Abstract
Summary
Background and objectives
The Oxford classification of IgA nephropathy (IgAN) may aid in predicting prognosis and providing therapeutic strategy but must be validated in different ancestry.
Design, setting, participants, & measurements
A total of 410 patients with IgAN, enrolled from one of the largest renal centers in China, were evaluated for the predictive value of the Oxford classification to prognosis defined as end stage renal disease. A total of 294 of these patients were prospectively treated with renin-angiotensin system blockade and immunosuppressants sequentially and were evaluated separately to assess the predictive value to therapeutic efficacy (defined as time-averaged proteinuria <1 g/d). Three pathologists reviewed specimens independently according to the Oxford classification and were blinded to clinical data.
Results
Segmental glomerulosclerosis and tubular atrophy and interstitial fibrosis were independent predictive factors of end stage renal disease. Patients who had >25% of glomeruli with endocapillary hypercellularity showed higher proteinuria, lower estimated GFR, and higher mean BP than patients with less endocapillary hypercellularity. Immunosuppressive therapy showed a protective effect to prognosis of endocapillary hypercellularity in patients with endoncapillary hypercellularity could benefit from immunosuppressive therapy. Mesangial hypercellularity and tubular atrophy and interstitial fibrosis were independent factors of inefficiency of renin-angiotensin system blockade alone. Crescents were not significant in predicting prognosis or in therapeutic efficacy.
Conclusions
The Oxford classification may aid in predicting prognosis and providing a therapeutic strategy in Chinese patients with IgAN.
Introduction
IgA nephropathy (IgAN), which is characterized by IgA deposition in the glomerular mesangium and is associated with a wide variability of clinical and pathologic presentations, is the most common glomerular disease worldwide (1–3). Histopathologic lesions have been reported as risk factors for the progression of IgAN (4). In the past few decades, a variety of histologic classifications have been used to attempt to predict prognosis for patients with IgAN (5–7), but none has gained wide acceptance as ideal in clinical practice. Whether the pathologic features in IgAN provide additional prognostic information when clinical data at both presentation and follow-up are available remains controversial.
The Oxford classification of IgAN, developed by an international consensus working group (8,9), was established by analyzing the biopsies of 265 adults and children and aimed to establish a consensus on specific pathologic features that reliably predict risk for progression of IgAN and to create for nephrologists and pathologists a standardized platform to improve evaluation of patient prognosis. Four histopathologic features—mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubular atrophy and interstitial fibrosis (T)—were identified as histopathologic predictors of renal prognosis of IgAN independent of the clinical features in the new Oxford classification.
The Oxford classification requires validation in other cohorts of patients. The majority of the original cohort was of European origin, and only 23% were of Asian origin. However, patients with IgAN in Asia are more prevalent and are more likely to be treated with immunosuppressive therapy; therefore, we studied the predictive value of the Oxford classification in a large Asian population. Also, patients with rapidly progressive IgAN, who are most likely to have extensive crescent formation, were excluded from the original Oxford classification study cohort, which might explain why crescents did not have prognostic significance in the Oxford study cohort. The predictive value of the Oxford classification should be validated in a cohort that includes more patients with crescentic lesions. In addition, which histopathologic lesions identified in the Oxford classification can predict both the efficacy of therapy and the progression of renal disease remains to be clarified.
In this study, we re-evaluated the predictive value of the Oxford classification, using the same definitions of the pathologic features, in a cohort of patient with IgAN from one of the largest renal centers in China.
Materials and Methods
Patients
Patients who had renal biopsy–proven primary IgAN and were registered in the Peking University First Hospital IgAN-Database (www.renal-online.org) from January 1990 through January 2008 were enrolled in this study. Patients with fewer than eight glomeruli per biopsy section were excluded. Twenty patients who had been involved in the initial Oxford classification (8) were excluded. All patients were regularly followed up for at least 1 year. The study protocol was reviewed and approved by the Ethics Committee of Peking University First Hospital, and written informed consent was provided by all participants.
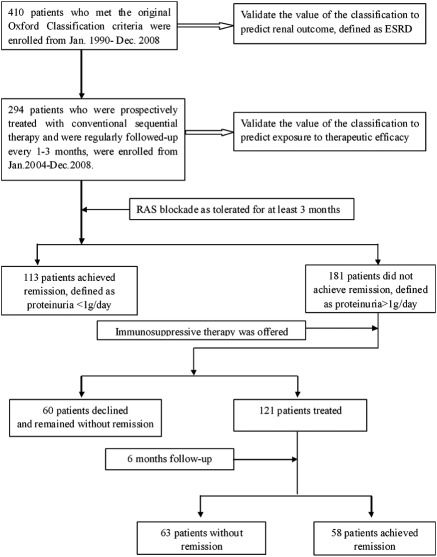
A total of 410 patients who met the original inclusion criteria of the Oxford classification (8,9) were evaluated for the prognostic significance of the Oxford classification. Of these, 294 were recruited from January 2004 through January 2008, were prospectively treated with uniform therapy using a renin-angiotensin system (RAS) blockade and immunosuppressive therapy sequentially, and were followed up every 1 to 3 months (range 12 to 60 months). These 294 patients were evaluated for the predictive value of Oxford classification as to therapy (Figure 1).
Figure 1.
Study scheme. Sequential therapy was carried out as RAS blockade therapy for at least 3 months, and then steroids were offered when time-averaged proteinuria was still >1 g/d.
