Abstract
Summary
Background and objectives
Hyperphosphatemia and subclinical endotoxemia are important sources of inflammation in HD. Proinflammatory cytokines are strong correlates of soluble CD14 (sCD14) concentrations, an independent predictor of mortality in this population. We evaluated the effects of calcium acetate and sevelamer hydrochloride on serum inflammatory profile, endotoxin concentrations, and sCD14 levels in HD patients.
Design, setting, participants, & measurements
Prospective, randomized, open-label, parallel design trial. Fifty-nine stable HD patients, 30 receiving sevelamer, and 29 receiving calcium acetate were evaluated. Serum levels of inflammatory parameters (high-sensitivity C-reactive protein [hs-CRP], TNF-α, interleukin (IL)-1, -6, -10, and -18), as well as endotoxin and sCD14 concentrations, were measured at baseline and after 3 months of therapy.
Results
Serum IL-6 increased in patients receiving calcium acetate, whereas hs-CRP and IL-6 significantly decreased in subjects treated with sevelamer, with IL-10 experiencing a trend to increase (P = 0.052). Serum endotoxin and sCD14 levels did not change after treatment with calcium acetate. However, these parameters decreased by 22.6% and 15.2%, respectively (P < 0.01), in patients receiving sevelamer. Multiple regression analysis showed that variation in serum endotoxin concentrations was the strongest factor associated with IL-6 change, whereas the only variables independently associated with changes in sCD14 levels were the variations in serum IL-6 and endotoxin concentrations.
Conclusions
Administration of the noncalcium phosphate binder sevelamer to maintenance HD patients is associated with a significant decrease in hs-CRP, IL-6, serum endotoxin levels and sCD14 concentrations.
Introduction
Inflammation, a prevalent condition in hemodialysis (HD) patients, plays a pivotal role in diverse complications (1), and it is a strong predictor of mortality (2–4). One relevant source of inflammation is subclinical endotoxemia, due to exposure to soluble bacterial products such as lipopolysaccharide fragments (LPS, endotoxins) (5). In blood, endotoxins are transferred to CD14, a key molecule acting as a pattern-recognition receptor with a critical role in proinflammatory signaling (6,7). Two forms of CD14 are present: membrane bound (mCD14; constitutively expressed on the surface of monocytes, macrophages, and neutrophils) and soluble (sCD14), derived from both secretion and enzymatic cleavage of mCD14 (6–8). Increased CD14 expression and sCD14 serum concentrations have been reported in HD patients (9). Recent studies have shown that proinflammatory cytokines are the strongest correlates of sCD14, this parameter being an independent predictor of mortality (10,11).
Hyperphosphatemia is a relevant mineral metabolism abnormality in chronic kidney disease (CKD) associated with increased morbidity and mortality (12,13). In addition, serum phosphorus is direct and independently correlated with inflammatory parameters, indicating that hyperphosphatemia may be a contributing factor to inflammation (14). Therefore, adequate management of hyperphosphatemia is a critical issue in renal patients.
In addition to its phosphate-binding effect, sevelamer hydrochloride, a nonabsorbable noncalcium-based hydrogel, has been shown to exert a raft of beneficial pleiotropic actions, including anti-inflammatory effects (15). sevelamer may bind endotoxins and sequester bile acid-LPS complexes in the intestinal tract (16). Clinically, in a cross-sectional study, plasma endotoxin levels were significantly lower among patients prescribed sevelamer compared with those receiving either calcium-based binders or no binders (17). More recently, in a prospective, noncontrolled trial, sevelamer treatment leads to a decrease in high-sensitivity C-reactive protein (hsCRP), which was accompanied by a parallel decrease in endotoxemia (18).
The aim of the present investigation was to evaluate, in a prospective, controlled, randomized study, the differential effects of a classical calcium-containing phosphate binder (calcium acetate) and a calcium-free phosphate binder (sevelamer hydrochloride) on serum profile of inflammatory cytokines, LPS concentration, and sCD14 levels in HD patients.
Materials and Methods
Study Design
This was a single-center, prospective, randomized, open-label, parallel design trial, registered at the European clinical trial database (EudraCT 2005–004052-12). This research was carried out in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines and applicable regulations. The protocol was approved by the institutional ethics committee, and patients provided written informed consent.
The primary end point was to analyze the differential influence of calcium-containing phosphate binders (calcium acetate) versus calcium-free phosphate binders (sevelamer) on the serum IL-6 concentrations after 3 months of therapy. Secondary end points were changes in serum inflammatory profile (hs-CRP and the inflammatory cytokines TNF-α, IL-1, IL-10, and IL-18), serum LPS and sCD14 concentrations, and the potential relationship between these parameters. After a washout/run-in phase of 2 to 3 weeks (all phosphate binders were discontinued), patients were randomized to receive calcium acetate (Royen®, 500 mg three times a day) or sevelamer hydrochloride (Renagel®, 1600 mg three times a day) for 12 weeks. Randomization was performed by a computer-generated series of random numbers. All laboratory parameters were determined blinded to treatment allocation.
