Abstract
Summary
Background and objectives
Control of serum concentrations of calcium (Ca), phosphorus (P), and parathyroid hormone (PTH) is essential for management of secondary hyperparathyroidism (SHPT).
Design, setting, participants, & measurements
This is a planned interim analysis of a longitudinal cohort study. The settings are dialysis facilities in Japan. Eligible patients comprise all those who were receiving hemodialysis at one of 86 participating facilities and who have SHPT. Using data from a random sample (n = 3276) of the participants from January 2008 through June 2009, we measured changes in the percentages of patients who were within the national guideline–specified target ranges of Ca (8.4 to 10 mg/dl), P (3.5 to 6.0 mg/dl), and intact PTH (iPTH) (60 to 180 pg/ml), and changes in prescriptions of drugs targeting SHPT. We used regression models to identify factors affecting the achievement of the guideline-specified targets.
Results
There were no notable changes in the percentage of patients who were within the guideline for Ca, P, or both. The percentage who were within the iPTH guideline increased from 14.5% to 43.3% (P < 0.001). There were no remarkable changes in the percentage of patients receiving vitamin D or phosphate binders. The percentage who received cinacalcet increased from 0% to 29%. Prescription of cinacalcet was associated with improvement or target-achievement for iPTH and for Ca by 16.8 percentage points (95% CI: 8.1 to 17.0) and by 12.6 percentage points (13.7 to 19.9), respectively.
Conclusions
In the routine care of hemodialysis patients, increasing use of cinacalcet was associated with better control of SHPT.
Introduction
Secondary hyperparathyroidism (SHPT) is a common complication of dialysis, and it increases the risk of bone disease (1,2) and cardiovascular diseases (3–7). Long-term disorders of bone-mineral metabolism can be seen as a systemic condition (chronic kidney disease–related mineral and bone disorders [CKD-MBD]) (8), and the prognosis for dialysis patients might be improved if SHPT is controlled (8,9). MBD is controlled by treatment with phosphate binders, vitamin D analogs, and calcimimetics, which are usually given in combination (10). In Japan, the calcimimetic agent cinacalcet hydrochloride has been manufactured and marketed by Kyowa Hakko Kirin Co., Ltd. since January 2008. Although phosphate binders and vitamin D analogs have the desirable effect of lowering parathyroid hormone (PTH) they can sometimes cause hypercalcemia (11,12). Calcimimetics can lower three indices of MBD (serum calcium, phosphorus, and PTH) and can increase the percentage of patients in whom MBD target levels are achieved (13,14).
Dialysis patients in Japan differ from those in other countries (15): mortality is much lower and the duration of dialysis is longer. Also, in the Japanese Society for Dialysis Therapy (JSDT) guidelines (16) the target range for intact PTH (iPTH) is lower than in guidelines used in other countries (9,17).
Outcomes of treatment of SHPT have been, and are being, studied (18–21). The Mineral and Bone Disorders Outcomes Study for Japanese CKD Stage 5D Patients (MBD-5D) (22,23) is an ongoing prospective observational study of patient characteristics and practice patterns in relation to mortality, hospitalization, and other clinical outcomes among dialysis patients in Japan who have SHPT. The MBD-5D started in January 2008, and here we present results of a planned interim analysis of MBD-5D data. We describe changes over 1.5 years in MBD management in Japan and identify factors associated with achievement of MBD management goals.
Materials and Methods
MBD-5D
The MBD-5D is a 3-year prospective case-cohort and cohort study investigating long-term outcomes of MBD management, cardiovascular disease, and death (22). The study adheres to the Declaration of Helsinki and the Japanese government's ethical guidelines for epidemiologic studies.
The study has a whole cohort comprising all patients enrolled and a subcohort comprising a randomly selected 40% of the whole cohort. Data are collected from large dialysis facilities (i.e., facilities with more than 100 patients) in Japan. From 86 facilities, 8229 patients were registered, and 3276 were selected into the subcohort. For the subcohort, data being collected prospectively are background and demographic information, dialysis prescription, results of laboratory tests, and prescriptions for medications (22). In the whole cohort, age, gender, and PTH are recorded, and the other detailed data for each patient are collected retrospectively after death (22).
Design and Data Collection
For the present study, we used subcohort data collected during the first 1.5 years of the MBD-5D. Eligible patients comprise all those who were receiving hemodialysis at a participating facility as of January 1, 2008, and who either had an iPTH of at least 180 pg/ml (106 pg/ml for whole PTH) or who were receiving intravenous active vitamin D sterols or an oral active vitamin D analog (falecalcitriol, the only oral drug approved in Japan for treatment of SHPT). Patients who had been undergoing hemodialysis for less than 3 months (at the time that they were evaluated) were not included.
Data were collected at the time of enrollment (visit 0) and every 3 months from January 2008 through June 2009 (visits 1 through 6). The number of patients from whom usable data were collected decreased during the study: 208 died, five underwent renal transplantation, one stopped hemodialysis to begin peritoneal dialysis, 56 underwent parathyroidectomy (PTx), four underwent percutaneous ethanol injection therapy, and 57 were lost to follow-up.
Serum whole PTH levels measured by third-generation PTH assay were converted to iPTH levels: iPTH = whole PTH × 1.7 (16). Serum calcium levels were corrected for albumin concentration (modified Payne method [24]). Data were excluded if they were judged to be probable measurement errors or data-entry errors.
