Abstract
Summary
Background and objectives
Despite modern treatment, the case fatality rate of hospital-acquired acute kidney injury (HA-AKI) is still high. We retrospectively described the prevalence and the outcome of HA-AKI without nephrology referral (nrHA-AKI) and late referred HA-AKI patients to nephrologists (lrHA-AKI) compared with early referral patients (erHA-AKI) with respect to renal function recovery, renal replacement therapy (RRT) requirement, and in-hospital mortality of HA-AKI.
Design, setting, participants, & measurements
Noncritically ill patients admitted to the tertiary care academic center of Lausanne, Switzerland, between 2004 and 2008 in the medical and surgical services were included. Acute kidney injury was defined using the Acute Kidney Injury Network (AKIN) classification.
Results
During 5 years, 4296 patients (4.12% of admissions) experienced 4727 episodes of HA-AKI during their hospital stay. The mean ± SD age of the patients was 61 ± 15 years with a 55% male predominance. There were 958 patients with nrHA-AKI (22.3%) and 2504 patients with lrHA-AKI (58.3%). RRT was required in 31% of the patients with lrHA-AKI compared with 24% of the patients with erHA-AKI. In the multiple risk factor analysis, compared with erHA-AKI, nrHA-AKI and lrHA-AKI were significantly associated with worse renal outcome and higher in-hospital mortality.
Conclusions
These data suggest that HA-AKI is frequent and the patients with nrHA-AKI or lrHA-AKI are at increased risk for in-hospital morbidity and mortality.
Introduction
Acute kidney injury (AKI) is a serious condition that often occurs in critically ill patients in the intensive care unit (ICU) setting (1). Hospital-acquired AKI (HA-AKI) has been reported in 1% to 7% of hospitalized patients on the basis of several single-center reports (2–7). One great challenge is to identify at-risk patients for HA-AKI, strategies for prevention and treatment of AKI in these patients, and appropriate use of healthcare resources (7–11). Several studies have shown an increasing incidence of HA-AKI among the elderly with higher mortality than non-AKI patients (6,7,11–15).
Despite the well recognized importance of HA-AKI, there are no clear data in non-ICU patients regarding the effect of a missed or a delayed diagnosis of HA-AKI and a late in-hospital referral to the nephrologist on outcomes such as need for renal replacement therapy (RRT), mortality, and hospital length of stay. On the basis of this, we hypothesized that the prevalence of HA-AKI in non-ICU patients remains high and the referral patterns to institutional nephrologists may be associated with the risk of in-hospital morbidity and mortality. In this retrospective study, the included patients were grouped as ones without referral to the nephrologist (nrHA-AKI), ones with late referral of HA-AKI (lrHA-AKI), and ones with a timely referral (erHA-AKI). We compared the rate of recovery of renal function during hospital stay, requirement for RRT, the length of hospital stay, and in-hospital mortality in these groups.
Patients and Methods
Patients
This study population consisted of patients who were hospitalized in the medical and surgical departments of the University Hospital of Lausanne (Centre Hospitalier Universitaire Vaudois), which is a tertiary care academic medical center in Switzerland between January 1, 2004, and December 31, 2008. Patients with AKI that was acquired in the ICU were excluded, and those coming from the ICU in the medicine or surgery wards had to present a stable serum creatinine (SCr) level for at least 48 hours to be included. Furthermore, patients from medicine or surgery wards who required a transfer to the ICU during the hospital stay were also excluded regardless of cause of transfer. The study was approved by the research ethics committee of the University of Lausanne and received the ClinicalTrials.gov Unique Identifier NCT01151514.
Patient demographic and laboratory data were downloaded from the hospital's electronic database (i.e., demographic data were retrieved from the electronic hospital database, laboratory data were retrieved from the laboratory database, and patient data were retrieved from the electronic hospital records). After merging data from the different sources, we performed automated and manual data verification (P.M., R.M.B., and B.V.). The patient data included demographic, administrative, physiologic, laboratory, and hospital outcome information.
The patient population included in this study was separated into three groups. The patients with undiagnosed or missed HA-AKI by the non-nephrologists and the patients with a proven or diagnosed HA-AKI with no referral to the nephrologist were grouped together in the nrHA-AKI group. The only interaction with a nephrologist in this group was at the time of RRT if the patient required dialysis. The patients in whom the diagnosis of HA-AKI was made by the non-nephrologists and referred late (>5 days after development of AKI as defined below) to the nephrologist were grouped as patients with lrHA-AKI. These groups were compared with the erHA-AKI group (referral to nephrology service within 5 days after development of AKI).
Assessment of HA-AKI and Comorbidities
SCr concentration before hospitalization was recorded and used as reference. Because of possible elevated SCr values before hospitalization and to exclude community-acquired AKI, we used the latest achievable outpatient SCr value (i.e., >30 days before the day of admission) according to the patient's file as basal renal function to improve sensitivity and to avoid misclassification of renal and patient outcome after AKI as recently demonstrated (16). SCr measurements performed in the emergency department as basal renal function on the day of admission were systematically excluded. We also alternatively defined baseline eGFR using the average of all (nonemergency department) measurements observed between 30 and 365 days before index hospitalization (if achievable). AKI status was determined for each patient by evaluating available clinical and laboratory data from the patient's file.
Conditions were classified as AKI in patients with a prior history of chronic kidney disease (CKD) if they met criteria for CKD as defined below. All remaining conditions were considered as AKI in patients without a prior history of CKD. As a general rule, SCr was measured at the first hospitalization day giving an estimated GFR (eGFR) by the abbreviated four-variable Modification of Diet in Renal Disease equation (17). When an increase in SCr level was noted as defined below (i.e., using the latest outpatient SCr measurement), the patient's chart was reviewed. Variables assessed were age; race; gender; location of hospitalization (medical or surgical departments); duration of hospitalization; type of primary disease (clinical or surgical); Physician Current Procedural Terminology Coding System (for patients admitted in the surgery ward); type of AKI (prerenal [functional], renal [intrinsic], or postrenal [obstructive]); use of nephrotoxic drugs; and need for, duration, and type of dialysis. Relevant coexisting conditions before and during index hospitalization were assessed using Charlson Comorbidity Score (CCS) on the basis of the International Classification of Diseases, Ninth Revision, codes (18). This index scored l for all forms of coronary artery disease as well as congestive heart failure, peripheral vascular and cerebrovascular diseases, dementia, chronic pulmonary disease, connective tissue disorder, peptic ulcer disease, mild liver disease, and diabetes. Hemiplegia, diabetes with organ damage, any tumor, leukemia, and lymphoma were scored as 2. Moderate or severe liver disease was scored as 3, and acquired immunodeficiency syndrome or metastatic solid tumor was scored as 6. We a priori stratified the CCS into three levels (<4, 4 to 6, and >6) according to Hemmelgarn et al. (19). Primary International Classification of Diseases, Ninth Revision, codes for each admission were obtained and used to classify hospitalizations into cardiovascular (390 to 459), respiratory (460 to 519), gastrointestinal (520 to 579), infectious disease (001 to 139), cancer (140 to 239), and all others. The data from patients with HA-AKI were analyzed from the altered kidney function as described until patient discharge or death.
