Abstract
Summary
Background and objectives
Mounting evidence suggests that 1,25-dihydroxyvitamin D prevents the progression of chronic kidney disease (CKD). It is not clear whether “nutritional” forms of vitamin D affect GFR.
Design, setting, participants, & measurements
We tested whether serum 25-hydroxyvitamin D concentration (25(OH)D), a measure of total vitamin D intake from cutaneous synthesis and dietary consumption, is associated with loss of estimated GFR among 1705 older adults with predominantly normal baseline kidney function participating in the Cardiovascular Health Study. Baseline 25(OH)D was measured by HPLC-tandem mass spectrometry. GFR was estimated at baseline and 4 years later using the CKD-EPI formula, with rapid GFR loss defined as 12 ml/min per 1.73 m2 or more over 4 years.
Results
Rapid GFR loss was observed for 207 participants (12%). Each 10 ng/ml lower 25(OH)D was associated with a 25% greater risk of rapid GFR loss (95% confidence interval [CI] 5%, 49%, P = 0.01), adjusting for potential confounding characteristics. Compared with 25(OH)D ≥30 ng/ml, 25(OH)D concentrations 15 to 29 ng/ml and <15 ng/ml were associated with 29% (95% CI −13%, 91%) and 68% (95% CI 1%, 177%) greater adjusted risks of rapid GFR loss, respectively. Magnitudes of association were largest among participants with diabetes. Results were similar evaluating a composite outcome of rapid GFR loss, end stage renal disease, and death.
Conclusions
Insufficient 25(OH)D may be a modifiable risk factor for early GFR loss. We recommend clinical trials to determine whether vitamin D supplementation prevents the development and progression of CKD.
Introduction
Impaired GFR is a cardinal manifestation of kidney disease and a strong risk factor for adverse health outcomes (1). Lower GFR, even within its “normal” range, is associated with markedly increased risks of cardiovascular disease (CVD) and death (2–4). Moreover, members of community-based populations who lose GFR more rapidly over time, i.e., more than 3 ml/min per 1.73 m2 per year, are at particularly high risks of adverse CVD events (5–7). A robust literature suggests that GFR loss may be causally related to the development of CVD by promoting oxidative stress, anemia, disturbed mineral metabolism, and other unique metabolic abnormalities (8). Thus, GFR loss may be a therapeutic target for the prevention of CVD and mortality in the general population.
Vitamin D receptor agonists, i.e. 1,25-dihydroxyvitamin D (calcitriol, the active vitamin D hormone) and its analogues, prevent kidney damage and GFR loss in animal-experimental models (9). These agents potently suppress renin secretion and downstream activation of the renin-angiotensin-aldosterone system, which plays a key role in the pathogenesis of chronic kidney disease (CKD) by increasing blood pressure and stimulating fibrosis within the kidney (10,11). Vitamin D receptor agonists also reduce the expression of inflammatory mediators by monocytes and T-cells, promote survival of podocytes by inducing differentiation and preventing apoptosis, and reduce albuminuria and glomerulosclerosis in animal models (12–20). Despite these promising data, however, the role of vitamin D receptor agonists in the prevention and treatment of human kidney disease remains unclear. Moreover, 1,25-dihydroxyvitamin D and its analogues are not appropriate for prevention of GFR loss in large populations with relatively preserved kidney function because they are expensive and carry known potential adverse events, such as hypercalcemia.
“Nutritional” forms of vitamin D, i.e., cholecalciferol and ergoclaciferol, could offer a relatively cost-efficient and low-risk opportunity to prevent GFR loss. Vitamin D deficiency is common, and vitamin D supplements are widely available, inexpensive, and relatively nontoxic. As a result, cholecalciferol or ergocalciferol may be suitable for CKD prevention on a large scale (21). Observational studies of circulating 25-hydroxyvitamin D (25(OH)D) concentration are a useful method of evaluating the potential benefits of nutritional vitamin D interventions. Serum 25(OH)D is widely accepted as a marker of total vitamin D intake from both cutaneous synthesis and dietary consumption, because it rises proportionally to ultraviolet light exposure and dietary supplementation (22).
It is not known whether low 25(OH)D is a risk factor for the development and progression of early CKD stages, which are highly prevalent and are associated with increased risks of CVD and death (2,3,23). Therefore, we tested whether serum 25(OH)D is associated with change in estimated GFR in the Cardiovascular Health Study, a community-based cohort of ambulatory older adults.