Patient Population
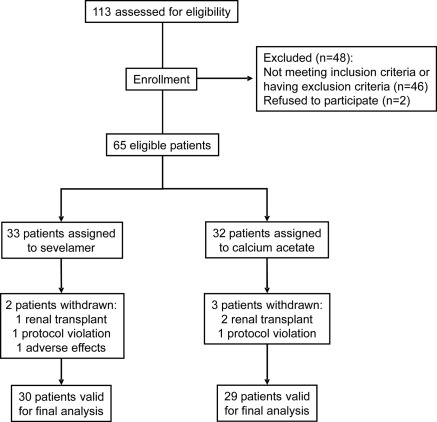
Stable adult patients with CKD stage 5-D, on maintenance HD (4 h/session, 3 sessions/wk, no dialysis filter reuse) for at least 3 months were enrolled in the study. Eligibility criteria were as follow: patients of either sex with age >18 years old, requiring therapy with a phosphate binder, and not receiving vitamin D or calcimimetics. Exclusion criteria included severe gastrointestinal disease; current smoking habit; alcohol dependence or drug abuse; history of immunologic or tumoral disease; an acute inflammatory or infectious episode in the previous month; hepatitis B, C, or HIV positivity; prior transplantation; and patients on immunotherapy or immunosuppressive treatment. A total of 113 patients were initially screened. Fifty-four were excluded and 65 were finally eligible and randomized to the two treatment regimens. Six patients were withdrawn: three received a renal transplant, two discontinued intervention, and one patient withdrew consent for adverse events. Fifty-nine patients completed the study (30 received sevelamer and 29 calcium acetate; Figure 1).
Figure 1.
Flow chart of patients included in the study.
Study Parameters
Blood samples were drawn after an 8-hour fasting period, before the midweek HD session. Serum was obtained and frozen at −80°C for biochemical analysis and for measurements of inflammatory parameters, endotoxins, and sCD14. Routine biochemical parameters were measured using standard methods. Serum hs-CRP was measured by a high sensitivity particle-enhanced immunoturbidimetric fully automated assay (Roche Diagnostics GmbH, Mannheim, Germany) in a Cobas 6000 analyzer from the same manufacturer (functional sensitivity was 0.3 mg/L and the intra- and interassay precision was 1.6 and 8.4, respectively). Levels of TNF-α, IL-1, IL-6, IL-10, and IL-18 were measured by a high-sensitive immunoenzymatic ELISA method (Quantikine Human, R&D Systems, Minneapolis, MN) in a DSXTM 4 Plate ELISA Processor (Vitro SA, Spain). Minimum detectable concentrations were 0.10 pg/ml, 0.05 pg/ml, 0.70 pg/ml, 0.50 pg/ml, and 12.5 pg/ml, respectively. Intra- and interassay coefficients of variability were <10.8%. Serum endotoxin concentration was quantified by chromogenic limulus amebocyte lysate test (QCL-1000; Cambrex Bioscience Inc., Walkersville, MD). The lower limit of detection was 0.01 endotoxin unit (EU)/ml, and the inter- and intra-assay variation coefficients were <10%. Samples used for endotoxin determinations were stored in LPS-free tubes, and all materials used for the assay were rendered LPS-free. sCD14 were determined by ELISA (Quantikine ELISA Kit; R&D Systems, Minneapolis, MN); the minimum detectable level was 125 pg/ml, and the inter- and intra-assay coefficients of variations <7.5% and 6.5%, respectively.
Statistical Analysis
Sample size calculation was based on IL-6, due to the superiority of this cytokine as a predictor of all-cause and cardiovascular mortality in dialysis patients (19,20). The assumed SD for IL-6 for the power calculation was 2.6 pg/ml (21). The sample size calculation to detect a 25% relative difference in the serum IL-6 concentration at the end of the study between both groups for an α value of 0.05 and a β value of 0.90 showed a need for a minimum of 26 individuals in each group.
Data are presented as mean ± SD, except for hs-CRP, cytokines, serum endotoxin, and sCD14, which are presented as median and interquartile range. Variables were tested for normality using the Kolmogorov–Smirnov test. Since serum endotoxin, sCD14, and inflammatory parameters were not normally distributed, these variables were logarithmically transformed. The primary analysis of the study was to compare the change in serum concentrations of hs-CRP, TNF-α, and interleukins 1, 6, 10, and 18. A general linear mixed-effect model was used. Subject number was entered as a random factor, treatment group was entered as a fixed factor, and age and dialysis vintage were entered as covariables. The paired and unpaired t tests were used to compare intra- and intergroup differences, as appropriate. The chi-squared test was used for differences in proportions and linear trends. Correlation analysis was performed to evaluate the association between the inflammatory parameters and the other variables. A forward stepwise multiple regression analysis was performed to determine the association between diverse independent variables and IL-6, serum endotoxin level, and sCD14 concentration as the dependent variables. Bonferroni correction was used for multiple analyses. A P value <0.05 was considered significant. Data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Fifty-nine patients (29 males and 30 females, mean age 61 ± 15 years, mean HD vintage 29 ± 10 months, 42% diabetics) were finally analyzed (Table 1). Patients used polyacrilonitrile (60%) or polysulfone membranes (40%). Regarding vascular access, 72% had an arteriovenous fistula, whereas 28% had grafts or central dialysis catheters. Most patients used antihypertensive therapy (calcium channel blockers, 51%; angiotensin converting enzyme inhibitors or angiotensin receptor blockers, 42%; β-blockers, 24%; diuretics, 19%; and α-blockers, 7%). Fifty-one patients (86%) used statins, and 90% received erythropoiesis-stimulating agents. Baseline characteristics and medication use were similar between groups.