In the effort to provide associative evidence supporting a link between HA-AKI and outcome, we referred to length of hospital stay including the period from admission to the occurrence of HA-AKI and then the period after the HA-AKI occurred. The time to discharge from HA-AKI occurrence was also assessed.
Assessment of AKI, CKD, and ESRD
For this retrospective study, we used the Acute Kidney Injury Network (AKIN) classification system (Table 1) (20). CKD was assessed by evaluating available clinical and laboratory data and history from the patient's file as defined above. Patients were considered to have CKD if they had evidence of altered eGFR (i.e., eGFR <60 ml/min per 1.73 m2) calculated by the Modification of Diet in Renal Disease equation according to the Kidney Disease Outcomes Quality Initiative guidelines (21). At hospital discharge, we considered patients as totally recovered if the SCr decrease was >75% of the difference (Δ) between the highest SCr level minus the baseline SCr level (outpatient SCr before admission) (ΔSCr). Patients were designated as partially recovered if the SCr decrease was between 25% and 75% of ΔSCr, and they were considered as nonrecovered if the SCr decrease was <25% of ΔSCr (10).
Table 1.
AKIN definition and staging for acute kidney injury (20)
| Stage | SCr Criteria | Urinary Output Criteriaa |
|---|---|---|
| 1 | Increase in SCr of ≥0.3 mg/dl (≥26.4 μmol/L) or increase to ≥150% to 200% (1.5- to 2-fold) from baseline | <0.5 ml/kg per hour for >6 hours |
| 2 | Increase in SCr to more than 200% to 300% (>2- to 3-fold) from baseline | <0.5 ml/kg per hour for >12 hours |
| 3 | Increase in SCr to more than 300% (>3-fold) from baseline (or SCr of ≥4.0 mg/dl (≥354 μmol/L) with an acute increase of at least 0.5 mg/dl (44 μmol/L) | <0.3 ml/kg per hour for 24 hours or anuria for 12 hours |
AKIN, Acute Kidney Injury Network; SCr, serum creatinine.
In the study, oliguria was defined as urinary output <500 ml/24 h.
We also focused on patients with dialysis-requiring AKI, which is known to be associated with high rates of in-hospital morbidity and mortality (2–4,9). ESRD was defined as being on maintenance dialysis. We ascertained development of ESRD within 90 days of discharge among patients who had CKD on admission complicated by AKI during hospitalization. We chose a 90-day window given the uncertainty inherent in the start date of ESRD therapy. Furthermore, progression to ESRD at the time of hospital discharge or any time within this 90-day window was judged to be of similar importance clinically. Long-term RRT was defined as if the patients needed hemodialysis (HD) or peritoneal dialysis for >6 months.
All analyses excluded patients who were identified as “not to be resuscitated,” patients who received a previous kidney transplant, or those who were on maintenance dialysis at the time of admission to the hospital.
Statistical Analyses
In this descriptive observational study, we determined the proportion of non-ICU patients with nrHA-AKI and lrHA-AKI who died during the index hospitalization and, among survivors, who recovered or not, and those who were dialysis dependent. This population was compared with erHA-AKI patients. Normally or near normally distributed variables were reported as means with SD and compared using t test or ANOVA. Non-normally distributed continuous data were reported as medians with interquartile ranges and compared using the Mann–Whitney U test or the Kruskal–Wallis test. The chi-squared test was used to compare dichotomous variables. Categorical data were compared using the Fisher exact test. Population-based incidence rates were calculated and reported as odds ratios (ORs) with exact 95% confidence intervals (CIs). We also analyzed the data regarding need for RRT, length of hospital stay, and in-hospital mortality. A multivariable logistic regression model was developed to determine factors associated with in-hospital mortality. The model included selected variables known to potentially confound and/or modify the association of HA-AKI and in-hospital death, including but not limited to age, gender, AKIN stage, CCS, and admission type (medical versus surgical wards) (16,19). To correct for multiple comparisons, a Bonferroni adjustment was used. This was applied to the primary outcomes (i.e., recovery of renal function during hospital stay, requirement for RRT, length of hospital stay, and in-hospital mortality), resulting in an accepted level of significance of 0.05/4 = 0.0125 for these outcomes. When nonspecified, two-tailed P < 0.05 was considered significant. The Statistical Package for the Social Sciences, version 12.0 for Windows (SPSS, Inc., Chicago, IL), was used for analysis.
Results
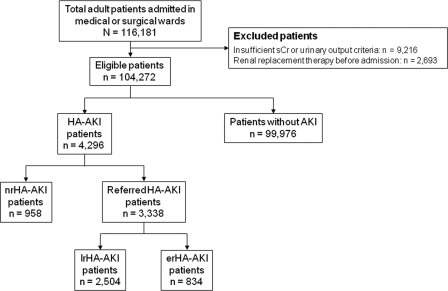
Of the 116,181 patients admitted to the medicine or surgery ward during the study period, the following were excluded: 9216 patients with insufficient SCr or urinary output criteria data and 2693 patients who received RRT before admission or within the first 48 hours of hospitalization (Figure 1). The final cohort consisted of 104,272 patients, and 4296 (4.12%) of these had HA-AKI. From the final cohort, 39,783 patients were hospitalized more than once.
Figure 1.
Patients included and excluded in the analysis. SCr, serum creatinine; HA-AKI, hospital-acquired acute kidney injury; nrHA-AKI, patients with HA-AKI with no referral to the nephrologist; lrHA-AKI, late-referred HA-AKI patients; erHA-AKI, early-referred patients with HA-AKI.