Histopathologic Methods
All renal biopsy specimens were processed routinely for immunofluorescence microscopy, light microscopy, and electron microscopy. Sections were stained with hematoxylin and eosin, periodic acid-Schiff together with silver methenamine and Masson's trichrome. Three pathologists reviewed the slides independently according to the definition of the Oxford classification (8,9). Interobserver reproducibility of each pathologic lesion among pathologists was evaluated by the values of the intraclass correlation coefficient and determined to be acceptable (Table 1). Pathologists were blinded to the clinical data.
Table 1.
Intraclass correlation coefficient (ICC) scores of pathologic parameters between pathologists A/B and A/Ca
| Pathologists | M | E | S | T |
|---|---|---|---|---|
| A/B | 0.68 | 0.56 | 0.75 | 0.75 |
| A/C | 0.63 | 0.67 | 0.60 | 0.74 |
M, mesagnial hypercellularity; E, endocapillary hypercellularity; S, segmental glomerulosclerosis; T, tubular atrophy/interstitial fibrosis.
The reproducibility of the Oxford classification was acceptable in renal biopsy sections stained with hematoxylin and eosin, periodic acid-Schiff together with silver methenamine and MST.
To analyze further the predictive value of crescents, we also used a semiquantitative method, which we previously reported (10). The extracapillary glomerular activity index (exGAI) was scored for both segmental and circumferential crescentic lesions (by percentage of glomeruli with these lesions: 0, 0%; 1, <10%; 2, 10% to 24%; 3, 25% to 50%; 4, >50%), and the score of circumferential cellular and fibrocellular crescents was weighted by a factor of 2 before it was added to the segmental score. Patients with exGAI ≥4 were more likely to progress to ESRD than patients with an exGAI <4. E25 was defined as >25% of glomeruli involved with endocapillary hypercellularity to analyze further the predictive value of endocapillary hypercellularity.
Definitions
ESRD was defined as estimated GFR (eGFR) <15 ml/min per 1.73 m2 according to the modified Modification of Diet in Renal Disease equation for the Chinese population (11) or for patients receiving renal replacement therapy. Mean arterial pressure (MAP) was defined as diastolic pressure plus one third of the pulse pressure. Follow-up MAP and proteinuria were calculated as the average of these values (12) and defined as time-averaged MAP and time-averaged proteinuria, respectively. RAS blockade indicated exposure to angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, or both. Immunosuppressive therapy was defined as receiving corticosteroids with or without a cytotoxic agent, and cyclophosphamide was mostly used at a total dosage of 6 to 8 g. Oral prednisone was started at 0.8 to 1.0 mg/kg per day for 6 to 8 weeks and then tapered to 5 to 10 mg every 2 weeks for up to 6 to 8 months.
ESRD was the end point to evaluate the predictive value of the pathologic lesions to renal outcome. Nonresponse to therapy, as another end point to evaluate therapeutic effect, was defined as time-average proteinuria >1 g/d.
Statistical Analysis
Normally distributed variables were expressed as mean ± SD and compared using the t test, one-way ANOVA, or the Pearson correlation. Nonparametric variables were expressed as median (range) and compared using the Mann-Whitney U test, Kruskal-Wallis test, or Spearman correlation. Categorical variables were expressed in percentages and compared using the chi-squared test.
Survival analysis using Cox regression was performed to test the association between pathologic lesion and clinical outcomes. Univariate Cox regression was used to determine factors predicting clinical outcomes; pathology variables associated with outcomes were further studied through stepwise multivariate Cox regression. Different relevant multivariate models were tested obeying the standard statistical rules. Results were expressed as hazard ratio (HR) with 95% confidence intervals (CIs).
P < 0.05 was considered statistically significant. All statistical analysis was performed with the statistical software SPSS 14.0 (SPSS, Chicago, IL).
Results
Clinical Features and Outcome
The clinical features of all 410 patients are summarized in Table 2. Proteinuria was 1.7 g/d (0.5 to 21.8 g/d), and eGFR was 85.8 ± 28.1 ml/min per 1.73 m2. Using the Kidney Disease Outcomes Quality Initiative classification, 43%, 38%, and 19% of patients had stages 1, 2, and 3 chronic kidney disease, respectively. The clinical features were compared with the Oxford cohort. We found that women were more common in our cohort, follow-up duration was shorter, immunosuppressive therapy was more prevalent, and fewer patients progressed to ESRD.
Table 2.
Baseline clinical characteristics of patients at the time of biopsy and at the end of follow-up, compared to the original Oxford study
| All Patients (n = 410) | Oxford Cohort (n = 265) 8 | |
|---|---|---|
| At time of biopsy | ||
| Age (yrs) | 31 ± 10.8 | 30 (4–73) |
| Female (%) | 51.0% | 28% |
| MAP (mmHg) | 94.0 ± 14.2 | 98 ± 17 |
| eGFR (ml/min per 1.73 m2) | 85.8 ± 28.1 | 83 ± 36 |
| Proteinuria (g/d) | 1.7 (0.5 to 21.8) | 1.7 (0.5 to 18.5) |
| Stage 1, 2, 3 (K-DOQI)(%) | 43, 38, 19% | 36, 38, 26% |
| Follow-up | ||
| duration of follow-up (months) | 38 (12 to 171) | 69 (12 to 268) |
| treated with RAS blockade (ACEi/ARB) (%) | 86.1% | 74% |
| treated with immunosuppressant (%) | 20.0% | 9% |
| treated with prednisone (%) | 42.7% | 29% |
| ESRD (%) | 7.3% | 13% |
eGFR, estimated GFR; MAP, mean arterial pressure; RAS, renin angiotensin system; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; ESRD, end-stage renal disease defined as eGFR <15 ml/min per 1.73 m2 or renal replacement therapy. K-DOQI, Kidney Disease Outcomes Quality Initiative.