Table 1.
Patient characteristics at enrollment
| Characteristics | Sevelamer (n = 30) | Calcium acetate (n = 29) |
|---|---|---|
| Sex (male/female) | 15/15 | 14/15 |
| Age (years) | 59.6 ± 16.9 | 62.8 ± 14.1 |
| Diabetics | 43% | 41% |
| Time on HD (months) | 30 ± 10 | 28 ± 11 |
| Calcium (mg/dl) | 9.0 ± 0.7 | 9.0 ± 0.5 |
| Phosphate (mg/dl) | 5.4 ± 1.0 | 5.1 ± 0.6 |
| Ca x P (mg2/dl2) | 49.9 ± 13.0 | 46.5 ± 6.4 |
| Intact PTH (pg/ml) | 247 ± 92 | 258 ± 145 |
| C-reactive protein (mg/L) | 6.9 (5 to 9.8) | 5.9 (4.7 to 6) |
| TNF-α (pg/ml) | 8 (6 to 8.4) | 6.4 (5.8 to 8.7) |
| Interleukin-1 (pg/ml) | 0.8 (0.5 to 1.2) | 1.2 (0.8 to 1.4) |
| Interleukin-6 (pg/ml) | 5 (3 to 9.1) | 4.9 (4 to 8.1) |
| Interleukin-10 (pg/ml) | 75 (52 to 98) | 77 (71 to 94) |
| Interleukin-18 (pg/ml) | 599 (514 to 635) | 526 (420 to 621) |
| Endotoxin (EU/ml) | 0.58 (0.51 to 0.60) | 0.60 (0.51 to 0.63) |
| Soluble CD14 (μg/ml) | 2.94 (1.76 to 5.29) | 2.88 (2.35 to 4.70) |
Values represent the mean ± the SDs, or the median and interquartile ranges in parentheses. HD, hemodialysis, PTH, parathyroid hormone.
Evolution of Mineral Metabolism and Inflammatory Parameters
At the end of the study, serum phosphorus, calcium-phosphorus product (CaxP), and serum parathyroid hormone (PTH) decreased significantly in both groups (Table 2). On the contrary, whereas serum calcium did not change in subjects receiving sevelamer, this parameter significantly increased in patients treated with calcium acetate. Mean reduction of CaxP was higher in the sevelamer group (4.5 versus 2.0 mg2/dl2, P = 0.02), whereas the decrease in serum phosphorus almost reached statistical significance (0.5 versus 0.3 mg/dl, P = 0.07). Mean percent reduction of PTH was similar: 20.9% in the sevelamer group and 13.4% in the calcium acetate group.
Table 2.
Change of study parameters after treatment
| Sevelamer |
Calcium acetate |
|||||
|---|---|---|---|---|---|---|
| Basal | Final | P value | Basal | Final | P value | |
| Calcium (mg/dl) | 9.0 ± 0.7 | 9.1 ± 0.5 | 0.33 | 9.0 ± 0.5 | 9.3 ± 0.5 | <0.01 |
| Phosphate (mg/dl) | 5.4 ± 1.0 | 4.9 ± 1.0 | <0.001 | 5.1 ± 0.6 | 4.7 ± 0.7 | <0.001 |
| Ca x P (mg2/dl2) | 49.9 ± 13.0 | 45.3 ± 11.7 | <0.001 | 46.5 ± 6.4 | 44.5 ± 7.8 | <0.001 |
| Intact PTH (pg/ml) | 247 ± 92 | 196 ± 94 | <0.001 | 258 ± 145 | 217 ± 136 | <0.001 |
| C-reactive protein (mg/L) | 6.9 (5.9 to 8) | 5.9 (5.1 to 10) | 0.01 | 5.9 (4.7 to 6) | 5.4 (4 to 7.1) | 0.45 |
| TNF-α (pg/ml) | 8 (6 to 8.4) | 7.1 (5.9 to 7.6) | 0.58 | 6.4 (5.8 to 8.7) | 7 (6.1 to 8.1) | 0.26 |
| Interleukin-1 (pg/ml) | 0.8 (0.5 to 1.2) | 0.8 (0.51 to 1.0) | 0.56 | 1.2 (0.8 to 1.4) | 1.1 (0.9 to 1.2) | 0.92 |
| Interleukin-6 (pg/ml) | 5 (3 to 9.1) | 4.9 (2.68 to 8.6) | 0.001 | 4.9 (4 to 8.1) | 4.8 (4.2 to 7.8) | 0.03 |
| Interleukin-10 (pg/ml) | 75 (52 to 98) | 71 (69 to 96) | 0.059 | 77 (71 to 94) | 80 (65 to 96) | 0.64 |
| Interleukin-18 (pg/ml) | 599 (514 to 635) | 601 (499 to 658) | 0.74 | 526 (420 to 621) | 574 (428 to 620) | 0.34 |
| Endotoxin (EU/ml) | 0.58 (0.51 to 0.60) | 0.42 (0.27 to 0.54) | 0.001 | 0.60 (0.51 to 0.63) | 0.60 (0.48 to 0.69) | 0.57 |
| Soluble CD14 (μg/ml) | 2.94 (1.76 to 5.29) | 2.57 (1.36 to 4.52) | 0.001 | 2.88 (2.35 to 4.70) | 2.56 (2.21 to 0.98) | 0.93 |
Values are means ± SDs or median and interquartile range in parentheses. PTH, parathyroid hormone.