Materials and Methods
Study Population
The Cardiovascular Health Study (CHS) is a prospective, community-based cohort designed to study risk factors for the development and progression of CVD in people aged 65 years and older (24). Participants were recruited from four U.S. communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Eligible participants were sampled using Medicare eligibility lists, were not institutionalized, and were expected to remain in the area for at least 3 years. Persons who were wheelchair-bound in the home or were receiving hospice treatment, radiation therapy, or chemotherapy for cancer were excluded. The original CHS cohort of 5201 participants was enrolled in 1989 to 1990, with an additional 687 predominantly African-American participants enrolled in 1992 to 1993. We measured serum 25(OH)D concentration at the 1992 to 1993 study visit for CHS participants who had no clinical evidence of CVD at that time (n = 2323). Of these, 1705 participants underwent repeat measurement of serum creatinine at the 1996 to 1997 study visit and were included in primary analyses of the current study.
25-Hydroxyvitamin D
Fasting serum was collected from CHS participants at the 1992 to 1993 study visit and stored at −70°C. We thawed stored serum to measure total 25(OH)D (including 25-hydroxyvitamins D2 and D3) using HPLC-tandem mass spectrometry on a Waters Quattro Micro mass spectrometer. Interassay coefficient of variation was <3.4%. 25(OH)D, assessed using a different assay, was demonstrated to be stable for long periods of time at −70°C (25). Serum 1,25-dihydroxyvitamin D was not measured in this study.
Study Outcomes
The primary study outcome was change in GFR estimated from serum creatinine using the CKD-EPI equation (26). Serum creatinine was measured at the 1992 to 1993 and 1996 to 1997 study visits using the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, New York, colorimetric method) and calibrated to Cleveland Clinic values. Mean coefficient of variation was 1.94% (range 1.16% to 3.60%) (27). We defined rapid GFR loss as change in estimated GFR (eGFR) < −12 ml/min per 1.73 m2 over the 4 years of follow-up because this magnitude of change is three times the expected rate previously described in aging studies and because this definition was associated with increased risks of CVD and death in previous CHS studies (5,6,28). We also assessed change in GFR estimated from serum cystatin C (29).
To assess whether results for change in eGFR were impacted by the competing risk of death, we evaluated a combined secondary end point. This dichotomous end point was defined as rapid GFR loss, development of ESRD before the scheduled 1996 to 1997 study visit, or death before the scheduled 1996 to 1997 study visit. For this combined end point only, we included all 2323 participants for whom 25(OH)D was measured in 1992 to 1993. ESRD cases were ascertained through linkage with U.S. Renal Data Systems files. Mortality was ascertained by examination of death certificates, inpatient records, nursing home or hospice records, physician questionnaires, and autopsy reports (3).
Covariates
Covariates were ascertained at the 1992 to 1993 CHS study visit. Age, gender, race (Caucasian or African American), attained education, smoking status, and overall health status were defined by self-report. Medication inventories were completed by CHS staff using participants' prescription and nonprescription medication bottles (24). Leisure time physical activity, a measure of activity specifically associated with change in eGFR in this population, was quantified in kilocalories per week using validated questionnaires assessing a broad range of common activities (30). Diabetes was defined as use of insulin or oral hypoglycemic agents and/or fasting blood glucose ≥126 mg/dl (31). Hypertension was defined as use of antihypertensive medications, systolic BP ≥140 mmHg, or diastolic BP ≥90 mmHg (32). Body mass index (BMI) was calculated as weight (kg) divided by height (m2). C-reactive protein was measured with an ELISA developed at the CHS central laboratory. Intact serum parathyroid hormone (PTH) was quantified using a second generation two-site immunoassay on a Beckman Unicell DxI clinical analyzer.
Data Analysis
We evaluated serum 25(OH)D concentration primarily as a continuous variable, adjusting for CHS site and season, to account for seasonal variation in 25(OH)D to the best of our ability (33). 25(OH)D was scaled to 10 ng/ml, because this increment is readily attained using a moderate dose of cholecalciferol supplement (34). In addition, we categorized 25(OH)D using common thresholds: deficient (<15 ng/ml), insufficient (15 to 30 ng/ml), and sufficient (≥30 ng/ml). These thresholds are based on reports that concentrations less than 15 ng/ml are associated with increased risk of incident CVD (35–37) and that 25(OH)D and PTH concentrations are inversely correlated below a 25(OH)D threshold near 30 ng/ml (38).