Predictive Evaluation for Renal Outcome
Mesangial hypercellularity (M1), endocapillary hypercellularity (E1), and segmental glomerulosclerosis (S1) were found in 56%, 57%, and 75% of patients, respectively. Tubular atrophy and interstitial fibrosis, 0% to 25% (T0), 26% to 50% (T1), and >50% (T2), were found in 78%, 14%, and 8% of patients, respectively. Crescents (C1) were found in 60% of patients. The median number of glomeruli with crescents was 16%. Eight patients had crescents involved in >50% of the glomeruli. Eighty-one (19.7%) of 410 patients were scored as having exGAI ≥4. All patients with crescents had been tested for anti-neutrophil cytoplasmic antibodies, and all were negative. Necrosis was found in 34 patients. Arterial lesions, mostly mild, were found in all patients.
Correlations of pathologic lesions and clinical features are shown in Table 3. E1 was not correlated with clinical features. E25 was further analyzed. M1, E25, S1, T1/T2, C1, and exGAI ≥4, as well as artery scores, were associated with initial eGFR. E25 and T1/T2 were also associated with initial proteinuria and MAP. These findings were similar to the results of the Oxford cohort; however, M1 and S1 were not associated with initial proteinuria in our cohort, as was found in the Oxford cohort.
Table 3.
Comparison of baseline clinical features between different pathologic lesions of Oxford Classification in 410 enrolled patients, and compared to the original Oxford study
| Pathologic lesion | Present Study |
Oxford Study(8) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | MAP mmHg | P | eGFR ml/min per 1.73 m2 | P | Urine Protein g/day | P | MAP mmHg | P | eGFR ml/min per 1.73 m2 | P | U-pro g/day | P | |
| Mesangial hypercellularity score ≤0.5 | 44 | 94.8 ± 13.0 | 0.82 | 90.1 ± 25.7 | 0.01 | 1.5 (0.5 to 18.8) | 0.69 | 100 | >0.1 | 84 | >0.1 | 1.4 | 0.01 |
| Mesangial hypercellularity score >0.5 | 56 | 93.1 ± 15.0 | 81.7 ± 28.7 | 1.7 (0.5 to 21.8) | 98 | 82 | 2.0 | ||||||
| No endocapillary hypercellularitya | 43 | 93.4 ± 14.3 | 0.53 | 86.6 ± 28.4 | 0.89 | 1.8 (0.5 to 18.8) | 0.94 | 101 | 0.08 | 76 | 0.01 | 1.5 | 0.01 |
| Any endocapillary hypercellularity | 57 | 94.6 ± 14.1 | 85.2 ± 27.3 | 1.7 (0.5 to 21.8) | 95 | 92 | 2.0 | ||||||
| Endocapillary hypercellularity ≤25% | 83 | 93.0 ± 15.0 | <0.01 | 88.9 ± 27.5 | <0.01 | 1.6 (0.5 to 18.9) | 0.01 | ||||||
| Endocapillary hypercellularity >25% | 17 | 99.2 ± 14.1 | 70.5 ± 17.5 | 2.4 (0.5 to 21.8) | |||||||||
| No extracapillary proliferation | 39 | 93.4 ± 14.9 | 0.81 | 91.1 ± 28.0 | 0.03 | 1.7 (0.5 to 18.9) | 0.20 | 98 | >0.1 | 84 | >0.1 | 1.5 | 0.02 |
| Any extracapillary proliferation | 61 | 94.6 ± 13.7 | 82.4 ± 27.2 | 1.8 (0.5 to 21.8) | 98 | 80 | 2.2 | ||||||
| Extracapillary glomerular activity indexb<4 | 80 | 94.3 ± 14.4 | 0.84 | 87.3 ± 27.8 | 0.06 | 1.7 (0.5 to 21.8) | 0.79 | ||||||
| Extracapillary glomerular activity index ≥4 | 20 | 92.6 ± 13.1 | 77.9 ± 26.8 | 1.8 (0.5 to 15.7) | |||||||||
| No segmental glomerulosclerosis | 25 | 94.0 ± 12.8 | 0.76 | 92.5 ± 29.4 | 0.05 | 1.8 (0.5 to 21.8) | 0.58 | 94 | 0.04 | 95 | 0.03 | 1.5 | 0.04 |
| Any segmental glomerulosclerosis | 75 | 94.1 ± 14.6 | 83.5 ± 26.8 | 1.7 (0.5 to 15.7) | 100 | 79 | 1.9 | ||||||
| Tubular atrophy and interstitial fibrosis | |||||||||||||
| mild (0 to 25%) | 78 | 92.6 ± 13.2 | <0.01 | 92.9 ± 24.1 | <0.01 | 1.5 (0.5 to 21.8) | 0.04 | 99 | 0.03 | 86 | <0.01 | 1.7 | 0.03 |
| moderate (26 to 50%) | 14 | 100.3 ± 17.9 | 65.2 ± 26.6 | 2.3 (0.7 to 13.7) | 100 | 59 | 1.8 | ||||||
| severe (51%+) | 8 | 100.3 ± 10.3 | 53.2 ± 27.8 | 2.4 (0.5 to 15.7) | 105 | 46 | 3.0 | ||||||
| Artery score | |||||||||||||
| mild | 91 | 93.9 ± 14.3 | 0.89 | 87.1 ± 27.4 | 0.06 | 1.7 (0.5 to 21.8 | 0.73 | 104 | 0.02 | 67 | <0.01 | 1.5 | >0.1 |
| moderate | 6 | 96.0 ± 13.4 | 74.1 ± 26.4 | 2.0 (0.6 to 8.3) | 101 | 70 | 1.6 | ||||||
| severe | 3 | 95.8 ± 10.2 | 65.9 ± 34.5 | 2.3 (0.5 to 8.2) | 102 | 72 | 1.7 | ||||||
eGFR, estimated GFR; demonstrated as mean ml/min per 1.73 m2. MAP, mean arterial pressure, demonstrated as mean mmHg. Proteinuria, demonstrated as median g/day.