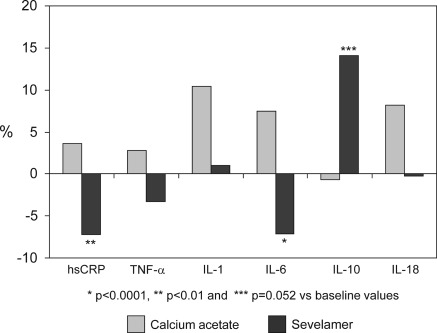
The evolution of serum inflammatory profile was significantly different between the sevelamer and the calcium acetate groups. Percent variation of serum hs-CRP and IL-6 were, respectively, −7.8% (95% confidence interval [CI] −13 to −2.5) versus +3.6% (95% CI 1.8 to 9.2; P < 0.01), and −7.1% (95% CI −12 to −2.3) versus +7.5% (95% CI 3 to 12; P < 0.001). Evolution of inflammatory parameters is shown in Table 2. In patients receiving calcium acetate, serum IL-6 experienced a mild but significant increase. On the contrary, hs-CRP and IL-6 significantly decreased in subjects treated with sevelamer, whereas serum levels of IL-10 showed a mean percent increase of 14.1% respect to baseline (P = 0.052). Serum TNF-α, IL-1 and IL-18 did not change. Mean percent variations of hs-CRP and serum cytokines are shown in Figure 2. Finally, the balance between pro- and anti-inflammatory forces was evaluated by the evolution of the ratios of TNFα, IL-1, IL-6, and IL-18 to the anti-inflammatory cytokine IL-10. We observed an opposite effect: Where all these ratios increased in subjects treated with calcium acetate (increase between 14% and 29%), in patients receiving sevelamer, these ratios experienced a mean percent reduction between 5.6 and 11.7% (Figure 4).
Figure 2.
Mean percent variation respect to baseline in serum concentration of inflammatory parameters in the calcium acetate and sevelamer groups at the end of the study. hsCRP, high-sensitivity C-reactive protein.
Figure 4.
Comparison of the mean percent variation of the ratios of proinflammatory (TNF-α, IL-1, IL-6 and IL-18) to the anti-inflammatory cytokine (IL-10) between the calcium acetate and sevelamer groups at the end of the study.
Evolution of Endotoxin Levels and sCD14
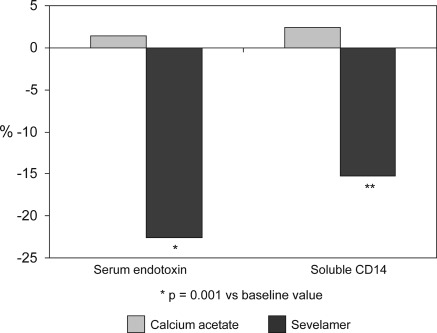
Serum endotoxin concentrations and sCD14 levels did not change after treatment with calcium acetate, whereas they significantly decreased (P < 0.01) in patients receiving sevelamer (Table 2). Serum endotoxin and sCD14 experienced a mild, nonsignificant increase in subjects receiving calcium acetate (1.4% and 2.4%, respectively), whereas they decreased by 22.6% and 15.2% in patients treated with sevelamer (P < 0.01 and P < 0.0001, respectively versus calcium acetate group; Figure 3).
Figure 3.
Mean percent variation respect to baseline in serum endotoxin concentration and soluble CD14 levels in the calcium acetate and sevelamer groups at the end of the study.