Clinical Characteristics of Patients
Table 2a shows the clinical characteristics of patients hospitalized in the medicine and surgery wards who presented an episode of HA-AKI during their stay. From the 4296 HA-AKI patients, 2444 patients were hospitalized in the medicine ward and 1852 were hospitalized in the surgery ward.
Table 2a.
Characteristics of the patients in the medicine and surgery wards
| Characteristics | nrHA-AKI (n = 958) | lrHA-AKI (n = 2504) | erHA-AKI (n = 834) | Pa |
|---|---|---|---|---|
| Age, years | 62 ± 16 | 61 ± 13 | 60 ± 17 | 0.93 |
| 20 to 39 | 73 (7.6) | 300 (12) | 217 (26) | <0.01 |
| 40 to 59 | 297 (31) | 626 (25) | 325 (39) | 0.03 |
| 60 to 79 | 469 (49) | 1302 (52) | 234 (28) | 0.02 |
| ≥80 | 119 (12.4) | 275 (11) | 58 (7) | 0.01 |
| Women | 412 (43) | 1127 (45) | 367 (44) | 0.81 |
| Men | 546 (57) | 1402 (55) | 467 (56) | 0.85 |
| Hospitalization | ||||
| medicine ward | 642 (67) | 1527 (61) | 275 (33) | 0.002 |
| surgery ward | 316 (33) | 977 (39) | 559 (67) | 0.003 |
| Comorbidity | ||||
| hypertension | 489 (51) | 1227 (49) | 417 (50) | 0.76 |
| diabetes mellitus | 240 (25) | 626 (25) | 192 (23) | 0.69 |
| cardiovascular disease | 220 (23) | 526 (21) | 183 (22) | 0.71 |
| acute infection | 172 (18) | 551 (22) | 158 (19) | 0.59 |
| neoplasia | 77 (8) | 225 (9) | 83 (10) | 0.51 |
| chronic obstructive pulmonary disease | 96 (10) | 250 (10) | 67 (8) | 0.64 |
| liver disease | 48 (5) | 150 (6) | 50 (6) | 0.69 |
| AIDS | 48 (5) | 100 (4) | 42 (5) | 0.73 |
| Charlson Comorbidity Score | 6.5 ± 2.7 | 5.1 ± 3.2 | 5.3 ± 2.6 | 0.03 |
| Median baseline SCr (μmol/L) | 131 (75 to 246) | 121 (68 to 237) | 131 (71 to 256) | 0.12 |
| Median baseline eGFR (ml/min per 1.73 m2) | 59 (121 to 24) | 61 (103 to 29) | 60 (123 to 24) | 0.19 |
| AKIN stage | ||||
| 1 | 572 (60) | 952 (38) | 218 (26) | 0.01 |
| 2 | 357 (37) | 825 (33) | 396 (47) | 0.02 |
| 3 | 29 (3) | 727 (29) | 220 (26) | <0.01 |
| Median SCr at first nephrologic evaluation (μmol/L) | NA | 345 (127 to 1542) | 292 (143 to 1845) | 0.01 |
| Mean SCr at first dialysis (μmol/L) | NA | 735 ± 213 | 532 ± 105 | 0.01 |
| RRT patients during hospital stay | NA | 776 (31) | 200 (24) | 0.01 |
| Oliguriab | 48 (5) | 426 (17) | 158 (19) | 0.03 |
| Mortality rate during hospital stay | 211 (22) | 526 (21) | 100 (12) | 0.01 |
Values are expressed as number (percent), mean ± SD, or median (range). nrHA-AKI, hospital acquired-acute kidney injury without nephrology referral; lrHA-AKI, late-referred HA-AKI; erHA-AKI, early-referred HA-AKI; eGFR: estimated GFR by the abbreviated four-variable Modification of Diet in Renal Disease equation; AKIN, Acute Kidney Injury Network; RRT, renal replacement therapy; NA, not assessed.
Group-wise differences were calculated using ANOVA with Bonferroni correction when necessary.
Oliguria was defined as urinary output <500 ml/24 h.
The erHA-AKI patients were more likely to be younger (i.e., 20 to 39 years; P < 0.001) compared with the nrHA-AKI and the lrHA-AKI patient populations. The nrHA-AKI patients had a higher CCS index compared with lrHA-AKI and erHA-AKI patients with 6.5 ± 2.7, 5.1 ± 3.2, 5.3 ± 2.6 CCS indices, respectively (adjustment for multiple comparisons; P = 0.03).
SCr and eGFR at baseline were comparable between the three groups. As expected, median SCr at the time of the first nephrology consultation was significantly higher in lrHA-AKI patients than in erHA-AKI patients (P = 0.01). Baseline kidney function was not associated with change in SCr level. Indeed, 1031 patients (24%) were classified as CKD patients (eGFR <60 ml/min per 1.73 m2). In this population, the median SCr rise was 126 ± 85 μmol/L (AKIN stage 1), whereas the SCr rise in non-CKD patients (n = 3265) was 217 ± 79 μmol/L (AKIN stage 2). Missing data represented approximately 6% of all collected data and were censored.
The proportion of patients with HA-AKI using the AKIN criteria after hospital admission was analyzed in the medicine and surgery wards. HA-AKI occurred in 1742 patients (40.5%) with stage 1, in 1578 patients (36.7%) with stage 2, and in 976 patients (22.7%) with stage 3.
The 4296 patients experienced 4727 episodes of HA-AKI during the hospital stay. As shown in Table 2a, 958 patients were classified as nrHA-AKI (22.3%). There were 3338 patients with HA-AKI (77.7%) referred to the nephrologist after altered kidney function, and of these 75% was referred late (2504 patients with lrHA-AKI) and 25% were referred early (834 patients with erHA-AKI).
Eighty-six percent of the patients hospitalized in the surgery ward were operated on (n = 1593). The rest of the patients (i.e., 14%; n = 259) were not and nonetheless presented an episode of HA-AKI during their stay. Using the Physician Current Procedural Terminology coding system, we identified those who underwent general, urologic, vascular, thoracic, cardiac, neurosurgery, or orthopedic surgery (Table 2b). Compared with patients with programmed surgery regardless of type, patients who required emergency surgery were more likely to present lrHA-AKI (17% versus 8%; P = 0.01). Furthermore, although patients from the surgery ward were more likely to be referred to the nephrologist early than those from the medicine ward (67% versus 33%; P = 0.001), the type of surgery showed a significant difference in the HA-AKI referral pattern between the groups as shown in Table 2b. A higher proportion of patients planned for urological, vascular, or orthopedic surgery were addressed to the institutional nephrologist late compared with other types of surgery (adjustment for multiple comparisons; P < 0.001). Twenty-seven percent of the patients who needed cardiac surgery regardless of type were not addressed (nrHA-AKI) to the nephrologist when they were in the cardiosurgery ward. This was mainly because these patients had usually stayed in the ICU (96%) long enough to be presented to the nephrologist and treated during their stay before integrating the ward. Because strict exclusion criteria were implemented for patients coming from the ICU, only 9% of the cardiosurgery patients in the ward were referred late.