We calculated change in eGFR from 1992 to 1993 to 1996 to 1997 for each participant as the difference between these two measures. We constructed a cubic spline model to describe the continuous association of 25(OH)D with change in eGFR and used linear regression to quantify this association and to test statistical significance. We used logistic regression to test associations of 25-OHD concentration with binary study outcomes. We created serial multivariable regression models: (1) adjusted for age, gender, race, CHS site, and season of measurement; (2) additionally adjusted for covariates which may plausibly confound associations of 25(OH)D with GFR (income, education, smoking, diabetes, physical activity, use of antihypertensive medications in general, use of angiotensin-converting enzyme inhibitors specifically, and BMI); and (3) additionally adjusted for potential confounders and/or mediators of this association (systolic BP, diastolic BP, and C-reactive protein).
We assessed for heterogeneity of the 25(OH)D change in eGFR association by incorporating interaction terms for covariate (categorical variable) with 25(OH)D (continuous variable), testing statistical significance using the likelihood ratio test. When appropriate (i.e., for diabetes), stratified analyses were also performed using the covariate-25(OH)D interaction term. All analyses were performed using S-Plus (release 8.0, Insightful Inc., Seattle, Washington) and SPSS statistical software (release 16.0.1, SPSS Inc., Chicago, Illinois).
Results
Participant Characteristics
Of the 2323 participants with 25(OH)D measured at the 1992 to 1993 study visit, 1705 underwent repeat eGFR measurement at the 1996 to 1997 study visit (73%). These participants tended to be younger (mean age 74 versus 76 years), were more likely to be women (70% versus 68%), were less likely to be African American (13% versus 17%) or to have eGFR that was <60 ml/min per 1.73 m2 (14% versus 27%), and tended to have slightly higher 25(OH)D concentration (mean 26 versus 25 ng/ml), compared with participants without repeat eGFR measurement. Lower 25(OH)D was associated with female gender, African-American race, lower attained education, winter season of measurement, higher BMI, smoking, lower physical activity, diabetes, hypertension, and more frequent use of antihypertensive medications (Table 1). 25(OH)D correlated weakly with baseline GFR estimated from creatinine (r = −0.08) or cystatin C (r = −0.05).
Table 1.
Characteristics of 1705 participants in the Cardiovascular Health Study (1992 to 1993 study visit)
| 25-hydroxyvitamin D (ng/ml) |
P (trend) | |||
|---|---|---|---|---|
| ≥30 | 15 to 29 | <15 | ||
| N | 526 | 911 | 268 | |
| Demographics | ||||
| Age (years) | 73 (4) | 74 (5) | 74 (5) | 0.03 |
| Gender (male) | 219 (42%) | 237 (26%) | 53 (20%) | <0.01 |
| Race (African American) | 23 (4%) | 104 (11%) | 101 (38%) | <0.01 |
| Education | ||||
| none to grade 9 | 50 (10%) | 148 (16%) | 51 (19%) | 0.43 |
| high school | 190 (36%) | 320 (35%) | 109 (41%) | |
| professional/vocational | 284 (54%) | 441 (48%) | 107 (40%) | |
| Household income | 0.40 | |||
| <$8000 | 36 (7%) | 94 (10%) | 54 (21%) | |
| $8000 to $34,999 | 303 (58%) | 518 (57%) | 139 (53%) | |