Analyses
MBD Management Status
For the concentrations in serum of phosphorus, calcium, and iPTH, the percentages of patients who were within JSDT-specified target ranges at each visit were computed. The JSDT-specified target ranges are 3.5 to 6.0 mg/dl for phosphorus, 8.4 to 10.0 mg/dl for calcium, and 60 to180 pg/ml for iPTH (16).
Treatment of MBD
Data on five classes of drugs were analyzed: intravenous vitamin D derivatives (calcitriol and maxacalcitol), oral vitamin D derivatives (falecalcitriol, calcitriol, and alphacalcidol), calcium-based phosphate binder (calcium carbonate), non-calcium-based phosphate binders (sevelamer hydrochloride and lanthanum carbonate), and cinacalcet. Percentages of patients to whom the drug was prescribed were computed for each visit. Lanthanum carbonate became available in Japan at the end of the 1.5 years (April, 2009) and was prescribed to only a few patients and only at visit 6. Cinacalcet became available at the beginning of the 1.5 years (January 2008).
Exploratory Analyses of Variables Likely to Affect Achievement of Guideline-Specified Targets
The analytic model is shown in Figure 1. Outcomes included improvement in iPTH (at least a one-category decrease in iPTH, when categorized as less than or equal to 180, 181 to 300, 301 to 500, or greater than 500 pg/ml, based on the target goals in the JSDT guidelines [16], the Kidney Disease Outcomes Quality Initiative (K/DOQI) [9], and the recommended level for PTx [16]), achievement of the calcium goal (8.4 to 10.0 mg/dl), achievement of the phosphorus goal (3.6 to 6.0 mg/dl), and simultaneous achievement of those two goals.
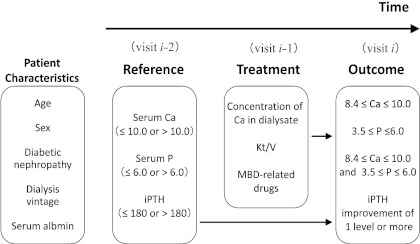
Figure 1.
Regression model used in the analyses of factors likely to affect the achievement of guideline-specified targets: The model assumes that laboratory values may cause changes in treatment, which may in turn modify later laboratory values. Patients' characteristics included age (<65 or ≥65 years), gender (male or female), diabetic nephropathy (no or yes), number of years of dialysis treatment (<10 or ≥10 years), and serum albumin (<3.5 or ≥3.5 g/dl). Reference values included phosphorus in serum at baseline (≤6.0 or >6.0 mg/dl), calcium in serum at baseline (≤10.0 or >10.0 mg/dl), and iPTH in serum at the reference visit (≤ 180 or >180 pg/ml). Treatments included calcium in dialysate (<3.0 or ≥3.0 mEq/L), Kt/V (<1.2, 1.2 to <1.6, or ≥1.6), an intravenous vitamin D receptor activator (none, oral VD alone, or intravenous VD), a phosphate binder (none, calcium-based phosphate binder alone, non-calcium-based phosphate binder alone, or both calcium-based and non-calcium-based phosphate binders), and cinacalcet (no or yes). Outcomes were defined in reference to the JSDT guidelines for calcium, for phosphorus, for both calcium and phosphorus, and for iPTH.
Independent variables included prescription of the drugs mentioned above and MBD-related laboratory values. To ensure that the results would not reflect any influence of those drugs on MBD-related laboratory values, we used the values recorded one visit before the drugs were prescribed. Thus, values of the dependent variables come from data collected at the last of three consecutive visits: reference, treatment, and outcome. To take full advantage of the 1.5 years of data, we used the data as repeated measurements: the data collected during the seven visits were handled as five imbricated sets of measures consisting of three consecutive visits. We did not include changes in treatment within each set of measurements in the analytic model. That is, only the drug prescriptions at the treatment visit, not those at the reference visit, were incorporated in the model. In addition, some variables regarding patient characteristics at baseline were included because cross-sectional analyses indicated that they were associated with achievement of guideline-specified targets.
From 3276 patients, 15,197 sets of measurements were available (mean: 4.6 sets per patient). For each index of MBD management, the therapeutic goal, treatment, and proportion in whom the goal was achieved all depend on whether that index was or was not already within the target range. Therefore, in the regression analyses we analyzed separately the sets of measurements in which the target had already been met at the reference visit and the sets in which it had not: iPTH greater than 180 pg/ml versus less than or equal to 180 pg/ml; calcium less than 8.4, from 8.4 through 10.0, and greater than 10; phosphorus less than 3.5, from 3.5 through 6.0, and greater than 6.0.
Absolute differences in percentages of patients who achieved the target or improved were used in risk-difference regression models (25), because stratified analyses suggested that the data are compatible with risk-difference models rather than risk-ratio models. Using risk-difference models, we can compute absolute effect sizes and we can estimate how many patients would benefit from treatment, neither of which would be possible if we used a relative indicator such as the odds ratio. No interaction term was included. Using generalized estimating equations, we estimated the parameters from intraindividual repeated measurements. We also did sensitivity analyses including only patients with complete follow-up, to examine the effect of dropouts. Results were expressed as point estimates and their 95% confidence intervals. All analyses were done with SAS 9.1.3 (SAS Institute, Cary, North Carolina).