Endocapillary hypercellularity involving no more than or more than 25% glomeruli defined as endocapillary hypercellularity ≤25% or >25%.
Extracapillary glomerular activity index was defined to evaluate the severity of crescent, which was scored by a double weight of >50% circumferential cellular or fibrocellular crescents.
Pathologic Prediction of Renal Outcome
Correlations between pathologic parameters in the Oxford classification and renal outcome were analyzed for 410 patients. By univariate analysis, M1, S1, C1, and T1/T2 were significantly associated with ESRD, whereas E25 and exGAI ≥4 were not. Multivariate Cox regression showed that patients with S1 were at higher risk for ESRD, compared with the reference S0 (HR 5.22; 95% CI 1.14 to 23.94; P = 0.03), as well as patients with T1 (HR 4.78; 95% CI 1.41 to 16.20; P < 0.001) or T2 (HR 11.02; 95% CI 2.99 to 40.64; P < 0.001), compared with the reference T0 (Table 4). Results were similar regardless of whether clinical data and treatment were taken into account.
Table 4.
Histopathologic features of Oxford Classification of IgA nephropathy as predictors of ESRD in 410 patients by Cox regression model
| ESRD Risk |
||
|---|---|---|
| Univariate Hazard Ratioa (95%CI) | Multivariate Hazard Ratiob (95%CI) | |
| Mesangial hypercellularity score | ||
| ≤0.5 | reference | reference |
| >0.5 | 4.82 (2.03 to 11.47) | 1.85 (0.66 to 5.22) |
| P<0.001 | P = 0.12 | |
| Segmental glomerulosclerosis | ||
| absent | reference | reference |
| present | 7.51 (1.78 to 31.77) | 5.22 (1.14 to 23.94) |
| P = 0.001 | P = 0.03 | |
| Tubular atrophy/interstitial fibrosis | ||
| 0 to 25% | reference | reference |
| 26 to 50% | 9.50 (3.69 to 24.44) | 4.78 (1.41 to 16.20) |
| >50% | 31.97 (11.99 to 85.21) | 11.02 (2.99 to 40.64) |
| P<0.001 | P<0.001 | |
| Extracapillary hypercellularity | ||
| absent | reference | reference |
| present | 3.60 (1.38 to 9.41) | 1.85 (0.70 to 4.88) |
| P = 0.009 | 0.22 | |
| Extracapillary glomerular activity index | ||
| <4 | reference | |
| ≥4 | 1.60 (0.73 to 3.52) | |
| 0.24 | ||
| Endocapillary hypercellularity | ||
| ≤25% present | reference | |
| >25% present | 1.28 (0.54 to 3.02) | |
| 0.57 | ||
CI, confidence interval; ESRD, end stage renal disease defined as estimated GFR <15 ml/min per 1.73 m2 or received renal replacement therapy. Extracapillary glomerular activity index and was defined to evaluate the severity of crescent, which was scored by a double weight of >50% circumferential cellular or fibrocellular crescent.
Endocapillary and arterial lesions were not associated with ESRD in univariate analysis.
Multivariate Model: histopathologic features, including mesangial hypercellularity, segmental glomerulosclerosis, endocapillary hypercellularity, extracapillary hypercellularity and tubular atrophy/interstitial fibrosis, and clinical features, including initial eGFR, MAP, and proteinuria and treatment were in the model.
Correlation of Pathologic Lesions and Treatment
Therapeutic strategies might confound correlations between pathologic lesions and renal outcome; therefore, we analyzed the correlation between two major treatments, RAS blockade and immunosuppressants, with pathologic lesions and the correlation between treatments and renal outcome. Patients with E25, C1, and exGAI ≥4 were more likely to be treated with immunosuppressants (Table 5), which was similar to the Oxford study. However, in our cohort, patients with M1 and T1/T2 were also likely to be treated with immunosuppressants.
Table 5.