Table 2b.
Characteristics of the operated patients in the surgery ward
| Characteristics | nrHA-AKI n (%) 272 (17) | lrHA-AKI n (%) 840 (53) | erHA-AKI n (%) 481 (30) | Pa |
|---|---|---|---|---|
| Age, years | 60 ± 13 | 61 ± 10 | 62 ± 15 | 0.91 |
| Women | 122 (45) | 395 (47) | 221 (46) | 0.82 |
| Men | 150 (55) | 445 (53) | 260 (54) | 0.69 |
| Emergency | 25 (9) | 143 (17) | 24 (5) | 0.02 |
| admission source (%) | ||||
| home | 94.8 | 95.1 | 94.7 | 0.86 |
| hospital | 3.8 | 3.4 | 3.9 | 0.93 |
| chronic care | 1.4 | 1.5 | 1.4 | 0.61 |
| Type of surgery | ||||
| general | 94 (19) | 33 (7) | 362 (74) | <0.01 |
| urologic | 26 (10) | 27 (10) | 208 (80) | <0.01 |
| vascular | 18 (9) | 26 (13) | 150 (77) | <0.01 |
| thoracic | 17 (15) | 8 (7) | 90 (78) | <0.01 |
| cardiac | 101 (27) | 33 (9) | 247 (65) | <0.01 |
| neurosurgery | 8 (9) | 8 (9) | 69 (81) | <0.01 |
| orthopedic | 8 (12) | 8 (12) | 52 (76) | <0.01 |
| Wound infection | 17 (6.3) | 97 (11.6) | 15 (3.1) | 0.01 |
Values are expressed as number (percent), mean ± SD, or median (range). nrHA-AKI, hospital-acquired acute kidney injury; without nephrology referral; lrHA-AKI, late-referred HA-AKI; erHA-AKI, early-referred HA-AKI.
Group-wise differences were calculated using ANOVA with Bonferroni correction when necessary.
Oliguria was defined as urinary output <500 ml/24 h.
RRT and Renal Outcome
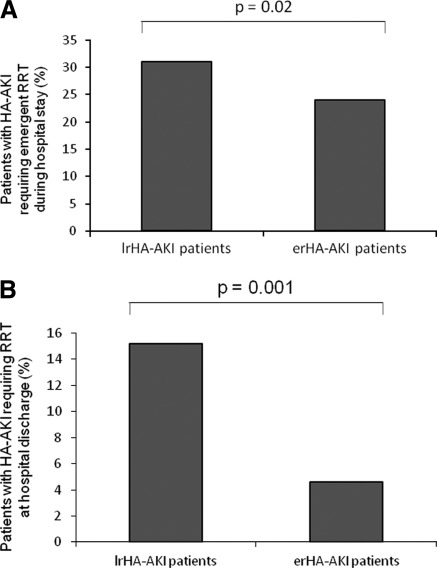
Emergency RRT (i.e., conventional HD) was required in 976 (55%) of the hospitalized patients. This corresponded to 34.5% of all AKIN stage 2 and 3 patients. Among them, as shown in Figure 2A, HD was required more frequent in lrHA-AKI patients compared with erHA-AKI patients (P = 0.02). These late-referred patients started RRT in emergency mainly because of a lack of awareness (86%). The other causes were lack of biologic controls (11%) and other causes (3%), including loss to follow-up and unchecked or late-checked blood samples (data not shown). This was explained by the inability to distinguish true delayed-start HD, in which patients were carefully followed up and HD therapy was started when AKIN criteria were reached, from high-risk individuals who urgently started HD therapy in the context of acute medical illnesses or acute surgical procedures without adequate predialysis care. All started HD with central venous catheter. When only adjusted for age and comorbidities, HD was associated with poor in-hospital outcome (see below). The requirement for emergency RRT was evenly distributed among groups (symptomatic uremia [24%], hyperkalemia [38%], severe metabolic acidosis [16%], and pulmonary edema [22%]; P = 0.09 between patient groups). Among patients with HA-AKI who required acute RRT during their hospital stay, the percentage of patients remaining on HD at hospital discharge was significantly higher in patients with lrHA-AKI compared with patients with erHA-AKI (P = 0.001) (Figure 2B). In the nrHA-AKI and lrHA-AKI populations, 7.2% of the patients (n = 249) required long-term (>6 months) RRT (i.e., HD or peritoneal dialysis) compared with 2.6% in the erHA-AKI group (n = 22; P = 0.001). Patients who were not referred to the institutional nephrologist (nrHA-AKI) and who required acute RRT during their hospital stay were not considered in the analysis because this patient group was very small (1.3%; n = 12). Practically, these patients were managed by the nephrology team once they were identified according to classical criteria for RRT (see Methods) by primary care physicians in both wards considered in this study. In these patients, no nephrology consult was asked for, and these patients were dialyzed in emergency. All of the patients of the nrHA-AKI group who required emergency RRT did not recover their renal function at hospital discharge (data not shown).
Figure 2.
(A) Percentage of emergent renal replacement therapy (RRT) (hemodialysis [HD]) for non-intensive care unit (ICU) patients (i.e., medicine and surgery wards) in hospital-acquired acute kidney injury (HA-AKI) patients according to their status. The x-axis shows the percentage of nrHA-AKI and early-referred (erHA-AKI) patients who received emergency RRT. The y-axis reflects the percentage of patients studied. (B) Percentage of patients with HA-AKI requiring RRT (HD or peritoneal dialysis) at the time of hospital discharge. The x-axis shows the percentage of nrHA-AKI and erHA-AKI patients who required RRT at hospital discharge. The y-axis reflects the percentage of patients studied.