Results
Characteristics of the study patients are shown in Table 1. Compared with all Japanese dialysis patients in 2007 (26), the study participants were slightly younger, a higher proportion of them had diabetic nephropathy as the cause of end-stage renal disease, and they had higher levels of iPTH (all had SHPT). They did not differ from the overall cohort (mean age 62.0 years, 37.7% women, and mean iPTH 341 pg/ml) (23).
Table 1.
Characteristics of the patients in this study and in Japan as a whole
| This Study | Japanese Hemodialysis Population (2007)a | |
|---|---|---|
| Number of patients | 3,276 | 275,242 |
| Age (years), mean (SD) | 61.9 (12.7) | 64.9 (12.7) |
| Percentage of women | 38.5 | 38.6 |
| Cause of end-stage renal disease (%) | ||
| glomerulonephritis | 45.1 | 42.4 |
| diabetic nephropathy | 24.2 | 33.4 |
| nephrosclerosis | 6.2 | 6.5 |
| polycystic kidney disease | 4.4 | 3.4 |
| others | 10.3 | 7.0 |
| unknown | 9.8 | 7.4 |
| Indices of CKD-MBD, mean (SD)b | ||
| calcium in serum (mg/dl) | 9.5 (0.9) | 9.3 (0.9)/9.4 (0.9) |
| phosphorus in serum (mg/dl) | 5.5 (1.4) | 5.3 (1.5)/5.2 (1.5) |
| iPTH (pg/dl) | 334 (255) | 193 (203)/202 (225) |
| Number of years of dialysis treatment (“vintage”) (years), mean (SD) | 10.2 (8.3) | NA |
CKD-MBD, chronic kidney disease-related mineral and bone disorders; iPTH, intact PTH; NA, not available.
Results of a survey carried out by the Japanese Society for Dialysis Therapy, in December 2007 (26).
Indices of CKD-MBD in men and women are shown separately: men/women.
There were no notable changes in the percentage of patients who were within the guidelines for calcium or for phosphorus or in the mean values for calcium and phosphorus (Table 2). The percentage who were within the guideline for iPTH increased markedly, from 14.5% to 43.9% (P < 0.001), and the serum iPTH decreased from 334 pg/ml to 227 pg/ml (P < 0.001). With the patients stratified by iPTH values at visit 0, the percentages of each stratum in whom iPTH was less than or equal to 180 pg/ml at visit 6 were as follows: baseline 181 to 300 pg/ml, 56.4%; 301 to 500 pg/ml, 48.5%; greater than 500 pg/ml, 35.1%. The percentage of patients who were within the guidelines for all three measures (calcium, phosphorus, and iPTH) also increased, from 7.0% to 22.9% (P < 0.001).
Table 2.
Changes over 1.5 years in achievement of MBD management targets specified by the JSDT guideline
| Visit number | Period | Number of Patients | Serum Ca |
Serum P |
Serum Ca and Serum P (%Achievement of Target)c | iPTH |
Serum Ca, Serum P, and iPTH (%Achievement of Target)e | |||
|---|---|---|---|---|---|---|---|---|---|---|
| %Achievement of Targeta | Mean (SD) (mg/dl) | %Achievement of Targetb | Mean (SD) (mg/dl) | %Achievement of Targetd | Mean (SD) (pg/ml) | |||||
| 0 | - Dec07 | 3276 | 65.4 | 9.45 (0.90) | 63.2 | 5.53 (1.37) | 41.9 | 14.5 | 334 (255) | 7.0 |
| 1 | Jan08 to Mar08 | 3192 | 68.8 | 9.44 (0.86) | 62.2 | 5.55 (1.40) | 43.2 | 25.3 | 309 (241) | 12.7 |
| 2 | Apr08 to Jun08 | 3173 | 69.2 | 9.51 (0.85) | 65.3 | 5.45 (1.36) | 45.8 | 33.4 | 265 (212) | 17.1 |
| 3 | Jul08 to Sep08 | 3083 | 67.3 | 9.58 (0.85) | 65.3 | 5.32 (1.37) | 44.4 | 40.5 | 227 (193) | 19.4 |
| 4 | Oct08 to Dec08 | 3051 | 70.1 | 9.54 (0.81) | 64.9 | 5.43 (1.40) | 45.8 | 38.4 | 240 (201) | 20.5 |
| 5 | Jan09 to Mar09 | 2956 | 71.9 | 9.45 (0.84) | 60.9 | 5.58 (1.42) | 44.5 | 37.9 | 243 (205) | 19.8 |
| 6 | Apr09 to Jun09 | 2906 | 70.6 | 9.51 (0.80) | 64.7 | 5.42 (1.39) | 46.5 | 43.9 | 227 (214) | 22.9 |
| P valuef | <0.001 | 0.003 | 0.22 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
MBD, mineral and bone disorders; JSDT, Japanese Society for Dialysis Therapy; iPTH, intact PTH.
Target of serum Ca: 8.4 to 10.0 mg/dl.
Target of serum P: 3.5 to 6.0 mg/dl.
Achievement of both serum Ca and serum P % achievement of target.
Target of iPTH: 60 to180 pg/ml.
Achievement of all of serum Ca, serum P, and iPTH % achievement of target.
For the percent of patients who achieved a target, differences between visit 0 and visit 6 were tested with the chi-squared test. For the mean values of serum Ca, serum P, and iPTH, differences between visit 0 and visit 6 were tested with the t test.
