH, Yin WD, An K, Feng DJ, Cui XL, Qi FC, Ju CJ, Zhang YH, Guo ZJ, Chen PY, Chen Z, Yan KM, Wang Y: Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375: 56–66, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Kumar D, Morris MI, Kotton CN, Fischer SA, Michaels MG, Allen U, Blumberg EA, Green M, Humar A, Ison MG, AST Infectious Diseases Community of Practice and Transplant Infectious Diseases Section of TTS: Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am J Transplant 10:18–25, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Scharpé J, Evenepoel P, Maes B, Bammens B, Claes K, Osterhaus AD, Vanrenterghem Y, Peetermans WE: Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant 8: 332–337, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Candon S, Thervet E, Lebon P, Suberbielle C, Zuber J, Lima C, Charron D, Legendre C, Chatenoud L: Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am J Transplant 9: 2346–2354, 2009 [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization: WHO Manual on Animal Influenza Diagnosis and Surveillance Rev. 1. World Health Organization, Geneva, Switzerland, 2002 [Google Scholar]
- 11. Salles MJC, Sens YAS, Boas LSV, Machado CM: Influenza virus vaccination in kidney transplant recipients: Serum antibody response to different immunosuppressive drugs. Clin Transplant 24: E17–E23, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL: Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med 361: 2405–2413, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M: Immune response after a single vaccination against 2009 influenza A H1N1 in USA: A preliminary report of two randomised controlled phase 2 trials. Lancet 375:41–48, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, Zhang XF, Pan HX, Meng FY, Hu YM, Liu WD, Li CG, Li W, Zhang X, Hu JM, Peng WB, Yang BP, Xi P, Wang HQ, Zheng JS: A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 361: 2414–2423, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I: Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 361: 2424–2435, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Beyer WE, Versluis DJ, Kramer P, Diderich PP, Weimar W, Masurel N: Trivalent influenza vaccine in patients on hemodialysis: Impaired seroresponse with differences for A-H3N2 and A-H1N1 vaccine components. Vaccine 5: 43–48, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Stavroulopoulos A, Stamogiannos G, Aresti V: Pandemic influenza H1N1 virus vaccination: Compliance and safety in a single hemodialysis center. Renal Failure 32: 1044–1048, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Danziger-Isakov L, Cherkassky L, Siegel H, McManamon M, Kramer K, Budev M, Sawinski D, Augustine JJ, Hricik DE, Fairchild R, Heeger PS, Poggio ED: Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation 89: 838–844, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meier S, Bel M, L'huillier A, Crisinel PA, Combescure C, Kaiser L, Grillet S, Pósfay-Barbe K, Siegrist CA: H1N1 Epidemiology Study Group of Geneva: Antibody responses to natural influenza A/H1N1/09 disease or following immunization with adjuvanted vaccines, in immunocompetent and immunocompromised children. Vaccine 29: 3548–5357, 2011 [DOI] [PubMed] [Google Scholar]