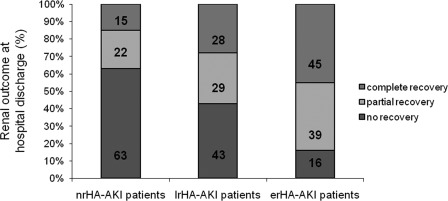
Figure 3 shows the percentage of patients with HA-AKI who presented total, partial, or no recovery of renal function determined by SCr level evolution at hospital discharge. Most patients with erHA-AKI had complete (45%) or partial (39%) recovery of renal function determined by the ΔSCr compared with the other patient groups (P = 0.001). At the time of hospital discharge, nonrecovery of renal function was more common in nrHA-AKI and lrHA-AKI patients than erHA-AKI patients (nrHA-AKI [67%], lrHA-AKI [43%], erHA-AKI [16%]; P = 0.001). Patients who died during the hospital stay were recorded and appear in the results until their death but were not identified in the final analysis of patients discharged from the hospital.
Figure 3.
Outcome of renal function determined by SCr level evolution in each patient group in medicine and surgery wards with HA-AKI at hospital discharge. For details, see Methods.  , percentage of patients with complete recovery (>75% ΔSCr);
, percentage of patients with complete recovery (>75% ΔSCr);  , percentage of patients with partial recovery (25% to 75% ΔSCr); ■, percentage of patients with no recovery (<25% ΔSCr). P = 0.001. nrHA-AKI, hospital-acquired acute kidney injury; without nephrology referral; lrHA-AKI, late-referred HA-AKI; erHA-AKI, early-referred HA-AKI.
, percentage of patients with partial recovery (25% to 75% ΔSCr); ■, percentage of patients with no recovery (<25% ΔSCr). P = 0.001. nrHA-AKI, hospital-acquired acute kidney injury; without nephrology referral; lrHA-AKI, late-referred HA-AKI; erHA-AKI, early-referred HA-AKI.
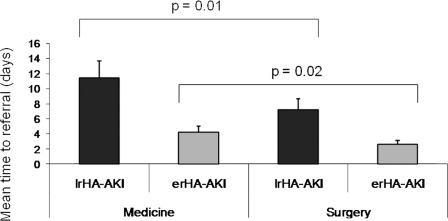
Mean Time to Referral
The mean time (± SD) to nephrologist referral was 7.8 ± 3.4 and 3.6 ± 1.2 days for the patients with lrHA-AKI and those with erHA-AKI, respectively (P = 0.004). Among the 2504 lrHA-AKI patients, 1527 patients were hospitalized in the medicine ward (61%) and 977 patients in the surgery ward (39%). The mean time (± SD) to referral in lrHA-AKI patients staying in the medicine and surgery ward was 11.6 ± 3.8 and 7.2 ± 3.4 days, respectively (P = 0.01) (Figure 4). There were no significant differences between the two groups with reference to age, gender, or cause of HA-AKI. However, there was a significant difference in CCS between these two groups (5.7 ± 2.1 versus 3.8 ± 2.4; P = 0.02). Furthermore, comorbidity and age were associated with referral pattern to the nephrologist. Indeed, patients with the lowest score (i.e., CCS <4) had an OR of 0.14 (95% CI, 0.12 to 0.17; P < 0.001) compared with more severely ill patients (CCS > 6) for referral to a nephrologist during their hospital stay, and compared with the oldest age category, 20- to 39-year-olds had a 5-fold chance of being referred to the nephrologist.
Figure 4.
Timing to nephrology referral. Mean time in days ± SD before the patients with hospital-acquired acute kidney disease (HA-AKI) was referred to the nephrologist. ■, late-referred (lrHA-AKI) patients to the nephrologist (>5 days after SCr increase as defined according the Acute Kidney Injury Network [AKIN] criteria) hospitalized in the medicine and surgery wards; [GRAPHIC], early-referred (erHA-AKI) patients to the nephrologist (≤5 days after SCr increase as defined) hospitalized in the medicine and surgery wards.
Length of Hospital Stay
Generally, HA-AKI was associated with a longer hospital stay compared with no HA-AKI (Figure 5). Mean length of hospital stay of patients with nrHA-AKI, lrHA-AKI, and erHA-AKI was 10 ± 5, 24 ± 6, and 15 ± 3 days, respectively (P = 0.001). Moreover, increasing severity of HA-AKI according to the AKIN classification was associated with an increasing length of hospital stay for all patients (data not shown).
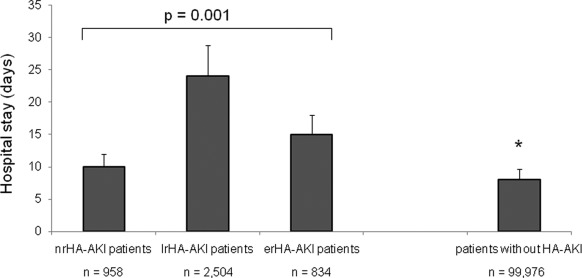
Figure 5.
Length of hospital stays for patients with hospital-acquired acute kidney injury (HA-AKI). Mean times in days ± SD for hospital stay for patients with HA-AKI in the medicine and surgery wards with or without RRT (HD). *P = 0.002, patients without any HA-AKI during hospital stay compared with HA-AKI patient groups (nrHA-AKI, lrHA-AKI, and erHA-AKI). nrHA-AKI, HA-AKI without nephrology referral; lrHA-AKI, late-referred HA-AKI; erHA-AKI, early-referred HA-AKI.
Except for nrHA-AKI patients (mainly in the surgery ward [68%]) who had a shorter stay than the other patient groups (nrHA-AKI, 10 ± 3 days; lrHA-AKI, 24 ± 5 days; erHA-AKI, 15 ± 3 days, P = 0.001), the hospital stay for patients with erHA-AKI was significantly shorter than that of the lrHA-AKI patients (P = 0.01). Renal function assessed in the surgery ward was not taken into account because the SCr level was not seen, because SCr determination was not done, or because the criteria of AKIN were not known. Indeed, <15% of the sample had three or more SCr determinations in the surgery ward compared with 89% of the sample that had three or more SCr determinations in the medicine ward. Some of these patients (3.6% from the surgery ward, 2.1% from the medicine ward) were discharged early with a missed HA-AKI and were then addressed to the nephrology outpatient clinic by their private general practitioner with a delay from 5 to 12 days after hospital discharge (data not shown).
lrHA-AKI patients who needed RRT stayed longer in the hospital than those who were hemodialized but referred to the nephrologist early (P = 0.001). For comparison, the average hospital stay for patients who did not present renal dysfunction during their hospital stay was significantly shorter (8 ± 2 days; P = 0.002). The reason for a longer hospital stay for RRT lrHA-AKI patients was uremia-related morbidity, illness, or complications. Subjects who received early care from a nephrologist had a lower mean SCr concentration at initiation of dialysis (532 ± 105 μmol/L; P = 0.01).