| ≥$35,000 | 150 (29%) | 241 (27%) | 55 (21%) | |
| Season of measurement | <0.01 | |||
| January to March | 91 (17%) | 213 (23%) | 126 (47%) | |
| April to June | 78 (15%) | 228 (25%) | 74 (28%) | |
| July to September | 222 (42%) | 244 (27%) | 34 (13%) | |
| October to December | 135 (26%) | 226 (25%) | 34 (13%) | |
| Comorbidity and lifestyle | ||||
| diabetes | 39 (7%) | 87 (10%) | 56 (21%) | <0.01 |
| hypertension | 256 (49%) | 462 (51%) | 170 (63%) | <0.01 |
| current smoking | 45 (9%) | 69 (8%) | 35 (14%) | 0.07 |
| physical activity (kcal/wk)a | 1509 [625, 2733] | 960 [398, 1988] | 536 [171, 1140] | <0.01 |
| antihypertensive medications | 185 (35%) | 338 (37%) | 131 (49%) | 0.01 |
| ACE inhibitors | 37 (7%) | 74 (8%) | 25 (9%) | 0.24 |
| beta blockers | 55 (10%) | 73 (8%) | 21 (8%) | 0.14 |
| calcium channel blockers | 52 (10%) | 76 (8%) | 43 (16%) | 0.04 |
| diuretics | 89 (17%) | 186 (20%) | 83 (31%) | <0.01 |
| Physical examination | ||||
| body mass index (kg/m2) | 25.7 (3.8) | 27.2 (4.7) | 27.7 (5.2) | <0.01 |
| systolic BP (mmHg) | 134 (19) | 135 (21) | 138 (20) | 0.01 |
| diastolic BP (mmHg) | 72 (10) | 71 (11) | 72 (12) | 0.87 |
| Laboratory | ||||
| estimated GFR (ml/min per 1.73 m2) | 73 (14) | 75 (15) | 76 (17) | 0.01 |
| calcium (mg/dl) | 9.5 (0.3) | 9.5 (0.4) | 9.5 (0.4) | 0.90 |
| phosphorous (mg/dl) | 3.6 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 0.32 |
| parathyroid hormone (pg/ml) | 48 (21) | 56 (25) | 71 (40) | <0.01 |
| C-reactive protein (mg/L)a | 2.06 [0.97, 4.71] | 2.30 [1.11, 5.02] | 2.95 [1.30, 5.97] | 0.02 |
| albumin (g/dl) | 3.93 (0.26) | 3.91 (0.27) | 3.91 (0.28) | 0.24 |
Cell contents are mean (SD) or n (%), except when indicated as amedian with interquartlie range in square brackets. ACE, angiotensin-converting enzyme.
Change in GFR
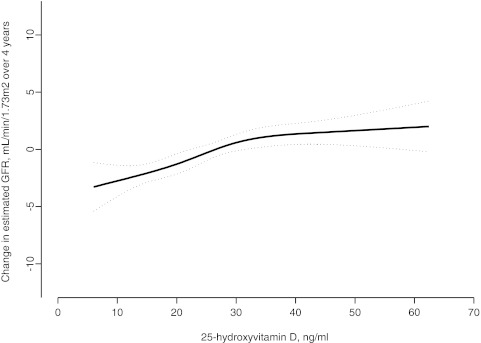
Over 4 years of follow-up, mean (SD) loss of eGFR was 0.4 (10.7) ml/min per 1.73 m2, and 207 participants (12%) lost 12 ml/min per 1.73 m2 or more. Lower 25(OH)D concentrations were monotonically associated with more rapid eGFR loss, particularly when 25(OH)D was less than approximately 30 ng/ml (Figure 1). In fully adjusted multivariable models, each 10 ng/ml lower 25(OH)D was associated with a 25% greater risk of rapid GFR loss (95% confidence interval [CI] 5%, 45%, P = 0.01). Compared with 25(OH)D ≥30 ng/ml, 25(OH)D concentration <15 ng/ml was associated with a 68% greater adjusted risk of rapid GFR loss (Table 2 and Figure 2). In comparison, in the same model, diabetes and hypertension were associated with 31% (95% CI −16%, +106%) and 21% (95% CI −23%, +89%) greater risks of rapid GFR loss, respectively. In parallel models, lower 25(OH)D was associated with excess GFR loss, evaluated as a continuous outcome (Table 2). Similar results were obtained when GFR was estimated from serum cystatin C, particularly focusing on 25(OH)D as a continuous variable (Supplementary Table) (39).
Figure 1.
Unadjusted association of serum 25-hydroxyvitamin D with change in estimated GFR over 4 years of follow-up among 1705 participants in the Cardiovascular Health Study, assessed using a restricted cubic spline. Dotted lines represent 95% confidence interval.
Table 2.