Treatment of 410 enrolled patients in relation to pathologic features during follow-up, compared to the original Oxford study
| Pathologic Lesion | Present Study |
Oxford Study |
||||||
|---|---|---|---|---|---|---|---|---|
| % RAS Blockade | P | % Immunosuppressive Therapy | P | % RAS Blockade | P | % Immunosuppressive Therapy | P | |
| Mesangial hypercellularity score | ||||||||
| ≤0.5 | 82 | 33 | 71 | 21 | ||||
| >0.5 | 89 | 0.08 | 50 | <0.01 | 75 | >0.01 | 30 | >0.01 |
| Segmental glomerulosclerosis | ||||||||
| absent | 81 | 46 | 54 | 28 | ||||
| present | 88 | 0.04 | 42 | 0.31 | 81 | <0.01 | 29 | >0.01 |
| Endocapillary hypercellularitya | ||||||||
| absent | 85 | 40 | 76 | 17 | ||||
| present | 87 | 0.31 | 45 | 0.52 | 72 | >0.01 | 45 | <0.01 |
| Endocapillary hypercellularity | ||||||||
| ≤25% present | 86 | 41 | ||||||
| >25% present | 88 | 0.59 | 52 | <0.01 | ||||
| Extracapillary hypercellularityb | ||||||||
| absent | 85 | 37 | 72 | 20 | ||||
| present | 88 | 0.42 | 47 | 0.04 | 78 | >0.01 | 39 | 0.02 |
| Extracapillary glomerular activity index | ||||||||
| <4 | 87 | 0.91 | 36 | <0.01 | ||||
| Extracapillary glomerular activity index | ||||||||
| ≥4 | 86 | 74 | ||||||
| Tubular atrophy/interstitial fibrosis | ||||||||
| 1 to 25% | 87 | 0.12 | 39 | 0.05 | 76 | 0.03 | 28 | >0.01 |
| 26 to 50% | 88 | 61 | 84 | 24 | ||||
| >50% | 81 | 50 | 85 | 50 | ||||
RAS, renin angiotensin system.
Endocapillary hypercellularity involving no more than or more than 25% glomeruli defined as endocapillary hypercellularity ≤25% or >25%.
Extracapillary glomerular activity index was defined to evaluate the severity of crescent, which was scored by a double weight of >50% circumferential cellular or fibrocellular crescent.
Seventy-one patients with E25, 44 of 71 were treated with immunosuppressive therapy. Initial proteinuria in patients who received immunosuppressants was higher than in patients who did not (4.1 ± 3.9 versus 2.2 ± 1.9 g/d; P = 0.01). C1 was more common in patients who received immunosuppressants (14% versus 6%; P = 0.02), whereas there was no significant difference in the level of eGFR and MAP or in the severity of M, S, and T between patients with and without immunosuppressive therapy. These findings indicate that patients who had E25 and were receiving immunosuppressants were more severe clinical and pathological features than those who had E25 and were not receiving immunosuppressants. However, patients' progression to ESRD was similar between the groups. Patients with E25 might benefit from immunosuppressive therapy. The benefit of immunosuppressive therapy to patients with C1 or exGAI ≥4 could not be shown in our cohort.
Relationship between Pathologic Features and Therapeutic Strategies
RAS Blockade.
The outcome of the 294 patients who were regularly followed up and treated with conventional therapy is shown in Figure 1. After at least 3 months of RAS blockade therapy, 181 patients' disease showed no response and presented with lower initial eGFR (81.1 ± 27.8 versus 89.3 ± 22.9 ml/min per 1.73 m2; P = 0.009) and higher proteinuria (2.79 ± 2.34 versus 1.33 ± 0.85 g/d; P < 0.001) as well as a higher percentage of M1 (68.5% versus 55.8%; P = 0.03) and more severe tubular atrophy and interstitial fibrosis (T0: 70.7% versus 88.6% [P < 0.056]; T1: 16.6% versus 7.9% [P < 0.05]; T2: 12.7% versus 3.5% [P < 0.05]) than 113 patients whose disease responded (Table 6). Correlation between pathologic parameters and nonresponse to RAS blockade was analyzed. By univariate analysis, nonresponse to RAS blockade was associated with M1, S1, T1/T2, and C1. Multivariate Cox analysis showed that M1 and T1/T2 were independent predictive factors for nonresponse to RAS blockade. Correlations between the combination of pathologic parameters and nonresponse to RAS blockade were analyzed; patients with M0T0 were the reference. Multivariate Cox analysis showed that the disease of patients with M1T0 (HR 1.66; 95% CI 1.14 to 2.40; P = 0.008), M1T1 (HR 1.81; 95% CI 1.09 to 3.01; P = 0.023), and M1T2 (HR 2.81; 95% CI 1.70 to 4.65; P < 0.001) was less likely to respond to RAS blockade than that of patients with M0T0 (Table 7). Initial proteinuria was also an independent predictor of nonresponse to RAS blockade. The disease of patients with proteinuria >2 g/d was less likely to respond to RAS blockade (Table 7).
Table 6.