Changes in Treatments of MBD
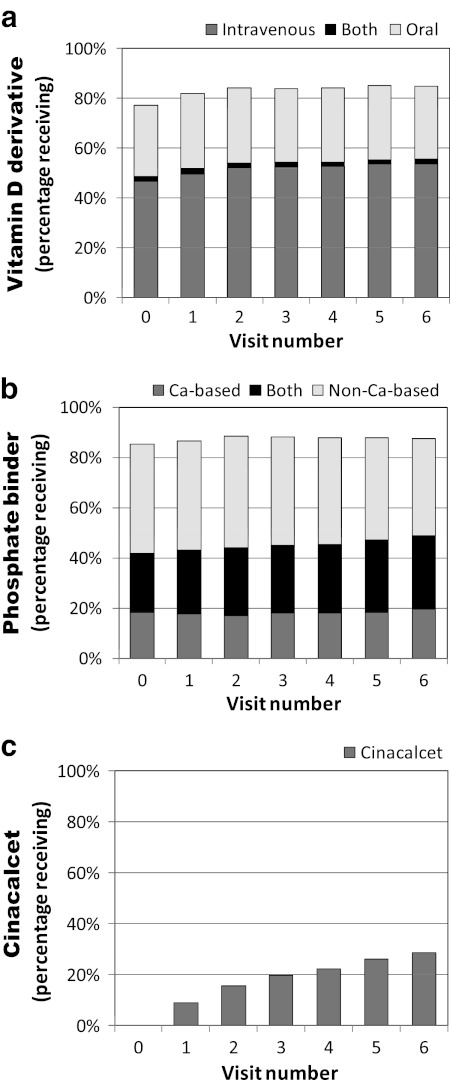
The percentage of patients who received vitamin D derivatives (VDs) increased slightly (from 77% to 85%), and approximately two-thirds of them received intravenous VDs (Figure 2a). There was no major change in the percentage of patients who received phosphate binders, but the percentage who received both calcium-based and non-calcium-based types increased (Figure 2b). The percentage of patients receiving cinacalcet increased. By the end of the observation period it had reached 29% (Figure 2c).
Figure 2.
Changes over 1.5 years in the percentages of patients to whom MBD-related drugs were prescribed. (a) Percentages of patients to whom intravenous vitamin D (calcitriol or maxacalcitol) or oral vitamin D (falecalcitriol, calcitriol, or alphacalcidol) were prescribed. (b) Percentages of patients to whom calcium-based phosphate binder (calcium carbonate) or non-calcium-based phosphate binder (sevelamer hydrochloride or lanthanum carbonate) were prescribed. Lanthanum carbonate became available in Japan at the end of the 1.5 years (April, 2009) and was prescribed to only a few patients and only at visit 6. (c) Percentages of patients to whom cinacalcet was prescribed. Cinacalcet became available at the beginning of the 1.5 years (January, 2008).
Associations between Treatments and Achievement of MBD Management Goals
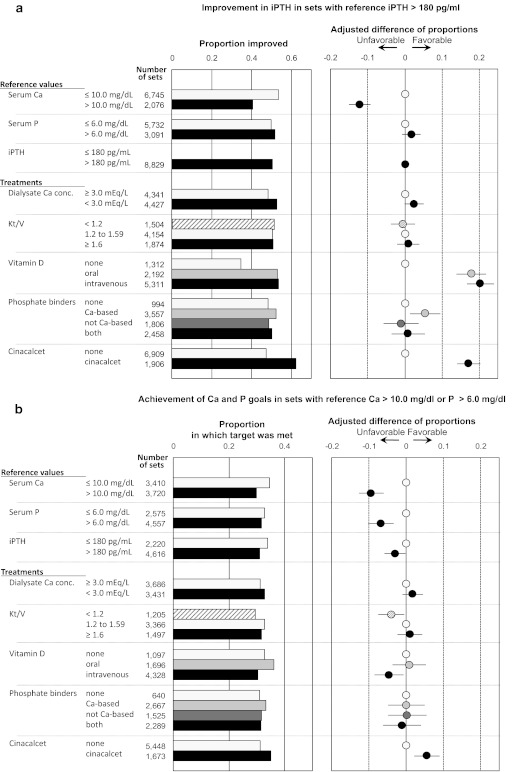
For these analyses, improvement within any given set of measurements was defined using the difference between that set's reference measurement and its outcome measurement. A total of 8829 sets of measurements with a reference iPTH greater than 180 pg/ml were available for analysis (Figure 3a). In 50.5% of those sets, the iPTH concentration improved by at least one category (for iPTH there were four categories: less than or equal to 180, 181 to 300, 301 to 500, and greater than 500 pg/ml). Five treatments were associated with improvement in iPTH by at least one category: intravenous VDs, oral VDs, cinacalcet, the calcium-based phosphate binder, and a low concentration of calcium in dialysate. One factor appeared to inhibit improvements in iPTH: a high concentration of serum calcium at the reference visit.
Figure 3.
Proportions of measurement sets with favorable results for iPTH, calcium, and phosphorus, with sets stratified on the basis of Japanese Society for Dialysis Therapy (JSDT) guidelines. (a and b) The leftmost column indicates the independent variable, the next column shows the categories used, the next column shows the number of sets of measurements, the next column (the bar graph) shows the crude results, and the next column shows the point estimate and 95% confidence interval of the difference in proportions after adjustment for covariates in the regression model. The covariates were all of the variables listed in the leftmost column, in addition to age, gender, presence or absence of diabetic nephropathy as the cause of renal failure, number of years of dialysis, and concentration of albumin in serum. (a) Proportion of sets in which intact PTH (iPTH) improved when the reference iPTH was >180 pg/ml. (b) Proportion of sets in which both the calcium goal and the phosphorus goal were met, when the reference calcium was >10.0 mg/dl or the reference phosphorus was >6.0 mg/dl.