Associations of serum 25-hydroxyvitamin D concentration with change in estimated GFR among 1705 participants in the Cardiovascular Health Study
| Rapid GFR Loss (dichotomous outcome) |
Change in Estimated GFR (continuous outcome, ml/min per 1.73 m2/4 years) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Participant Group |
N (%) | Adjusted Odds Ratio (95% CI) |
Unadjusted Change, Mean (SD) | Adjusted Difference (95% CI) |
||||
| 25(OH)D Concentration | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | ||
| All participants | 207 (12%) | −0.4 (10.7) | ||||||
| ≥30 ng/ml | 43 (8%) | 1 (ref) | 1 (ref) | 1 (ref) | 1.2 (9.8) | 0 (ref) | 0 (ref) | 0 (ref) |
| 15–29 ng/mL | 111 (12%) | 1.33 (0.91, 1.95) | 1.28 (0.87, 1.89) | 1.29 (0.87, 1.91) | −0.7 (10.6) | −1.4 (−2.6, −0.2) | −1.2 (−2.1, −0.1) | −1.2 (−2.4, −0.1) |
| <15 ng/ml | 53 (20%) | 1.82 (1.12, 2.96) | 1.66 (1.01, 2.74) | 1.68 (1.01, 2.77) | −2.7 (12.4) | −2.1 (−3.9, −0.4) | −1.7 (−3.5, 0.02) | −1.7 (−3.4, 0.1) |
| per 10 ng/ml* | 1.30 (1.09, 1.54) | 1.26 (1.02, 1.50) | 1.25 (1.05, 1.49) | −0.7 (−1.2, −0.2) | −0.6 (−1.1, −0.1) | −0.6 (−1.0, −0.1) | ||
| P* | 0.03 | 0.01 | 0.01 | 0.05 | 0.02 | 0.02 | ||
| Participants without diabetes | 172 (11%) | −0.2 (10.3) | ||||||
| per 10 ng/ml* | 1.22 (1.02, 1.46) | 1.21 (1.00, 1.45) | 1.20 (1.00, 1.44) | −0.6 (−1.0, −0.1) | −0.5 (−1.0, −0.1) | −0.5 (−1.0, −0.03) | ||
| P* | 0.03 | 0.05 | 0.05 | 0.02 | 0.03 | 0.04 | ||
| Participants with diabetes | 35 (19%) | −2.2 (13.9) | ||||||
| per 10 ng/mL* | 2.11 (1.17, 3.82) | 2.04 (1.04, 4.00) | 2.19 (1.10, 4.37) | −2.1 (−4.2, 0.1) | −1.9 (−4.0, 0.2) | −2.0 (−4.1, 0.1) | ||
| P* | 0.01 | 0.04 | 0.03 | 0.06 | 0.07 | 0.06 | ||
Rapid GFR loss is defined as change in estimated GFR < −12 mL/min/1.73 m2 over four years, with numbers greater than one representing increased risk of rapid GFR loss. Difference in change in estimated GFR is reported in mL/min/1.73 m2 over the four years of follow-up, with numbers less than zero representing excess GFR loss compared with the reference group.
Evaluates 25(OH)D as a continuous variable, per 10 ng/mL lower 25(OH)D concentration.
Model 1 adjusted for age, gender, race, site, and season.
Model 2 additionally adjusted for attained education, household income, diabetes, smoking, physical activity, antihypertensive medication use, angiotensin-converting enzyme inhibitor use, body mass index, and serum albumin.
Model 3 additionally adjusted for variables in Model 2, systolic and diastolic blood pressures, and C-reactive protein.
Abbreviations: GFR, glomerular filtration rate; 25(OH)D, 25-hydroxyvitamin D; ref, reference.
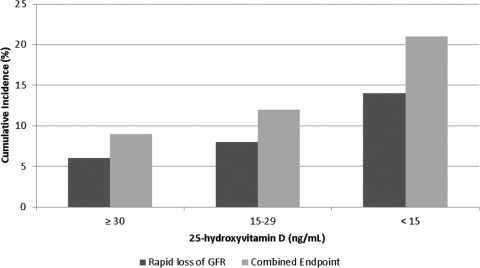
Figure 2.
Cumulative incidence of rapid estimated GFR (eGFR) loss (■, 346 cases, 1705 at risk) and of the combined end point of rapid GFR loss, incident ESRD, or death ( , 559 cases, 2323 participants at risk) in the Cardiovascular Health Study, by serum 25-hydroxyvitamin D concentration. Adjusted for age, gender, race, site, season, attained education, household income, diabetes, smoking, physical activity, antihypertensive medication use, angiotensin-converting enzyme inhibitor use, body mass index, systolic and diastolic BP, and C-reactive protein.
, 559 cases, 2323 participants at risk) in the Cardiovascular Health Study, by serum 25-hydroxyvitamin D concentration. Adjusted for age, gender, race, site, season, attained education, household income, diabetes, smoking, physical activity, antihypertensive medication use, angiotensin-converting enzyme inhibitor use, body mass index, systolic and diastolic BP, and C-reactive protein.
In a sensitivity analysis restricted to participants reporting good or better health (n = 1525), each 10 ng/ml lower 25(OH)D was associated with a 23% greater risk of rapid GFR loss (95% CI 2%, 49%, P = 0.03). In a second sensitivity analysis adjusting for baseline eGFR in addition to all covariates in model 3, each 10 ng/ml lower 25(OH)D was associated with a 23% greater risk of rapid GFR loss (95% CI 3%, 46%, P = 0.02). With the addition of serum calcium, phosphorus, and PTH concentrations to the fully adjusted model 3, each 10 ng/ml lower 25(OH)D was associated with a 24% greater risk of rapid GFR loss (95% CI 3%, 48%, P = 0.03).