Hospital Mortality
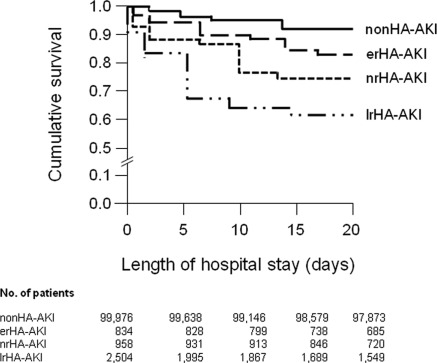
The overall in-hospital mortality rate for the patients with HA-AKI was 19.5% (Table 2), and they experienced greater in-hospital mortality relative to patients without HA-AKI (5.2% patients in medicine ward and 3.7% patients in surgery ward; P < 0.001) (Figure 6). Patients with stage 1, 2, or 3 AKIN score had in-hospital mortality rates of 7.6%, 10.5%, and 25.3%, respectively. The mortality rate was significantly higher in patients who were not referred or were referred to the nephrologist late (nrHA-AKI [22%], lrHA-AKI [21%]) compared with erHA-AKI patients (12%; P = 0.01). Similarly, lrHA-AKI patients requiring RRT had a higher in-hospital mortality rate compared with erHA-AKI patients requiring acute dialysis during their hospital stay (38% versus 17%, respectively; P = 0.008). Patients with fully recovered HA-AKI during their hospital stay had lower mortality compared with those who remained HD dependent (6.9% versus 34.7%, P < 0.001). Finally, 78% of the overall HA-AKI patients regardless of referral status were discharged to home, and 22% were discharged to an extended-care facility.
Figure 6.
Kaplan–Meier curves for survival (in-hospital) by referral class during the first 20 days of hospitalization. Patients discharged alive were censored. Log-rank statistic, P < 0.001. erHA-AKI, early-referred hospital-acquired acute kidney injury; nrHA-AKI, hospital-acquired acute kidney injury without nephrology referral; lrHA-AKI, late-referred HA-AKI.
Table 3 lists the significant independent variables associated with mortality and included in the final model. The OR for hospital mortality for HA-AKI compared with non-HA-AKI was 1.31 (95% CI, 1.27 to 1.37; P < 0.001). nrHA-AKI and lrHA-AKI patients presented an OR of 2.07 (95% CI, 1.59 to 2.71; P < 0.001) and 1.95 (95% CI, 1.54 to 2.47; P < 0.001), respectively, to die during their hospital stay compared with erHA-AKI patients. Other variables contained in this model that significantly interfered with in-hospital mortality were presumed intrinsic cause of HA-AKI, high AKIN stage, need for RRT, presence of underlying CKD, old age, high CCS index, and admission to the medicine ward. However, gender did not show any influence on in-hospital mortality. We therefore suggest that the abovementioned variables can be used to adjust for the confounding effect of comorbidity on survival, provided that age is also included as an independent variable in the analysis. Moreover, the importance of adjusting for age was stressed by the finding that age alone already had strong prognostic value compared with the prognostic value of each of the comorbidity indices and age together.
Table 3.
Multivariate logistic regression analysis for factors associated with mortality among hospital-acquired acute kidney injury (HA-AKI) patients
| Variable | OR | 95% CI | P |
|---|---|---|---|
| HA-AKI versus non-HA-AKI | 1.31 | 1.27 to 1.37 | <0.01 |
| Cause of HA-AKI | <0.01 | ||
| prerenal (functional) | 1.75 | 1.46 to 1.98 | |
| renal (intrinsic) | 2.41 | 1.85 to 2.71 | |
| postrenal (obstructive) | Reference | ||
| AKIN score | <0.01 | ||
| AKIN stage 1 | 0.20 | 0.18 to 0.22 | |
| AKIN stage 2 | 0.17 | 0.16 to 0.19 | |
| AKIN stage 3 | Reference | ||
| nrHA-AKI versus erHA-AKI | 2.07 | 1.59 to 2.71 | <0.01 |
| lrHA-AKI versus erHA-AKI | 1.95 | 1.54 to 2.47 | <0.01 |
| Renal replacement therapy | 1.21 | 1.19 to 1.23 | <0.01 |
| Chronic kidney disease | 1.18 | 1.17 to 1.20 | <0.01 |
| Age (years) | <0.01 | ||
| 20 to 39 | 0.019 | 0.016 to 0.021 | |
| 40 to 59 | 0.048 | 0.043 to 0.054 | |
| 60 to 79 | 0.103 | 0.092 to 0.116 | |
| ≥80 | Reference | ||
| Male versus female gender | 0.91 | 0.77 to 1.07 | 0.09 |
| Charlson Comorbidity Score | <0.01 | ||
| <4 | 0.041 | 0.036 to 0.063 | |
| 4 to 5 | 0.163 | 0.132 to 0.194 | |
| >6 | Reference | ||
| Medicine versus surgery ward | 1.25 | 1.23 to 1.26 | <0.01 |
HA-AKI, hospital-acquired acute kidney injury; OR, odds ratio; CI, confidence interval; nrHA-AKI, referred hospital-acquired acute kidney injury; HA-AKI without nephrology referral; lrHA-AKI, late referral HA-AKI.
The retrospective study presented here did not allow for developing a standardized research consult template. Thus, the most likely of the HA-AKI causes was provided based on history, chart review, and available laboratory data. Prerenal cause (which included true volume depletion, but also decreased effective perfusion, such as congestive failure and liver disease) was the most common HA-AKI type in both patient groups (medicine ward, 74.3%; surgery ward, 72.9%). Laboratory data ordinarily used for differentiating types of AKI frequently were unavailable. Therefore, it was difficult to distinguish prerenal from early intrinsic disease (early renal). Intrinsic renal causes of HA-AKI were called with certainty when there was a clear cause (such as contrast or hypotensive shock) in addition to the overall clinical context. Finally, postrenal (obstructive) cause of HA-AKI was more easily determined and generally well described in the patient's file.