Among the 5029 sets with a reference iPTH less than or equal to 180 pg/ml, the low iPTH level was maintained in 69.8%. Three treatments were associated with maintenance of a low iPTH: use of oral VDs, intravenous VDs, and cinacalcet. Only one factor appeared to inhibit the maintenance of a low iPTH: a high concentration (>10 mg/dl) of calcium in serum at the reference visit.
Among the 3722 sets with a reference calcium greater than 10 mg/dl, a calcium within the target range of 8.4 to 10.0 mg/dl was achieved in 48.4%. Cinacalcet and a low concentration of calcium in dialysate were associated with a higher percentage achieving the calcium-concentration goal (Figure S1). Three factors appeared to inhibit the achievement of that goal: intravenous VDs, oral VDs, and a reference iPTH greater than 180 pg/ml. Even an iPTH between 181 and 300 pg/ml was associated with not achieving the calcium-concentration goal when subcategories of iPTH level were analyzed.
There were 10,339 sets with a reference calcium of 8.4 to 10 mg/dl, and in 78.5% of them calcium was maintained in that range. No variables were found to be associated with maintenance within that range. In contrast, five factors appeared to inhibit maintenance within that range: intravenous VDs, oral VDs, a reference phosphorus greater than 6.0 mg/dl, a reference iPTH greater than 180 pg/ml, and cinacalcet. Among the 14,061 sets with a reference calcium level greater than 8.4, hypocalcemia (Ca <8.4) occurred in 3.7% of those in which cinacalcet was prescribed and in 3.6% of those in which it was not prescribed. Among the 1066 sets with a reference calcium less than 8.4 mg/dl, intravenous and oral VDs were associated with a higher percentage of patients achieving the calcium-concentration goal.
There were 4559 sets with a reference phosphorus greater than 6.0 mg/dl, and among them a phosphorus within the target range of 3.5 to 6.0 mg/dl was achieved in 46.6%. Only the prescription of oral VDs was associated with a higher percentage achieving the phosphorus-concentration goal (Figure S2). Two factors appeared to inhibit the achievement of that goal: having a high reference calcium and having a Kt/V of less than 1.2, where K is clearance, t is time, and V is volume.
Among the 9728 sets with a reference phosphorus of 3.5 to 6.0 mg/dl, phosphorus was maintained in that range in 72.7%. Only prescription of the non-Ca-based phosphate binder was associated with maintenance of the phosphorus-concentration goal. Among the 857 sets with a reference phosphorus less than 3.5 mg/dl, achievement of that goal was positively associated with prescription of intravenous VDs and negatively associated with having an iPTH greater than 180 pg/ml.
Among the 7134 sets with either a reference calcium or phosphorus greater than 10 mg/dl or 6.0 mg/dl, respectively, both calcium and phosphorus concentrations within the target range were achieved in 32.1%. Only cinacalcet was associated with a higher percentage of patients achieving both of those goals. Five factors appeared to inhibit the achievement of those two goals: intravenous VDs, having a Kt/V less than 1.2, having a high reference calcium, having a high reference phosphorus, and having a high reference iPTH (Figure 3b).
For sensitivity analyses we used only data from those patients who had been followed for 1.5 years. The results did not differ from those described above.
Discussion
Focusing on patients with SHPT at dialysis facilities in Japan, these results document recent changes in treatment and in achievement of MBD management goals. The whole cohort of this study is not expected to be a representative sample of dialysis patients in Japan, so the generalizability of the results may be limited. However, the associations between independent variables and achievement of MBD management goals should also be applicable to patients outside the MBD-5D study (27). Although dialysis practice patterns differ between countries (15), this study's results are consistent with those reported previously from countries other than Japan. Therefore, these results are considered to be good estimates of the overall changes in treatment, the changes in outcomes, and the associations between outcomes and likely causes.
MBD Management Status and Treatment
We note that the iPTH goal was achieved with no decrease in the percentages of patients who were within the guidelines for calcium and for phosphorus. During the time that these outcomes improved, the largest change in treatment was the increasing use of cinacalcet (28,29). Within 1.5 years after it first became available (January, 2008) it was being prescribed to 29% of the patients in this study. Although the percentage of patients to whom VDs were prescribed slightly increased, there were no such changes in prescriptions for phosphate binders.
Thus, these changes in MBD management may reflect changes initiated by the use of cinacalcet. Addition of cinacalcet to other treatments has been reported to improve MBD management (13). A study of 10,337 patients with a mean iPTH level of 576 pg/ml found that by 2 years after cinacalcet had been introduced 32% of those patients had begun receiving it, their iPTH level had decreased to a mean of 453, and the percentage in whom indices of MBD were within K/DOQI guidelines had increased (30). Considering the difference in the initial iPTH levels, we interpret the present results as indicating that cinacalcet may be effective even in patients whose hyperparathyroidism is not severe.