The baseline and the outcome of two subgroups in 294 patients who were regularly followed and treated with RAS blockade alone at least 3 months
| Response Patients (n = 113) | Non-response Patients (n = 181) | P | |
|---|---|---|---|
| At time of biopsy | |||
| age (yrs) | 33.8 ± 10.6 | 33.2 ± 10.9 | 0.68 |
| female (%) | 59.2 | 43.6 | 0.009 |
| MAP (mmHg) | 89.3 ± 22.9 | 81.1 ± 27.8 | 0.16 |
| eGFR (ml/min per 1.73 m2) | 89.3 ± 22.9 | 81.1 ± 27.8 | 0.009 |
| proteinuria (g/day) | 1.3 ± 0.9 | 2.8 ± 2.3 | <0.001 |
| M1 (%) | 55.8 | 68.5 | 0.03 |
| E25 (%) | 13.3 | 17.7 | 0.32 |
| S1 (%) | 78.8 | 82.9 | 0.38 |
| T0 (%) | 88.6 | 70.7 | <0.05 |
| T1 (%) | 7.9 | 16.6 | <0.001 |
| T2 (%) | 3.5 | 12.7 | <0.001 |
| Follow-up | |||
| duration of follow-up (months) | 32 (12 to 171) | 36 (12 to 94) | 0.27 |
| TA-Up (g/day) | 0.6 ± 0.2 | 1.5 ± 1.1 | <0.001 |
| TA-MAP (mmHg) | 85.7 ± 11.3 | 89.0 ± 12.9 | 0.03 |
| ESRD (%) | 0 | 8.8 | 0.001 |
| eGFR slope (ml/min per 1.73 m2 per year) | 0 ± 0.7 | −0.2 ± 0.7 | 0.06 |
eGFR, estimated GFR; MAP, mean arterial pressure; TA-MAP, time-averaged MAP; TA-Up, time-averaged proteinuria; M1, mesangial hypercellularity score >0.5; E25, endocapillary hypercellularity involving more than 25% glomeruli; C1, crescent present; S1, segmental glomerulosclerosis present; T0, tubular atrophy/interstitial fibrosis range from 0 to 25%; T1, tubular atrophy/interstitial fibrosis range from 26 to 50%; T2, tubular atrophy/interstitial fibrosis >50%.
Table 7.
The lesion predictive value of the Oxford Classification for nonresponse to RAS blockade in 294 IgAN patients by Cox regression
| Pathologic lesion | Patients | Risk of No Response to RAS Blockade |
|
|---|---|---|---|
| Univariate Hazard Ratio (95% CI) | Multivariate Hazard Ratio (95% CI) | ||
| Mesangial hypercellularity score | |||
| ≤0.5 | 107 | reference | reference |
| >0.5 | 187 | 1.88 (1.37 to 2.59) | 1.74 (1.25 to 2.43) |
| P<0.001 | P = 0.001 | ||
| Segmental glomerulosclerosis | |||
| absent | 55 | reference | reference |
| present | 239 | 1.62 (1.09 to 2.40) | 1.17 (0.75 to 1.82) |
| P = 0.02 | 0.35 | ||
| Tubular atrophy/interstitial fibrosis | |||
| 0 to 25% | 228 | reference | reference |
| 26 to 50% | 39 | 1.31 (0.88 to 1.95) | 1.13 (0.75 to 1.71) |
| >50% | 27 | 2.04 (1.31 to 3.92) | 1.84 (1.15 to 2.94) |
| P = 0.002 | P = 0.02 | ||
| Extracapillary hypercellularity | |||
| absent | 116 | reference | reference |
| present | 178 | 1.38 (1.01 to 1.86) | 1.19 (0.85 to 1.65) |
| P = 0.04 | 0.43 | ||
| Combination of pathologic lesions (M0/1T0/1/2) | |||
| M0T0 | 96 | reference | |
| M1T0 | 128 | P = 0.008 | 1.66 (1.14 to 2.40) |
| M0T1 | 8 | 0.34 | 0.98 (0.42 to 2.30) |
| M1T1 | 31 | P = 0.02 | 1.81 (1.09 to 3.01) |
| M1T2 | 26 | P<0.001 | 2.81 (1.70 to 4.65) |
| Risk of No Remission to RAS Blockade | |||
| Clinical parameter | Univariate | Multivariate | |
| Patients | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |
| Proteinuria | 294 | 1.11 (1.05 to 1.16) | 1.10 (1.04 to 1.16) |
| P<0.001 | P = 0.001 | ||
| Proteinuria range | |||
| <1g/day | 85 | reference | |
| 1 to 1.99 g/day | 99 | P = 0.01 | 1.83 (1.14 to 2.94) |
| 2 to 3.5 g/day | 63 | P<0.001 | 2.40 (1.50 to 3.86) |
| >3.5 g/day | 47 | P<0.001 | 2.80 (1.70 to 4.62) |
M0, mesangial hypercellularity score <0.5; M1, mesangial hypercellularity score >0.5; T0, tubular atrophy/interstitial fibrosis range from 0 to 25%; T1, tubular atrophy/interstitial fibrosis range from 26 to 50%; T2, tubular atrophy/interstitial fibrosis >50%; CI, confidence interval; eGFR, estimated GFR; MAP, mean arterial pressure; RAS, renin angiotensin system.
Endocapillary, arterial lesions, and eGFR as well as MAP were not associated with inefficiency to RAS blockades therapy in univariate analysis.
Multivariate Model: multivariate with pathologic features, including mesangial hypercellularity segmental glomerulosclerosis tubular atrophy/interstitial fibrosis and extracapillary hypercellularity, and clinical features, including initial eGFR, MAP, and proteinuria.
Immunosuppressive Therapy
Of the 181 patients whose disease showed no response to RAS blockade therapy (at least 3 months), 121 received and 60 patients refused immunosuppressive therapy (Figure 1). Of the 121 patients who received immunosuppressants, the disease of 58 responded and that of 63 did not respond. There was no significant difference in the level of initial proteinuria, MAP, and eGFR or in the severity of pathologic parameters M1, E25, C1, S1, and T0/T1/T2 between patients whose disease did and did not respond. Patients whose disease responded had lower time-averaged proteinuria and time-averaged MAP, and fewer patients progressed to ESRD than patients whose disease did not respond (Table 8). Multivariable Cox analysis showed that patients who did not receive immunosuppressive therapy had a higher risk for nonresponse of proteinuria (HR 1.79; 95% CI 1.25 to 2.57; P = 0.002) than patients who received immunosuppressive therapy. Immunosuppressive therapy might be a protective factor for patients whose disease shows no response to RAS blockade therapy alone. No significant result could be found for the predictive value of pathologic features to the efficacy of immunosuppressant therapy in our cohort.