In patients with HA-AKI who were referred to the nephrologist during their hospital stay, time to referral was also associated with in-hospital mortality as shown in Table 4. Compared with referral to the nephrologist within 5 days of AKI diagnosis, the risk of death was 81% higher when the lrHA-AKI patients were referred between 6 and 10 days after SCr level elevation (95% CI, 1.36 to 2.42; P < 0.001). The OR was significantly higher when the patients were referred at days 11 to 15 after SCr level increase (OR, 2.44, 95% CI 1.89 to 3.15; P < 0.001), and finally the OR was 3.45 when the lrHA-AKI patients were referred after 15 days (95% CI, 2.68 to 4.43; P < 0.001). In the multivariate analysis of the risk factors for in-hospital mortality, we included AKIN score, baseline SCr, CKD, age, CCS, and ward admission. After adjustment, the in-hospital mortality was still associated with longer time to refer to the nephrologist (data not shown).
Table 4.
Time to refer HA-AKI patients and in-hospital mortality in medicine and surgery wards
| Time to Refer (days) | Mortality (OR) | 95% CI | P |
|---|---|---|---|
| ≤5 | Reference | ||
| 6 to 10 | 1.81 | 1.36 to 2.42 | <0.01 |
| 11 to 15 | 2.44 | 1.89 to 3.15 | <0.01 |
| >15 | 3.45 | 2.68 to 4.43 | <0.01 |
HA-AKI, hospital-acquired acute kidney injury; OR, odds ratio; CI, confidence interval.
Discussion
In this study, we aimed to characterize the referral patterns in patients with HA-AKI in a tertiary university center over a 5-year period. Our results clearly show that nrHA-AKI or lrHA-AKI represents a common condition among hospitalized patients and is associated with in-hospital mortality, recovery of renal function, and need for RRT. As one of the few studies examining the nephrology referral patterns for HA-AKI patients, our results may suggest that timely nephrologic interventions to prevent HA-AKI in medicine and surgical wards over time could benefit clinical and research studies aimed at improving renal outcomes as recently shown by Balasubramanian et al. (22). However, because the goal of our study was not to evaluate institutional nephrologists' activity per se, we could not determine whether the investigations were not done versus done and not available and whether the nephrologic care had modified patient outcome.
The potential reasons why 22.3% and 58.3% of the patients hospitalized were either not referred or referred to the nephrologist late, respectively, are multiple. One explanation may be the definition of AKI, which is neither uniformly known nor accepted in the non-nephrologic community. Although AKIN criteria provide a unique basis for epidemiologic and interventional outcome studies and describe a constellation of diverse pathophysiologic events, they are not commonly used in the clinical setting. SCr level alone is a relatively late and imprecise biomarker of kidney dysfunction, which may also lead to delayed referral. Other biomarkers are being investigated as potential earlier and more accurate markers of clinically important renal dysfunction (16,23–26). They could be a useful addition to criteria for AKI classification (i.e., AKIN or RIFLE criteria [Risk, Injury, Failure, Loss, and ESRD]). Finally, patients with AKI had more comorbidities, increasing the complexity of medical care and possibly increasing the time required to make treatment decisions. However, adjustment for comorbid conditions did not account for the treatment delays we noted. There are several other reasons why erHA-AKI is associated with better outcome. Some patients with HA-AKI who would have been assigned to the nrHA-AKI or lrHA-AKI groups might have died before initiating any nephrologic therapy. Alternatively, patients might not have had the same “opportunity” to be assigned to the erHA-AKI group. Because this descriptive study did not directly question referring primary care physicians, other referral biases may exist. For example, in-hospital primary care physicians may refer, usually late, only HA-AKI patients whom they consider too complex to be managed in the absence of nephrologic guidelines.
From these results, some clinical implications can be drawn. In-hospital outcomes for patients with HA-AKI during their stay in the medicine or surgery ward vis-à-vis length of stay and RRT requirements differ considerably by referral status. Thus, this prognostic stratification for patients with HA-AKI is crucial. Although mortality rates are high for nrHA-AKI and lrHA-AKI groups, patients with lrHA-AKI were more likely to die during their hospital stay according to the time to refer to the institutional nephrologist. Thus, nephrologists and in-hospital primary care physicians should be mindful of these findings and should intensify their collaboration. Because the indications for RRT in HA-AKI, optimal timing, dosage, and modality are all controversial, the tendency to administer lifesaving therapies such as RRT to patients most likely to have a favorable prognosis may explain why nrHA-AKI patients had a shorter hospital stay and a higher mortality rate. Indeed, these patients had a general worse clinical prognosis than the erHA-AKI patients and even the lrHA-AKI ones as demonstrated by the higher CSS. These patients were not presented to the nephrologist and thus did not have the opportunity to be dialyzed. Another implication of the nrHA-AKI or lrHA-AKI may be the presence of pre-existing CKD. Indeed, in the study presented here, 24% of the patients with HA-AKI had a pre-existing CKD, which is associated with increased postdischarge mortality and the risk of chronic dialysis (15,27–31). Among individuals with pre-existing CKD, an episode of HA-AKI and dialysis during hospital stay was associated with an approximate three-fold higher risk of a chronic dialysis. To avoid CKD misclassification, the delineation of CKD was based on history and laboratory data available before hospitalization. Thus, as previously shown, earlier identification of AKI among patients with prior CKD could have modified the process of care delivered to these patients, manifested by earlier nephrology consultation (28). Finally, in-hospital primary care physicians should not underestimate renal risk factors known to be responsible for HA-AKI, especially if these risk factors cumulate (e.g., old age with high basal SCr, emergency, radiologic procedures with iodine radiocontrast, nephrotoxic drugs) (29). When caring for patients at risk for AKI (e.g., those with multiple myeloma, diabetes mellitus, hypertension) or patients with mild to moderate CKD (i.e., 30 to 60 ml/min per 1.73 m2), awareness of AKI status is dependent on understanding of individual risk, which in turn depends on general knowledge of AKI (29,30). Indeed, the prevalence of awareness did not show signs of increasing over the period from 2004 to 2008. From the limited data available in our study, non-nephrologists did not consider age as a risk factor for HA-AKI, although older patients are at higher risk for CKD and thus at higher risk to develop AKI over CKD. In medicine and surgery wards, higher comorbidity score, assessed using the CCS, was associated with lower referral rate and even nonreferral to the institutional nephrologist. This may be explained by the hierarchy of the clinical problems to be solved, the understanding of HA-AKI and its risk factors, and the potential associated complications or consequences of HA-AKI patient management strategies to slow AKI progression. Indeed, <15% of the sample had three or more SCr determinations in the surgery ward, compared with 89% of the sample that had three or more SCr determinations in the medicine ward. In fact, recent studies have suggested that even minimal increases in SCr are associated with substantial increases in mortality, hospital length of stay, and cost (10–15).