Variables Associated with Meeting MBD Management Goals
The JSDT guidelines became available in Japanese in 2006, and they were published in English in 2008 (16), which may have changed MBD management practices. Specifically, the improvements in control of PTH measured in this study might have been caused both by the publication of the new guidelines and by the introduction of cinacalcet. Nonetheless, the control of PTH was better in those patients to whom cinacalcet was prescribed than in those to whom it was not, which we interpret as evidence that at least some of the improvement can be attributed to the drug.
When iPTH was greater than 180 pg/ml at the reference visit, the two variables associated with improvement were VDs and cinacalcet: iPTH improved in 62% of the sets in which cinacalcet was given but in only 47% of those in which it was not. Thus, the apparent advantage of receiving cinacalcet was an adjusted difference of about 15 percentage points. The apparent effect of VDs was similar. Consistent with previous findings (12–14), it is likely that VDs and cinacalcet contributed to the decrease in iPTH.
High concentrations of iPTH in serum at the reference visit (>180) also appeared to inhibit by about 10% the achievement of the calcium-concentration goal. This is consistent with the cross-sectional finding by Fukagawa et al. (23) that higher levels of iPTH were associated with being outside the guidelines for calcium and phosphorus, independent of prescriptions for VDs. As expected (12–14), cinacalcet and a low concentration of calcium in dialysate appeared to lower concentrations of calcium in serum, but VDs appeared to inhibit such improvement.
As mentioned above, increased awareness due to publication of the JSDT guidelines can account for some of the measured improvement. We also note that many of the patients who received cinacalcet were receiving VDs, so the present results can be interpreted as showing a biochemical benefit of giving cinacalcet as an addition to pre-existing treatment with VDs in dialysis patients whose MBD management is not good.
Disorders of mineral metabolism are associated with a higher risk of death in dialysis patients (3,4,7,31), and in one observational study consistent control of MBD was associated with longer survival (32). Therefore, to the extent that using cinacalcet helps to maintain indices of MBD within the guideline-specified ranges (14,30), it would also be expected to benefit dialysis patients with comorbid SHPT. In a previous observational study cinacalcet was found to reduce the risks of death and of cardiovascular disease (21), and it is likely also to result in longer survival of dialysis patients in Japan. Further information on cinacalcet, MBD management, and clinical outcomes is expected from analyses of data from the full 3 years of the MBD-5D, and from the EVOLVE, which is the first randomized controlled trial investigating associations between clinical outcomes MBD management with cinacalcet (19).
Having a high concentration of calcium in serum at the reference visit appeared to inhibit achievement of the phosphorus goal, possibly because clinicians have less leeway in prescribing calcium-based phosphate binders when the concentration of calcium in serum is high. Oral VDs were associated with decreases in phosphorus concentration, but intravenous VDs appeared to inhibit those decreases. The reason for that discrepancy between the effects of oral and of intravenous VDs is not clear.
Limitations
The MBD-5D data come from patients at relatively large dialysis facilities, so they may not be representative of Japan as a whole, and international differences in dialysis practices may also limit the generalizability of these results.
The results could also reflect some confounding by indication. For example, there was no apparent effect of phosphate binders on the concentration of phosphorus in serum, but patients given sevelamer probably already had SHPT that was relatively severe. Also, the analyses accounted for drug prescriptions at the treatment visit, but not changes within each set of measurements.
In each set of measurements the interval between “treatment” and “outcome” was only 3 months, but good outcomes depend on good MBD management that is maintained over longer periods (4,33). Information about longer-term maintenance within set goals and about clinical events will become available after the planned 3 years of this study have passed.
Conclusions
In Japanese hemodialysis patients with SHPT, during the 1.5 years starting in January 2008, the percentage of those who met the JSDT guidelines for calcium and phosphorus concentrations did not change, but the control of iPTH levels improved. By the end of that period, 29% of those patients were receiving prescriptions for cinacalcet. Consistent with results from randomized controlled trials, these results from regular daily practice show the benefits of cinacalcet in hemodialysis patients whose MBD management is not good (Figure 3).
Disclosures
The MBD-5D is supported by research grants from Kyowa Hakko Kirin (manufacturer of intravenous calcitriol, cinacalcet hydrochloride, and sevelamer hydrochloride), without restrictions on publications. T.A. has acted as a consultant for Kyowa Hakko Kirin, has received grants (research support) from Kyowa Hakko Kirin, and is a member of speakers' bureau of Kyowa Hakko Kirin. S.F. has acted as a scientific advisor for Kyowa Hakko Kirin and has received grants (research support) from Kyowa Hakko Kirin. M.F. has acted as a consultant for Kyowa Hakko Kirin, has received honoraria from Kyowa Hakko Kirin, and has received grants (research support) from Kyowa Hakko Kirin. Y.O., T.Y., T.H., R.K., and K.K. have no such potential competing interests to declare.