Table 8.
The baseline data and the renal outcome of two subgroups in 121 patients who were regularly followed and treated with RAS blockade together with immunosuppressive therapy after 3 months of RAS blockade therapy alone
| Response Patients (n = 58) | Non-Response Patients (n = 63) | P | |
|---|---|---|---|
| At time of biopsy | |||
| Age (yrs) | 32.6 ± 9.6 | 30.4 ± 11.4 | 0.26 |
| Female (%) | 51.7% | 32.2% | 0.04 |
| MAP (mmHg) | 93.30 ± 10.64 | 93.83 ± 9.02 | 0.77 |
| eGFR (ml/min per 1.73 m2) | 81.79 ± 24.58 | 83.11 ± 30.76 | 0.80 |
| Proteinuria (g/d) | 2.71 ± 2.59 | 3.55 ± 2.56 | 0.08 |
| M1 (%) | 67.2% | 74.6% | 0.30 |
| E25 (%) | 17.2% | 17.5% | 0.94 |
| C1 (%) | 69.0% | 66.1% | 0.74 |
| S1 (%) | 81% | 82.3% | 0.86 |
| T0 (%) | 72.4% | 69.8% | 0.84 |
| T1 (%) | 17.2% | 17.5% | 0.88 |
| T2 (%) | 10.4% | 12.7% | 0.76 |
| Follow-up | |||
| Duration of follow-up (months) | 32 (12 to 74) | 38 (12 to 86) | 0.32 |
| TA-Up (g/d) | 0.5 ± 0.3 | 2.0 ± 1.1 | <0.001 |
| TA-MAP (mmHg) | 86.2 ± 7.0 | 91.6 ± 9.1 | <0.001 |
| ESRD (%) | 0 | 11.3% | 0.01 |
| eGFR slope (ml/min per 1.73 m2 per year) | 0.2 ± 0.7 | –0.2 ± 0.7 | 0.002 |
eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure; TA-MAP, time-averaged MAP; TA-Up, time-averaged proteinuria; M1, mesangial hypercellularity score >0.5; E25, endocapillary hypercellularity involving more than 25% glomeruli; C1, crescent present; S1, segmental glomerulosclerosis present; T0, tubular atrophy/interstitial fibrosis range from 0 to 25%; T1, tubular atrophy/interstitial fibrosis range from 26 to 50%; T2, tubular atrophy/interstitial fibrosis >50%.
Discussion
The Oxford classification of IgAN provides a histopathologic grading system for the determination of the prognosis in IgAN (8,9). Here we report the validation of the Oxford classification in a large Chinese cohort, with features at the time of renal biopsy comparable with those of patients in the Oxford cohort, except that crescents and women were more common in our cohort. As was the case in the Oxford cohort, our study excluded patients with mild (proteinuria <0.5 g/d) or more severe (eGFR <30 ml/min per 1.73 m2) IgAN.
ESRD was the end point in our study. We found that pathologic lesions S1 and T1/T2 were predictive of ESRD independent of the clinical features and treatment, whereas M1 and E1 were not. Compared with the Oxford classification, the presence of E1 was not predictive of outcome and not associated with clinical data, but further analysis showed that E25 was associated with initial eGFR, proteinuria, and MAP. Hisano et al. (13) also found that the progression of glomerular sclerosis in repeated biopsy depends on the extent of endocapillary proliferation in the first biopsy in childhood IgAN. In our study, we found that patients with E25, especially those with heavy proteinuria and more crescents, were more likely to be treated with immunosuppressants; however, renal outcome was similar in patients with E25 and without immunosuppressants, which suggested that patients with E25 might benefit from immunosuppressive therapy for preservation of renal function. Recent published data from our center demonstrated that IgAN with diffuse endocapillary proliferation correlated with a better prognosis in patients with normal renal function, but it is a relative risk factor for ESRD in patients with abnormal renal function (14). Tumlin et al. (15) found that immunosuppressants reduced endocapillary proliferation in repeat renal biopsies, whereas interstitial fibrosis and tubule dropout remained unchanged. In that study, the rate of ESRD in the treated group was lower than that in the control subjects after 36 months. Further prospective, controlled studies are needed to confirm whether immunosuppressive therapy is a protective factor for patients with endocapillary proliferation.
Another difference between the results of the Oxford cohort and our study was the predictive function of M1. There is some evidence that immunosuppressive therapy can modify mesangial hypercellularity (16–19). Patients with M1 in our study were more likely to be treated with immunosuppressive therapy compared with the Oxford cohort, which may explain why our retrospective study did not identify M1 as an independent predictor of ESRD.