Correlates of Serum Endotoxin, IL-6, and sCD14 Changes
Table 3 lists bivariate regression results between change in serum LPS and sCD14 levels with variation in mineral metabolism and inflammatory parameters. Change in serum calcium, hsCRP, IL-6, and IL-18 correlated positively with changes in LPS levels, with variations in hsCRP and IL-6 showing the strongest correlation. Regarding sCD14, variations in CaxP, PTH, hsCRP, and IL-6 were directly correlated with changes in sCD14, whereas IL-10 variation showed a negative correlation. Similarly to LPS, changes in hsCRP and IL-6 had the strongest correlation with sCD14 variation.
Table 3.
Changes in serum endotoxin and sCD14 as the dependent variables and relationships with variation in mineral metabolism and inflammatory parameters
| Bivariate regression | Beta | 95% CI Beta | P value |
|---|---|---|---|
| Change in serum endotoxin concentration | |||
| Variable | |||
| Calcium | 0.30 | 0.05, 0.55 | 0.01 |
| Phosphate | 0.08 | −0.18, 0.34 | 0.53 |
| Calcium-Phosphate product | 0.22 | −0.03, 0.48 | 0.08 |
| Parathyroid hormone | 0.06 | −0.19, 0.33 | 0.61 |
| C-reactive protein | 0.50 | 0.27, 0.73 | <0.0001 |
| Tumor necrosis factor–α | 0.03 | −0.22, 0.30 | 0.77 |
| Interleukin-1 | 0.01 | −0.25, 0.27 | 0.93 |
| Interleukin-6 | 0.51 | 0.28, 0.74 | <0.0001 |
| Interleukin-10 | 0.15 | −0.42, 0.10 | 0.23 |
| Interleukin-18 | 0.28 | 0.03, 0.53 | 0.02 |
| Change in serum sCD14 level | |||
| Variable | |||
| Calcium | 0.20 | −0.05, 0.33 | 0.11 |
| Phosphate | 0.19 | −0.07, 0.45 | 0.14 |
| Calcium-Phosphate product | 0.25 | 0.02, 0.51 | 0.04 |
| Parathyroid hormone | 0.30 | 0.05, 0.55 | 0.01 |
| C-reactive protein | 0.59 | 0.37, 0.80 | <0.0001 |
| Tumor necrosis factor–α | 0.11 | −0.14, 0.38 | 0.37 |
| Interleukin-1 | 0.13 | −0.12, 0.39 | 0.30 |
| Interleukin-6 | 0.79 | 0.63, 0.95 | <0.0001 |
| Interleukin-10 | −0.41 | −0.65, −0.17 | 0.001 |
| Interleukin-18 | 0.23 | −0.02, 0.49 | 0.07 |
sCD14, soluble CD14; CI, confidence interval. Bivariate linear regression analysis was used to specify the potential interrelationships between changes in serum endotoxin and sCD14 concentrations and variations in mineral and inflammatory parameters.
Simple regression showed that the variation in calcium and CaxP were directly related to changes in serum IL-6 (r = 0.28 and r = 0.30, respectively, P < 0.05). Multiple regression analysis with age, gender, dialysis vintage, and variations in calcium, phosphate, CaxP, and PTH as the independent variables, demonstrated that age and variation in CaxP were independently related to change in serum IL-6. When variation in serum endotoxin concentrations was included in the model, this parameter had the strongest correlation with IL-6 change (Table 4).
Table 4.
Multivariate regression analysis between change in serum IL-6 concentrations and other variables
| Model number | Beta | 95% CI Beta | P value |
|---|---|---|---|
| Model 1 | |||
| Change in calcium-phosphate product | 0.35 | 0.13, 0.70 | 0.004 |
| Age | −0.35 | −0.57, −0.08 | 0.004 |
| Dialysis vintage | −0.14 | −0.30, 0.17 | 0.22 |
| Independent variables: age, gender, dialysis vintage, and changes in serum calcium, phosphate, calcium-phosphate product, and parathyroid hormone. | |||
| Model 2 | Beta | 95% CI Beta | P value |
| Change in serum endotoxin levels | 0.43 | 0.03, 0.87 | 0.0002 |
| Age | −0.30 | −0.42, −0.03 | 0.008 |
| Change in calcium-phosphate product | 0.24 | 0.02, 0.51 | 0.02 |
| Dialysis vintage | −0.14 | −0.32, 0.06 | 0.18 |
| Independent variables: Similar to model 1 + change in serum endotoxin levels. | |||
CI, confidence interval.
Finally, multiple regression analysis was performed using the changes in serum sCD14 as the dependent variable (Table 5). In Model 1, the independent variables were age, gender, dialysis vintage, and changes in parameters of mineral metabolism (calcium, phosphorus, CaxP, and PTH). Analysis showed that changes in serum calcium and PTH were independently associated with variations in sCD14. Model 2 was performed using age, gender, dialysis vintage, and changes in inflammatory parameters (hsCRP, TNF-α, IL-1, IL-6, IL-10, and IL-18) as the independent variables, showing that changes in sCD14 were independently and significantly associated only with the variation in serum IL-6, a relationship that remained significant when changes in calcium and PTH were included in the analysis (Model 3). Finally, when the variation in serum endotoxin levels was included (Model 4), the only variables independently associated with changes in sCD14 concentrations were the variations in serum IL-6 and LPS.