Combined End Point
In addition to the 207 participants with rapid eGFR loss, the combined end point included four participants with incident ESRD and 210 participants who died before their anticipated 1996 to 1997 study visit. Each 10 ng/ml lower 25(OH)D was associated with a 23% greater risk of the combined endpoint, with full adjustment (95% CI 8%, 39%, P = 0.002), Table 3 and Figure 2. This risk estimate is similar to that for rapid eGFR loss alone (25%).
Table 3.
Associations of serum 25-hydroxyvitamin D concentration with rapid GFR loss, ESRD, or death (composite outcome) among 2323 participants in the Cardiovascular Health Study
| Participant Group | No. of Eventsb | Adjusted Odds Ratioa |
||
|---|---|---|---|---|
| Model 1c | Model 2d | Model 3e | ||
| 25(OH)D concentration | ||||
| all participants | 419 (18) | |||
| ≥30 ng/ml | 91 (13) | 1 (ref) | 1 (ref) | 1 (ref) |
| 15 to 29 ng/ml | 216 (17) | 1.26 (0.96, 1.66) | 1.23 (0.92, 1.63) | 1.23 (0.92, 1.63) |
| <15 ng/ml | 112 (29) | 2.06 (1.45, 2.93) | 1.88 (1.31, 2.71) | 1.85 (1.29, 2.67) |
| per 10 ng/mlf | 1.27 (1.12, 1.44) | 1.23 (1.08, 1.39) | 1.23 (1.08, 1.39) | |
| Pf | <0.001 | 0.002 | 0.002 | |
| Participants without diabetes | 343 (17) | |||
| per 10 ng/mlf | 1.21 (1.06, 1.38) | 1.19 (1.04, 1.36) | 1.18 (1.04, 1.35) | |
| Pf | 0.005 | 0.01 | 0.01 | |
| Participants with diabetes | 76 (29) | |||
| per 10 ng/mlf | 1.62 (1.11, 2.36) | 1.63 (1.08, 2.45) | 1.80 (1.17, 2.77) | |
| Pf | 0.01 | 0.02 | 0.008 | |
Rapid GFR loss is defined as change in estimated GFR < −12 ml/min per 1.73 m2 over 4 years. 25(OH)D, 25-hydroxyvitamin D; CI, confidence interval.
95% CI in parentheses.
Percentage in parenthese.
Model 1 was adjusted for age, gender, race, site, and season.
Model 2 was additionally adjusted for attained education, household income, diabetes, smoking, physical activity, antihypertensive medication use, ACE inhibitor use, body mass index, and serum albumin.
Model 3 was additionally adjusted for variables in model 2, systolic and diastolic blood pressures, and C-reactive protein.
Evaluates 25(OH)D as a continuous variable, per 10 ng/ml lower 25(OH)D concentration.
Subgroup Analyses
Magnitudes of association of 25(OH)D with change in eGFR were larger among participants who had diabetes at baseline (Tables 2 and 3). P-values for interaction by diabetes status were 0.005 evaluating change in eGFR as a continuous outcome and 0.2 evaluating rapid eGFR loss as a dichotomous outcome. Associations of 25(OH)D with change in eGFR and rapid GFR loss did not differ by gender, race, use of antihypertensive medications, BMI, or serum PTH concentration (each P > 0.1).
Discussion
Lower 25(OH)D concentrations were associated with increased risk of eGFR loss in a community-based population of older adults with predominantly normal baseline kidney function. Results were independent of known potential confounding characteristics and were similar for a combined end point of rapid eGFR loss, incident ESRD, or death. Remarkably, low 25(OH)D concentration was associated with eGFR loss with a magnitude similar to or stronger than that for diabetes or hypertension, the most important traditional risk factors for CKD. These findings suggest that low 25(OH)D concentration may be a modifiable risk factor for early GFR loss.