Our study also provided information on the significance of crescents. In the Oxford classification, crescents did not predict outcome or response to treatment, most likely because very few patients with crescents were enrolled in that study, as the authors discussed (9). In our validation cohort, more patients had crescents, and the median number of glomeruli with crescents was much higher, indicating that more rapidly progressive cases were included in our cohort. However, we still did not find a correlation between crescents and renal outcome or the response to immunosuppressive treatment. Crescents in IgAN may vary in extent, size, and type (cellular, fibrocellular, or fibrous) (20). Although categorizing crescents by size did not provide additional information in the Oxford cohort (9), the pathologic classification that we established previously (10) demonstrated that exGAI independently correlated with ESRD and, furthermore, that an exGAI ≥4 could identify patients who could benefit from treatment with immunosuppressive agents, the same as the scoring system for lupus nephritis (21). In our study, we also used exGAI to analyze the significance of crescents in predicting renal outcome, but we could not find evidence for the predictive value of exGAI. We think that this might be refuted by immunosuppressive therapy in retrospective studies. Further prospective, randomized, controlled studies based on the Oxford classification are required to determine the role of crescents.
We also evaluated whether the pathologic parameters identified in the Oxford classification could predict response to treatment. Although retrospective, our study cohort is more informative because patients were recruited from a single center, were treated consistently with RAS blockade alone for at least 3 months, and immunosuppressive therapy was added for patients whose disease did not respond (Figure 1). Our data show that M1 and T1/T2 were independent risk factors for nonresponse to RAS blockade alone. The Cox regression model showed that the highest risk for nonresponse to RAS blockade was with the combination of M1 and T1/T2. We further found that immunosuppressive therapy was an independent predictive factor of the decrease of proteinuria. This result was consistent with our previous randomized, controlled trial, which showed that the addition of immunosuppressive therapy for those with incomplete response to RAS blockade achieved additional proteinuria reduction (22); therefore, it was suggested that patients with the pathologic classification of M1 or T1/T2 might benefit from immunosuppressive therapy in addition to RAS blockade.
There were limitations to this study. Our study was retrospective in a single center with a short follow-up time and had more women than in the Oxford study. A multicenter, prospective, controlled study with longer follow-up is needed to confirm the results.
Conclusions
Our validation of the Oxford classification in a large Chinese cohort confirmed that pathologic features could predict renal outcome and provide additional evidence for the role of clinical therapeutic options.
Disclosures
None.
Acknowledgments
This study was supported by a grant from the National Science Fund Committee (grant 30825021) and the Foundation of Ministry of Health of China (grant 200802052) to H.Z.
We thank Jie E and Xin Zheng from the Peking University First Hospital for technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Lee SM, Rao VM, Franklin WA, Schiffer MS, Aronson AJ, Spargo BH, Katz AI: IgA nephropathy: Morphological predictors of progressive renal disease. Hum Pathol 13: 314–322, 1982 [DOI] [PubMed] [Google Scholar]
- 2. Haas M: Histologic sub classification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis 29: 829–842, 1997 [DOI] [PubMed] [Google Scholar]
- 3. D'Amico G: Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24: 179–196, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Roufosse CA, Cook HT: Pathological predictors of prognosis in immunoglobulin A nephropathy: A review. Curr Opin Nephrol Hypertens 18: 212–219, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N, Ikeda K, Yanase T, Fujimi S: Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clin Nephrol 49: 1–8, 1998 [PubMed] [Google Scholar]
- 6. Manno C, Strippoli GF, D'Altri C, Torres D, Rossini M, Schena FP: A novel simpler histological classification for renal survival in IgA nephropathy: A retrospective study. Am J Kidney Dis 49: 763–775, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, Park SY, Han JS, Kim S, Lee JS: Histological grading of IgA nephropathy predicting renal outcome: Revisiting H.S. Lee's glomerular grading system. Nephrol Dial Transplant 20: 342–348, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D'Agati V, D'Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford Classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D'Agati V, D'Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Jiang L, Liu G, Lv J, Huang C, Chen B, Wang S, Zou W, Zhang H, Wang H: Concise semiquantitative histological scoring system for immunoglobulin A nephropathy. Nephrology 14: 597–605, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry: Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Hisano S, Kiyoshi Y, Tanaka I, Tokieda K, Niimi K, Tsuru N, Takebayashii S, Iwasaki H: Clinicopathological correlation of childhood IgA glomerulonephritis presenting diffuse endocapillary proliferation. Pathol Int 54: 174–180, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Liu LJ, Li GT, Zhou Y, Lv JC, Zhang H: Clinicopathologic features and outcomes in endocapillary proliferative IgA nephropathy. Nephron Clin Pract 115: c161–c167, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Tumlin JA, Lohavichan V, Hennigar R: Crescentic and proliferative IgA nephropathy: Clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant 18: 1321–1329, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hotta O, Furuta T, Chiba S, Tomioka S, Taguma Y: Regression of IgA nephropathy: A biopsy study. Am J Kidney Dis 39: 493–502, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Kuriki M, Asahi K, Asano K, Sakurai K, Eiro M, Suzuki H, Watanabe K, Katoh T, Watanabe T: Steroid therapy reduces mesangial matrix accumulation in advanced IgA nephropathy. Nephrol Dial Transplant 18: 1311–1315, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, Imai E, Hori M, Tsubakihara Y: Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis 35: 194–201, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Shima Y, Nakanishi K, Kamei K, Togawa H, Nozu K, Tanaka R, Sasaki S, Iijima K, Yoshikawa N: Disappearance of glomerular IgA deposits in childhood IgA nephropathy showing diffuse mesangial proliferation after 2 years of combination/prednisolone therapy. Nephrol Dial Transplant 26: 163–169, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Philibert D, Cattran D, Cook T: Clinicopathologic correlation in IgA nephropathy. Semin Nephrol 28: 10–17, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]