Our study had several limitations. First, some data were missing because of the retrospective study design. Sufficient laboratory data were unavailable for approximately 6% of patients admitted during the study period. Second, this study was based on unique kidney dysfunction criteria. No specimen to measure biomarkers during the AKI episode and after hospital discharge was drawn. Furthermore, the use of imputed or commonly used surrogate estimates of baseline kidney function can result in substantial misclassification of AKI and hinder adequate study of its associated outcomes. As already mentioned, we did not ascertain the nature of the AKI clinic experience, which does not demarcate prognoses on the basis of underlying causative pathophysiology and therefore cannot be used to determine precise mortality estimates for subgroups of patients. This limitation may hinder the creation of a uniform structure to resident learning in the AKI clinic (32). Because of major difficulty in avoiding heterogeneity in the population studied, this study did not analyze the economic aspect of nrHA-AKI or lrHA-AKI.
Our study has also several strengths. It was conducted over a period long enough to analyze many data in a tertiary hospital. The referral patterns were similar during this period of time. We used standardized definitions for AKI and CKD. We captured extensive data on comorbidity and severity of illness, which allowed us to adjust for these factors when considering nrHA-AKI, lrHA-AKI, and erHA-AKI outcomes such as recovery of renal function, requirement for RRT, length of hospital stay, and in-hospital mortality.
In summary, the results of our study show that HA-AKI is frequent, and compared with erHA-AKI, nrHA-AKI and lrHA-AKI are significantly associated with worse renal outcome and low in-hospital patient survival. An episode of HA-AKI requires prompt awareness and nephrologist referral to lower in-hospital morbidity and mortality. Our findings are robust across multiple subgroups, including CSS, age, and CKD and AKIN stages. However, these associations should be confirmed in other cohorts of hospitalized patients as well as in other populations at risk for HA-AKI. In addition to early diagnosis of HA-AKI by use of well known criteria and biomarkers, effective interventions that improve renal outcome should be largely widespread for preventing and/or attenuating HA-AKI.
Disclosures
None.
Acknowledgments
The authors are indebted to T. Alp Ikizler (Division of Nephrology, Department of Medicine, Vanderbilt University School of Medicine) for his support and helpful suggestions. Part of this work was presented in poster form at the Annual Renal Week meeting of the American Society of Nephrology; November 8 to 13, 2005; Philadelphia, PA. Part of the results were published in abstract form in the Journal of the American Society of Nephrology 18: 85A-FC081, 2005. This study was supported by intramural funds from the Centre Hospitalier Universitaire Vaudois and the Centre Hospitalier du Centre du Valais Sion (both public funds).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Management of Acute Kidney Injury: It's the Squeaky Wheel That Gets the Oil!,” on pages 2102–2104.
References
- 1. Ricci Z, Cruz D, Ronco C: The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73: 538–546, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Kwon SH, Noh H, Jeon JS, Kim Y, Han DC: An assessment of AKIN criteria for hospital-acquired acute kidney injury: A prospective observational cohort study. Nephron Clin Pract 116: 217–223, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT: Hospital-acquired renal insufficiency: A prospective study. Am J Med 74: 243–248, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G: Risk factors and outcome of hospital-acquired acute renal failure. Am J Med 83: 65–71, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Sesso R, Roque A, Vicioso B, Stella S: Prognosis of ARF in hospitalized elderly patients. Am J Kidney Dis 44: 410–419, 2004 [PubMed] [Google Scholar]
- 7. Chronopoulos A, Cruz DN, Ronco C: Hospital-acquired acute kidney injury in the elderly. Nat Rev Nephrol 6: 141–149, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Liaño F, Pascual F: Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Obialo CI, Okonofua EC, Tayade AS, Riley LJ: Epidemiology of de novo acute renal failure in hospitalized African Americans. Arch Intern Med 160: 1309–1313, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Chew SL, Lins RL, Daelemans R, Broe ME: Outcome in acute renal failure. Nephrol Dial Transplant 8: 101–107, 1993 [PubMed] [Google Scholar]
- 12. Lafrance JP, Miller DR: Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 21: 345–352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pascual J, Marcén R, Ortuño J: Incidence and prognosis of acute renal failure in older patients. J Am Geriatr Soc 38: 25–30, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Druml W, Lax F, Grimm G, Schneeweiss B, Lenz K, Laggner AN: Acute renal failure in the elderly, 1975–1990. Clin Nephrol 41: 342–349, 1994 [PubMed] [Google Scholar]
- 15. Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, Go AS, Parikh CR, Peterson JF: Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 77: 536–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 19. Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31–R34, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 22. Balasubramanian G, Al-Aly Z, Moiz A, Rauchman M, Zhang Z, Gopalakrishnan R, Balasubramanian S, El-Achkar TM: Early nephrologist involvement in hospital-acquired acute kidney injury: A pilot study. Am J Kidney Dis 57: 228–234, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Coca SG, Parikh CR: Urinary biomarkers for acute kidney injury: Perspectives on translation. Clin J Am Soc Nephrol 3: 481–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bonventre JV: Diagnosis of acute kidney injury: From classic parameters to new biomarkers. Contrib Nephrol 156: 213–219, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Nickolas TL, O'Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 148: 810–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edelstein CL: Biomarkers of acute kidney injury. Adv Chronic Kidney Dis 15: 222–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Devarajan P: Emerging biomarkers of acute kidney injury. Contrib Nephrol 156: 203–212, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Ahlström A, Tallgren M, Peltonen S, Räsänen P, Pettilä V: Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31: 1222–1228, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Khosla N, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini E, Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) Preexisting chronic kidney disease: A potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol 4: 1914–1919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agrawal V, Ghosh AK, Barnes MA, McCullough PA: Perception of indications for nephrology referral among internal medicine residents: A national online survey. Clin J Am Soc Nephrol 4: 323–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coca SG, King JT, Jr, Rosenthal RA, Perkal MF, Parikh CR: The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int 78: 926–933, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]