Table 5.
Multivariate regression analysis between changes in soluble CD14 and other variables
| Model number | Beta | 95% CI Beta | P value |
|---|---|---|---|
| Model 1 | |||
| Change in parathyroid hormone | 0.38 | 0.11, 0.75 | 0.005 |
| Change in calcium | 0.35 | 0.09, 0.70 | 0.006 |
| Age | −0.19 | −0.59, 0.16 | 0.11 |
| Change in phosphate | 0.15 | −0.19, 0.32 | 0.21 |
| Independent variables: age, gender, dialysis vintage, and changes in serum calcium, phosphate, calcium-phosphate product and parathyroid hormone. | |||
| Model 2 | |||
| Change in interleukin-6 | 0.78 | 0.48, 0.96 | 0.004 |
| Change in interleukin-18 | 0.13 | −0.03, 0.31 | 0.08 |
| Dialysis vintage | 0.08 | −0.16, 0.20 | 0.28 |
| Independent variables: age, gender, dialysis vintage, and changes in serum hsPCR and cytokines. | |||
| Model 3 | |||
| Change in interleukin-6 | 0.76 | 0.40, 0.94 | 0.004 |
| Change in interleukin-18 | 0.13 | −0.04, 0.32 | 0.08 |
| Dialysis vintage | 0.08 | −0.015, 0.20 | 0.30 |
| Independent variables: Similar to Model 2 + changes in parathyroid hormone and calcium. | |||
| Model 4 | |||
| Change in interleukin-6 | 0.60 | 0.28, 0.89 | <0.0001 |
| Change in serum endotoxin | 0.31 | 0.18, 0.66 | <0.001 |
| Change in interleukin-10 | −0.13 | −0.38, 0.09 | 0.09 |
| Independent variables: Similar to Model 3 + changes in serum endotoxin concentrations. | |||
CI, confidence interval; hsCRP, high-sensitivity C-reactive protein.
Discussion
This study shows that sevelamer treatment, in comparison with calcium-containing phosphate binders, is associated with anti-inflammatory effects, which are dependent of reduction in serum endotoxin levels. In addition, sevelamer is also associated with a significant decrease of sCD14 serum concentrations.
Hyperphosphatemia and inflammation are key pathogenic factors of prevalent complications in HD. Furthermore, increased serum phosphorus is an independent risk factor for the presence of a significant inflammatory state in CKD patients (14). In addition to the reduction of phosphorus levels, the noncalcium phosphate binder sevelamer has been associated with anti-inflammatory properties. Ferramosca et al. (22) showed that sevelamer treatment resulted in significant reductions of serum phosphorus and CRP levels. Likewise, Yamada et al. (23) observed a significant decrease of CRP during sevelamer therapy, which was correlated with the change of phosphorus. More recently, a subanalysis from the Nutritional and Inflammatory Evaluation of Dialysis Patients Study showed that patients receiving sevelamer were more likely to have a lower CRP concentration when compared with patients treated with calcium-based phosphate binders (24). In our study, serum hs-CRP and IL-6 significantly decreased in subjects treated with sevelamer, whereas inflammatory parameters did not change in subjects receiving calcium acetate. Of interest, multiple regression analysis demonstrated that changes in CaxP product was an independent predictor of variations in serum IL-6, even after adjusting for serum endotoxin concentrations, suggesting that control of hyperphosphatemia and CaxP with noncalcium phosphate binders may be associated with modulation of inflammation.
Exposure to soluble bacterial products causes immune activation and is a source of inflammation in the dialysis population. Plasma endotoxin levels are increased in dialysis patients, and correlate with levels of inflammatory markers and atherosclerosis (25,26). We found that endotoxemia is common in HD patients, with serum endotoxin concentrations similar to those recently reported by others (11,25). Furthermore, we observed that whereas patients treated with calcium acetate did not show any change, subjects receiving sevelamer experienced a significant 22.6% reduction in serum endotoxin levels. Our randomized prospective study confirms previous data regarding the properties of sevelamer in reducing the level of circulating endotoxins, with a parallel decrease in CRP (18). In that noncomparative, nonradomized study, endotoxin concentration decreased by 80%. The lower reduction in our study may be explained by several factors: the higher mean age of our patients (59 versus 51 years), the higher percent of diabetics (43% versus 15%), the higher dialysis vintage (30 versus 12 months), and the lower time of sevelamer therapy (3 versus 6 months). In addition, our study shows that reduction in serum endotoxin levels is the strongest factor associated with reduction of IL-6 concentrations.