Supplementary Material
Acknowledgments
We thank the MBD-5D study advisory investigators: Masashi Suzuki (Shinrakuen Hospital), Yoshindo Kawaguchi (Shiomidai Hospital), Akira Saito (International University of Health and Welfare Atami Hospital), Yoshiki Nishizawa (Osaka City University Graduate School of Medicine), Yusuke Tsukamoto (Shuwa General Hospital), Satoshi Kurihara (Tsukinomori Clinic), Takashi Akiba (Tokyo Women's Medical University), Eriko Kinugasa (Showa University Northern Yokohama Hospital), Yuzo Watanabe (Kasugai Municipal Hospital), Yoshihiro Tominaga (Nagoya Daini Red Cross Hospital), Takashi Shigematsu (Wakayama Medical University), Masaaki Inaba (Osaka City University Graduate School of Medicine), Jun Minakuchi (Kawashima Hospital), Hideki Hirakata (Fukuoka Red Cross Hospital), Keitaro Yokoyama (Jikei University School of Medicine), Naoki Kimata (Tokyo Women's Medical University), Fumihiko Koiwa (Showa University Fujigaoka Hospital), Ryoichi Ando (Musashino Red Cross Hospital), Junichiro J. Kazama (Niigata University), Takatoshi Kakuta (Tokai University School of Medicine), Hirotaka Komaba (Tokai University School of Medicine), Daijo Inaguma (Nagoya Daini Red Cross Hospital), Eiji Ishimura (Osaka City University Graduate School of Medicine), Hideki Tahara(Osaka City University Graduate School of Medicine), Kazuhiko Tsuruya (Kyushu University), and Akira Fujimori (Konan Hospital).
Appendix
The following investigators also participated in this study: Nobuo Hashimoto (H·N·MEDIC), Mari Ishida (Kitasaito Hospital), Toshiyuki Date (Date Clinic), Kiyotaka Yabuki (Yabuki Hospital), Hideki Tanida (Tendo Onsen Yabuki Clinic), Fumitoshi Yamauchi (San-ai Hospital), Mikihiko Fujishima (Yahaba Clinic), Tomohito Matsunaga (Eijinkai Hospital), Jun Urae (Ishinomaki Clinic), Hiroshi Kawaguchi (Iwaki Urological Hospital), Ikuo Takahashi (Kisen Hospital), Yoshiko Tanaka (Shinjuku-Koshin Clinic), Hideo Kobayashi (Suda Clinic), Maki Takahashi (Suda Naika Clinic), Tatsuya Nonaka (Seishokai Memorial Hospital), Hideto Emoto (Tokai Hospital), Kyosuke Nishio (Shinkoiwa Clinic), Atsushi Hayama (Moriyama Rehabilitation Hospital), Toshio Shinoda (Kawakita General Hospital Dialysis Center), Takashi Kono (Mihama Narita Clinic), Takahiro Mochizuki (Kameda Medical Center), Yasuo Kimura (Shin-kashiwa Clinic), Noriyoshi Murotani (Chiba Social Insurance Hospital), Satoshi Yamaguchi (Asahi Hospital), Taichi Nakanishi (Kurihama Clinic), Kiyoshi Ozawa (Yokosuka Clinic), Takashi Nagaoka (Sagamihara Clinic), Takao Suga (Bousei Hiratsuka Clinic), Masakazu Suda (Suda Medical Clinic), Yoshikazu Goto (Saiyu Soka Hospital), Michio Kuwahara (Shuwa General Hospital Hemodialysis Clinic), Hiromi Shimoyama (Yuai Clinic), Kimihiko Matsuyama (Misato Kenwa Clinic), Kazue Ueki (Toho Hospital), Kyoko Ito (Heisei Hidaka Clinic), Katsuhiko Miyamoto (Seseragi Hospital), Takashi Ishizu (Tukuba Central Hospital), Shuichi Kikuchi (Ohba Renal Clinic), Masaki Kobayashi (Tokyo Medical University Ibaraki Medical Center), Mitsuyoshi Furuhashi (Maruyama Hospital), Masanori Wakabayashi (Bousei Dai-ichi Clinic), Kazuyoshi Nakamura (Fujidaiichi Clinic), Hirotake Kasuga (Kaikoukai Central Clinic), Itsuo Yokoyama (Nagoya Memorial Foundation Narumi Clinic), Chikao Yamazaki (Masuko Clinic SUBARU), Kijun Nagata (Sawada Hospital), Yasumasa Kawade (Suzuka Kidney Clinic), Toshiaki Kawanaka (Ishikiriseiki Hospital), Yoshihiro Tsujimoto (Inoue Hospital), Mikio Okamura (Ohno Memorial Hospital), Shigeki Okada (Okada Clinic), Senji Okuno (Kidney Center Shirasagi Clinic), Harumi Nagayama (Nagayama Hemodialysis Clinic), Shuji Okazaki (Nagayama Hospital), Yoshinori Tone (Fujii Clinic), Ibuki Yajima (Ibuki Clinic), Kouji Shibuya (Sumiyoshigawa Hospital), Kunihiko Yoshiya (Hara Genitourinary Hospital), Morihiro Kondou (Otowa Kinen Hospital), Satoru Yamazaki (Tojinkai Hospital), Ryoichi Miyazaki (Fujita Memorial Hospital), Katsuhiko Arimoto (Shigei Medical Research Hospital), Misaki Moriishi (Nakajima Tsuchiya Clinic), Takahito Nasu (Tokuyama Central Hospital), Seiichi Obayashi (Kinashi Obayashi Hospital), Yuzuru Sato (Sato Junkankika Naika), Takao Tanaka (Ohji Hospital), Hidetoshi Nakamura (Kokura Daiichi Hospital), Nobuhiko Koga (Shin-Koga Clinic), Harumichi Higashi (St. Mary's Hospital), Kougi Yuu (Takahashi Naika Clinic), Asako Kitamura (Chikuho Social Insurance Hospital), Tomoji Matsumae (Murakami Memorial Hospital), Katsushige Abe (Jinikai Hospital), Masahiro Kawatomi (Kawatomi Internal Medicine Clinic), Motoko Tanaka (Akebono Clinic), Chisa Nogami (Kumamoto Urological Hospital), Etsuo Yoshidome (Ikeda Hospital), Shinyu Miyagi (Okinawa Daiichi Hospital), Satoshi Nakazato (Chibana Clinic), Yoshiki Shiohira (Tomishiro Central Hospital), and Kiyoyuki Tokuyama (Tokuyama Clinic).