Although a mounting literature suggests that 1,25(OH)2D and its analogs have renoprotective effects (15–20,40–44), the clinical relevance of nutritional vitamin D to GFR loss remains an important, unanswered question. In cross-sectional analyses of the Third Health and Nutrition Examination Survey (NHANES III), lower serum concentrations of 25(OH)D were associated with increased prevalence of albuminuria, but not with eGFR (40,45). However, low eGFR may represent a mix of stable and declining kidney function in cross-sectional studies, and longitudinal analysis was required to reveal an association of 25(OH)D with eGFR in our work. More recently, lower 25(OH)D concentrations were associated with increased risk of progression from moderate-to-severe stages of CKD to ESRD, and in NHANES III, 25(OH)D concentrations <15 ng/ml were associated with increased risk of incident ESRD (46,47). These important studies support the hypothesis that insufficient 25(OH)D contributes to GFR loss in advanced stages of CKD. In comparison, our study suggests that 25(OH)D may play a role in early stages of GFR loss, even accounting for the competing risk of death. In addition, our results suggest that the association of lower 25(OH)D with GFR loss is independent of serum PTH, calcium, and phosphorus.
The observation that lower 25(OH)D concentrations are associated with early changes in eGFR may have substantial public health importance because mild-to-moderate CKD is the most common CKD stage and is known to be strongly associated with CVD and death (2,3,23). In this respect, demonstrating associations of lower 25(OH)D with eGFR loss in an older, community-based population with predominantly normal baseline kidney function but high risk of CKD is novel and relevant. Vitamin D supplements are relatively safe and inexpensive, such that oral supplementation with cholecalciferol or ergocalciferol may be feasible in large populations. Before widespread implementation, however, effects of vitamin D supplements on manifestations of CKD require testing in well controlled clinical trials. The potential utility of vitamin D supplementation could also be further evaluated in additional observational studies and proof-of-concept clinical trials evaluating intermediate outcomes.
In our study, the association of lower 25(OH)D concentration with eGFR loss was strongest among participants with diabetes. These results should be viewed as hypothesis generating because, to our knowledge, this is the first study to report such an interaction, and because vitamin D has been observed to have renoprotective effects in both diabetic and nondiabetic animal models (15–19). Nonetheless, it is possible that lower 25(OH)D concentrations are particularly detrimental in the setting of renin-angiotensin- aldosterone system activation and hyperfiltration, which characterize diabetic kidney disease. Indeed, in animal models, vitamin D potently suppresses the Renin-angiotensin- aldosterone system, particularly in high-renin states such as treatment with angiotensin receptor blockers (10,16). Combined with knowledge that persons with diabetes are at high risk of kidney disease and with clinical trial results indicating that paricalcitol lowers albuminuria in diabetic kidney disease (44), our results suggest that future vitamin D intervention studies might target persons with diabetes.
Strengths of this study include its large, community-based population; longitudinal measurements of GFR estimated using both creatinine and cystatin C; and reduction of potential confounding by exclusion of participants with clinical CVD and adjustment for well defined potential confounding variables. This study also has limitations. First, we do not have direct measurements of GFR. Second, our ability to ascertain the full impact of 25(OH)D concentration on change in eGFR may have been limited by the use of only one follow-up measurement of eGFR and by a follow-up duration of only 4 years. Third, as with all studies of change in GFR, our study is subject to potential survival bias. However, our results for eGFR loss were consistent with those of a secondary combined end point which additionally included ESRD and death. Fourth, because urine albumin excretion was not measured at baseline, we cannot evaluate whether associations of 25(OH)D with change in eGFR are explained by albuminuria. For example, albuminuria could confound the association of low 25(OH)D concentration with GFR loss by causing both urinary losses of vitamin D–binding protein (48) and progressive kidney disease, particularly with diabetes; albuminuria could mediate effects of vitamin D on GFR; or decreased delivery of 25(OH)D to proximal tubular cells could mediate, in part, known associations of albuminuria with GFR loss. Most importantly, our study is observational in nature and subject to residual confounding, given that low 25(OH)D concentration is correlated with a number of metabolic abnormalities. In particular, fibroblast growth factor-23, which was not measured, may both lower serum 25(OH)D concentration by increasing its renal metabolism to 24,25-dihydroxyvitamin D and promote GFR loss (49,50). Therefore, observed associations do not necessarily represent causal relationships.
In conclusion, lower serum 25(OH)D concentrations were associated with increased risk of eGFR loss among community-dwelling older adults. We recommend clinical trials to determine whether vitamin D supplementation prevents the development and progression of CKD.
Disclosures.
None.