Another important finding in this study is the reduction of sCD14 in patients receiving sevelamer. CD14 acts as a major surface receptor for endotoxins as well as for different molecules from gram-positive and gram-negative bacteria (6). In blood, endotoxins are transferred to mCD14 on monocytes, resulting in cell activation (7). In addition to mCD14, sCD14 is present in serum, and elevated concentrations have been related to inflammation, metabolic disorders, aortic stiffness, and heart failure (27–29). Increased monocyte mCD14 expression and sCD14 serum concentrations have been reported in HD patients (9). More important, serum levels of sCD14 were negatively associated with nutritional indexes and were independent predictors of mortality (10,11). Our study shows that sevelamer administration was associated with a 15.2% significant reduction in sCD14 concentration, as compared with a nonsignificant increase in subjects receiving calcium acetate (P < 0.0001 between groups). When we analyze the correlates of sCD14 variations after sevelamer administration by multiple regression analysis modeling for changes in mineral metabolism parameters, inflammatory markers, and serum endotoxin levels, the only variables independently associated with changes in sCD14 were the variations in serum IL-6 and endotoxin concentrations.
The potential translation of results of the present study on clinical outcomes is of interest. Previous trials have shown that sevelamer, as compared with calcium-containing phosphate binders, is associated with a positive impact on vascular calcification (30–32), a process in which inflammation plays a key pathogenic role (33). Moreover, a post hoc analysis of the RIND (Renagel In New Dialysis) trial showed that sevelamer use conferred a significant survival benefit as compared with calcium acetate (35). These previous results are in agreement with our findings on the anti-inflammatory activity of sevelamer and the potential clinical translation of these effects. However, these observations contrast with the DCOR (Dialysis Clinical Outcomes Revisited) study (34), which showed that mortality rates were not significantly different between patients receiving sevelamer or calcium-based phosphate binders. This deserves several comments. First, inflammatory parameters were not measured in the DCOR study, and, therefore, significant differences regarding this profile cannot be excluded. Second, there are important differences regarding patients' characteristics. Thus, in the DCOR study, 50% of participants were diabetics, whereas in our study, this percent was substantially lower (42%). In addition, all patients in our study were Caucasians, whereas in the DCOR trial, more than half of subjects were of other races. A third difference is the dialysis vintage, which was substantially lower in our study (29 versus 38 months). Finally, in the DCOR study, a significant interaction between treatment and age was observed for mortality, with sevelamer treatment being associated with improved survival in subjects ≥65 years of age. After analysis by age, the older group (≥65 years old) in the DCOR trial had a higher percent of Caucasians (60.8% versus 38.1%), a lower proportion of diabetics (45.4% versus 53.2%), a lower dialysis duration (29.9 versus 44.9 months), and a lower serum phosphorus concentration (5.5 versus 6.1 mg/dl) than younger patients. Interestingly, all these characteristics in the older group of the DCOR study are similar to those observed in our patients.
Although presenting novel information, this study has several limitations. First, the prevalent nature of our patients, and their relatively long dialysis vintage, may represent a selection of subjects who have survived despite the presence of factors potentially contributing to increased cardiovascular risk, including subclinical endotoxemia and inflammation. Second, we based our determinations on single measurements of inflammatory markers that are subjected to certain variability. Finally, although CD14 genetic polymorphisms have been related to serum sCD14 levels (35,36), the influence of CD14 genotype on sCD14 and its effect on the response to sevelamer was not analyzed.
In conclusion, our study shows that the noncalcium phosphate binder sevelarmer is associated with a significant decrease in hs-CRP, IL-6, serum endotoxin levels, and sCD14 concentrations, parameters that have been shown as independent predictors of mortality in HD patients (10,11,37,38). Although future studies in HD need to clarify the relationships between inflammatory parameters, circulating endotoxin, and sCD14 levels, and evaluate their effects on vascular health and their contribution to morbidity, clinical practice strategies may reduce the level of inflammation in these patients and may, in turn, affect outcome in a positive manner.
Disclosures
None.