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL: Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM: PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney 47: 149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, Akizawa T, Saito A, Asano Y, Kurokawa K, Pisoni RL, Port FK: Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial Int 11: 340–348, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Nakai S, Akiba T, Kazama J, Yokoyama K, Fukagawa M, Tominaga Y, Iseki K, Tsubakihara Y: Patient Registration Committee of the Japanese Society for Dialysis Therapy, Tokyo, Japan: Effects of serum calcium, phosphorous, and intact parathyroid hormone levels on survival in chronic hemodialysis patients in Japan. Ther Apher Dial 12: 49–54, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Kidney Disease: Improving Global Outcomes (KDIGO): Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 9. National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[4 Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 10. Wetmore JB, Quarles LD: Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nat Clinc Pract Nephrol 5: 24–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Indridason OS, Quarles LD: Comparison of treatments for mild secondary hyperparathyroidism in hemodialysis patients. Durham Renal Osteodystrophy Study Group. Kidney Int 57: 282–292, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF: Meta-analysis: Vitamin D compounds in chronic kidney disease. Ann Intern Med 147: 840–853, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, Drüeke TB, Cunningham J, Sherrard DJ, McCary LC, Olson KA, Turner SA, Martin KJ: Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 67: 760–771, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW: Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270–3277, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Guideline Working Group, Japanese Society for Dialysis Therapy: Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial 12: 514–525, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 76: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Fishbane S, Shapiro WB, Corry DB, Vicks SL, Roppolo M, Rappaport K, Ling X, Goodman WG, Turner S, Charytan C: Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: The ACHIEVE study results. Clin J Am Soc Nephrol 3: 1718–1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chertow GM, Pupim LB, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, London GM, Mahaffey KW, Moe SM, Wheeler DC, Albizem M, Olson K, Klassen P, Parfrey P: Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE): Rationale and design overview. Clin J Am Soc Nephrol 2: 898–905, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Cannata-Andia JB, Fernandez-Martin JL, Zoccali C, London GM, Locatelli F, Ketteler M, Ferreira A, Covic A, Floege J, Gorriz JL, Rutkowski B, Memmos DE, Verbeelen D, Tielemans C, Teplan V, Bos WJ, Nagy J, Kramar R, Goldsmith DJ, Martin PY, Wuthrich RP, Pavlovic D, Benedik M: Current management of secondary hyperparathyroidism: A multicenter observational study (COSMOS). J Nephrol 21: 290–298, 2008 [PubMed] [Google Scholar]
- 21. Block GA, Zaun D, Smits G, Persky M, Brillhart S, Nieman K, Liu J, St Peter WL: Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 78: 578–589, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Fukuhara S, Akizawa T, Fukagawa M, Onishi Y, Yamaguchi T, Hasegawa T, Kurokawa K: Mineral and Bone Disorders Outcomes Study for Japanese Chronic Kidney Disease Stage 5D Patients (MBD-5D): Rationale and study design. Ther Apher Dial 15: 169–175, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Fukagawa M, Komaba H, Onishi Y, Fukuhara S, Akizawa T: Mineral metabolism management in hemodialysis patients with secondary hyperparathyroidism in Japan: Baseline data from the MBD-5D. Am J Nephrol 33: 427–437, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 4: 643–646, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spiegelman D, Hertzmark E: Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162: 199–200, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Nakai S, Masakane I, Shigematsu T, Hamano T, Yamagata K, Watanabe Y, Itami N, Ogata S, Kimata N, Shinoda T, Syouji T, Suzuki K, Taniguchi M, Tsuchida K, Nakamoto H, Nishi S, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Fujii N, Marubayashi S, Morita O, Wakai K, Wada A, Iseki K, Tsubakihara Y: An overview of dialysis treatment in Japan (as of Dec. 31, 2007). Ther Apher Dial 13: 457–504, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Rothman KJ, Greenland S, Lash TL: Validity in Epidemiologic Studies In: Modern Epidemiology, 3rd ed., edited by Rothman KJ, Greenland S, Lash TL: Philadelphia, Lippincott Williams & Wilkins, 2008, pp 146–147 [Google Scholar]
- 28. Fukagawa M, Yumita S, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, Koshikawa S: Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant 23: 328–335, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Komaba H, Fukagawa M: KRN1493 Study Group: Impact of cinacalcet hydrochloride on the achievement of the Japanese Society for Dialysis Therapy (JSDT) guideline targets: A post-hoc analysis of the KRN1493 study. Ther Apher Dial [Suppl 1]: S44–S49, 2008 [DOI] [PubMed] [Google Scholar]
- 30. St Peter WL, Li Q, Liu J, Persky M, Nieman K, Arko C, Block GA: Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 4: 354–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Danese MD, Belozeroff V, Smirnakis K, Rothman KJ: Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 3: 1423–1429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT: Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group: The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: Association with mortality in dialysis patients. Am J Kidney Dis 46: 925–932, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.