Acknowledgments
The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and Grant U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurologic Disorders and Stroke. Additional support came from National Institutes of Health Grants R01HL084443, R01AG027002, KL2RR025015, R01HL096875, and R01DK087726. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Results of this study were presented in part as an abstract at the American Society of Nephrology Renal Week in 2010 in Denver, Colorado.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266 2002 [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Boer IH, Katz R, Cao JJ, Fried LF, Kestenbaum B, Mukamal K, Rifkin DE, Sarnak MJ, Shlipak MG, Siscovick DS: Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care 32: 1833–1838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, Balasubramanian S, Nurutdinova D, Xian H, Stroupe K, Abbott KC, Eisen S: Rate of kidney function decline associates with mortality. J Am Soc Nephrol 21: 1961–1969, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarnak MJ: Cardiovascular complications in chronic kidney disease. Am J Kidney Dis 41: 11–17, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Li YC: Renoprotective effects of vitamin D analogs. Kidney Int 78: 134–139, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Durvasula RV, Shankland SJ: The renin-angiotensin system in glomerular podocytes: Mediator of glomerulosclerosis and link to hypertensive nephropathy. Curr Hypertens Rep 8: 132–138, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Giovannini L, Panichi V, Migliori M, De Pietro S, Bertelli AA, Fulgenzi A, Filippi C, Sarnico I, Taccola D, Palla R, Bertelli A: 1,25-Dihydroxyvitamin D(3) dose-dependently inhibits LPS-induced cytokines production in PBMC modulating intracellular calcium. Transplant Proc 33: 2366–2368, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Reichel H, Koeffler HP, Tobler A, Norman AW: 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A 84: 3385–3389, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panichi V, De Pietro S, Andreini B, Bianchi AM, Migliori M, Taccola D, Giovannini L, Tetta C, Palla R: Calcitriol modulates in vivo and in vitro cytokine production: A role for intracellular calcium. Kidney Int 54: 1463–1469, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K: 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286: F526–F533, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC: Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci U S A 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Makibayashi K, Tatematsu M, Hirata M, Fukushima N, Kusano K, Ohashi S, Abe H, Kuze K, Fukatsu A, Kita T, Doi T: A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol 158: 1733–1741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirata M, Makibayashi K, Katsumata K, Kusano K, Watanabe T, Fukushima N, Doi T: 22-Oxacalcitriol prevents progressive glomerulosclerosis without adversely affecting calcium and phosphorus metabolism in subtotally nephrectomized rats. Nephrol Dial Transplant 17: 2132–2137, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, Kong J, Shi H, Chang A, Li YC: Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int 77: 1000–1009, 2010 [DOI] [PubMed] [Google Scholar]
- 21. de Boer IH, Kestenbaum B: Vitamin D in chronic kidney disease: Is the jury in? Kidney Int 74: 985–987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Institute of Medicine of the National Academies, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium: Dietary Reference Intakes for Calcium and Vitamin D, Washington, D.C., The National Academies Press, 2011 [PubMed] [Google Scholar]
- 23. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. : The Cardiovascular Health Study: Design and rationale. Ann Epidemiol 1: 263–276, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Agborsangaya C, Toriola AT, Grankvist K, Surcel HM, Holl K, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M: The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr Cancer 62: 51–57 [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP: Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 41: 264–270, 1995 [PubMed] [Google Scholar]
- 28. Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B: Physical activity and rapid decline in kidney function among older adults. Arch Intern Med 169: 2116–2123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Standards of medical care in diabetes—2007. Diabetes Care 30[Suppl 1]: S4–S41, 2007 [DOI] [PubMed] [Google Scholar]
- 32. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, Bethesda, MD, National Institutes of Health, 2004 [Google Scholar]
- 33. Wang Y, Jacobs EJ, McCullough ML, Rodriguez C, Thun MJ, Calle EE, Flanders WD: Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: Insights from simulations based on serum 25-hydroxyvitamin D. Am J Epidemiol 170: 88–94, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ: Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77: 204–210, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS: Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503–511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giovannucci E, Liu Y, Hollis BW, Rimm EB: 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med 168: 1174–1180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS: 25-Hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol 20: 1805–1812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ: Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7: 439–443, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: A0828, 2000 [Google Scholar]
- 40. de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS: 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 50: 69–77, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, Raymond NT, Howie AJ, Cockwell P, Stewart PM, Hewison M: Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int 74: 1343–1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R: Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension 52: 249–255, 2008 [DOI] [PubMed] [Google Scholar]
- 44. de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Chonchol M, Scragg R: 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney Int 71: 134–139, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C: Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 75: 88–95, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P: 25-Hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol 20: 2631–2639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL: Enhanced excretion of vitamin D binding protein in type 1 diabetes: A role in vitamin D deficiency? J Clin Endocrinol Metab 96: 142–149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P: Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]