Acknowledgments
This study was supported in part by FUNCIS and ACINEF.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimburger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-a: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C: Inflammation enhances cardiovascular risk and mortality in hemodialysis. Kidney Int 55: 648–658, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Stenvinkel P: Inflammation in end-stage renal disease: The hidden enemy. Nephrology (Carlton) 11: 36–41, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Jofré R, Rodríguez-Benítez P, López-Gómez JM, Pérez-García R: Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol 17: S274–S280, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Sundaram S, Barrett TW, Meyer KB, Perella C, Neto MC, King AJ, Pereira BJ: Transmembrane passage of cytokine-inducing bacterial products across new and reprocessed polysulfone dialyzers. J Am Soc Nephrol 7: 2183–2191, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, Glauser MP, Tobias PS, Ulevitch RJ: CD14 is a pattern recognition receptor. Immunity 1: 509–516, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Heumann D, Roger T: Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta 323: 59–72, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Ulevitch RJ, Tobias PS: Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol 13: 437–457, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Nockher WA, Scherberich JE: Monocyte cell-surface CD14 expression and soluble CD14 antigen in hemodialysis: Evidence for chronic exposure to LPS. Kidney Int 48: 1469–1476, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Raj DSC, Shah VO, Rambod M, Kovesdy CP, Kalantar-Zadeh K: Association of soluble endotoxin receptor CD14 and mortality among patients undergoing hemodialysis. Am J Kidney Dis 54: 1062–1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raj DSC, Carrero JJ, Shah VO, Qureshi AR, Barany P, Heimburger O, Lindholm B, Ferguson J, Moseley PL, Stenvinkel P: Soluble CD14 levels, interleukin 6, and mortality among prevalent hemodialysis patients. Am J Kidney Dis 54: 1072–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Block GA, Klassen PS, Lazarus M, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Navarro-González JF, Mora-Fernández C, Muros M, Herrera H, García J: Mineral metabolism and inflammation in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1646–1654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nikolov IJ, Joki M, Maizel J, Lacour B, Drueke T, Massy ZA: Pleiotropic effects of the non-calcium phosphate binder sevelamer. Kidney Int 70: S16–S23, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Perianayagam MC, Jaber BL: Endotoxin-binding affinity of sevelamer hydrochloride. Am J Nephrol 28: 802–807, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Sun PP, Perianayagam MC, Jaber BL: Sevelamer hydrochloride use and circulating endotoxin in hemodialysis patients: A pilot cross-sectional study. J Ren Nutr 19: 432–438, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Stinghem AEM, Gonçalves SM, Bucharles S, Branco FS, Gruber B, Hauser AB, Pecoits-Filho R: Sevelamer reduces systemic inflammation in parallel to a reduction in endotoxemia. Blood Purification 29: 352–356, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Panichi V, Maggiore U, Taccola D, Migliori M, Manca Rizza G, Consani C, Bertini A, Sposini S, Pérez-García R, Rindi P, Palla R, Tetta C: Interleukin-6 is a stronger predictor of total and cardiovascular mortality than C-reactive protein in haemodialysis. Nephrol Dial Transplant 19: 1154–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Zoccali C, Tripepi G, Mallamachi F: Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 17[Suppl 3]: S169–S173, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Borazan A, Aydemir S, Sert M, Yilmaz A: The effects of hemodialysis and peritoneal dialysis on serum homocysteine and C-reactive protein levels. Mediators Inflamm 13: 361–364, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P: Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J 149: 820–825, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Yamada K, Tokura T, Fukudome K, Fukudome K, Ochiai H, Komatsu H, Sato Y, Hara S, Eto T: Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 27: 361–365, 2005 [PubMed] [Google Scholar]
- 24. Shantouf R, Budoff MJ, Ahmadi N, Tiano J, Flores F, Kalantar-Zadeh K: Effects of sevelamer and calcium-based phosphate binders on lipid and inflammatory markers in hemodialysis patients. Am J Nephrol 28: 275–279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK: Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 3: 431–436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hauser AB, Stingher AEM, Gonçalves SM, Bucharles S, Pecoits-Filho R: A gut feeling on endotoxemia: Causes and consequences in chronic kidney disease. Nephrol Clin Pract 118: c165–c172, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Amar J, Ruidavets JB, Bal Dit Sollier C, Bongard V, Boccalon H, Chamontin B, Drouet L, Ferrières J: Soluble CD14 and aortic stiffness in a population-based study. J Hypertens 21: 1869–1877, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Fernández-Real JM, Broch M, Richart C, Vendrell J, Lopez-Bermejo A, Ricart W: CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol Metab 88: 1780–1784, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ: Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol 79: 1426–1430, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Braun J, Asmus H-G, Holzer H, Brunkhorst R, Krause R, Schulz W, Neumayer HH, Raggi P, Bommer J: Long term comparison of a calcium free phosphate binder and calcium carbonate-phosphorus metabolism and cardiovascular calcification. Clin Nephrol 62: 104–115, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Mizobuchi M, Towler D, Slatopolosky E: Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol 20: 1453–1464, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK: Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72: 1130–1137, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Koenig W, Khuseyinova N, Hoffmann MM, Marz W, Frölich M, Hoffmeister A, Brenner H, Rothenbacher D: CD14 C(-260)→T polymorphism, plasma levels of soluble endotoxin receptor CD14, their association with chronic infections and risk of stable coronary artery disease. J Am Coll Cardiol 40: 34–42, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Losito A, Kalidas K, Santoni S, Errico R, Jeffrey S: Association of the –159C/T polymorphism of the endotoxin receptor (CD14) with carotid artery disease and cardiovascular mortality in dialysis patients. Blood Purif 23: 128–133, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Panichi V, Maggiore U, Taccola D, Migliori M, Manca Rizza G, Consani C, Bertini A, Sposini S, Pérez-García R, Rindi P, Palla R, Tetta C: Interleukin-6 is a stronger predictor of total and cardiovascular mortality than C-reactive protein in haemodialysis patients. Nephrol Dial Transplant 19: 1154–1160, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Tripepi G, Mallamachi F, Zoccali C: Inflammation markers, adhesion molecules, and all-cause and cardiovascular mortality in patients with ESRD: Searching for the best risk marker by multivariate modelling. J Am Soc Nephrol Suppl 16: S83–S88, 2005 [DOI] [PubMed] [Google Scholar]