Abstract
Pyridoxal 5'-phosphate enzymes are ubiquitous in the nitrogen metabolism of all organisms. They catalyze a wide variety of reactions including racemization, transamination, decarboxylation, elimination, retro-aldol cleavage, Claisen condensation, and others on substrates containing an amino group, most commonly α-amino acids. The wide variety of reactions catalyzed by PLP enzymes is enabled by the ability of the covalent aldimine intermediate formed between substrate and PLP to stabilize carbanionic intermediates at Cα of the substrate. This review attempts to summarize the mechanisms by which reaction specificity can be achieved in PLP enzymes by focusing on three aspects of these reactions: stereoelectronic effects, protonation state of the external aldimine intermediate, and interaction of the carbanionic intermediate with the protein side chains present in the active site.
Introduction
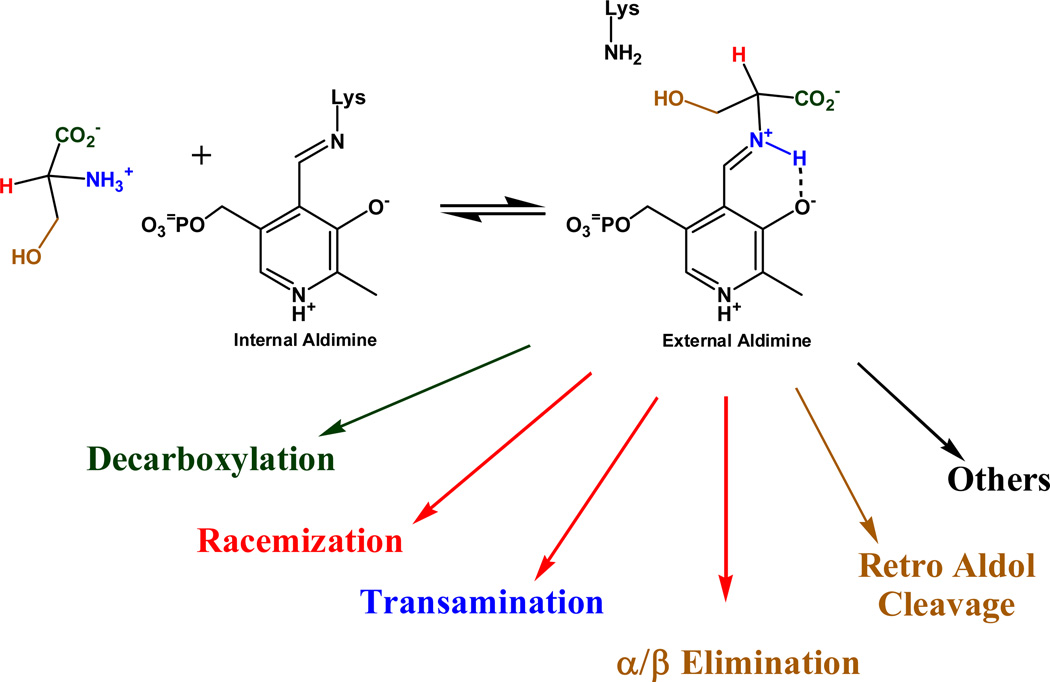
Pyridoxal phosphate (PLP) enzymes catalyze many different reaction types on amine and amino acid substrates.[1, 2] The first and common step in all pyridoxal phosphate catalyzed reactions is the formation of an external aldimine intermediate with the substrate. This occurs through a series of steps in which the unprotonated amino group of the substrate attacks the protonated Schiff base formed between a lysine side chain in the active site and the aldehyde group of PLP, followed by proton transfers and collapse to a Schiff base between the substrate and PLP, the external aldimine intermediate. All PLP dependent enzymes have the external aldimine intermediate in common, and it is from this intermediate that the different reaction types catalyzed by PLP diverge, as shown in Figure 1.
Figure 1.
Variety of reactions catalyzed by PLP. Several other reaction types are not shown for clarity.
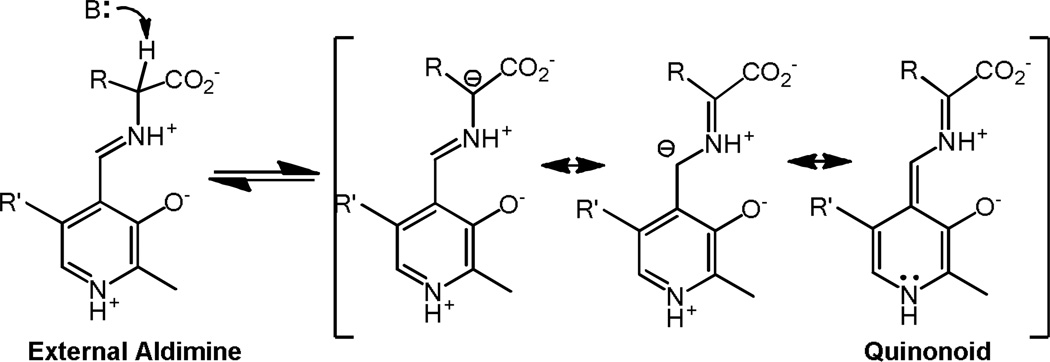
The majority of reactions catalyzed by PLP are initiated by abstraction of a proton from the carbon attached to the Schiff base nitrogen. Deprotonation is commonly carried out by the ε–amino group of the lysine that was liberated from the internal aldimine with PLP, resulting in the formation of a carbanionic intermediate. The latter is commonly called the quinonoid intermediate because of the quinone-like structure of one of its resonance forms. The carbanionic intermediate is resonance stabilized by the Schiff base and the pyridine ring, which may or may not be protonated depending on the environment. Deprotonation and resonance stabilization of the resulting carbanionic intermediate are illustrated in Figure 2.
Figure 2.
Resonance structures of carbanionic intermediates. The quinonoid structure is shown on the right.
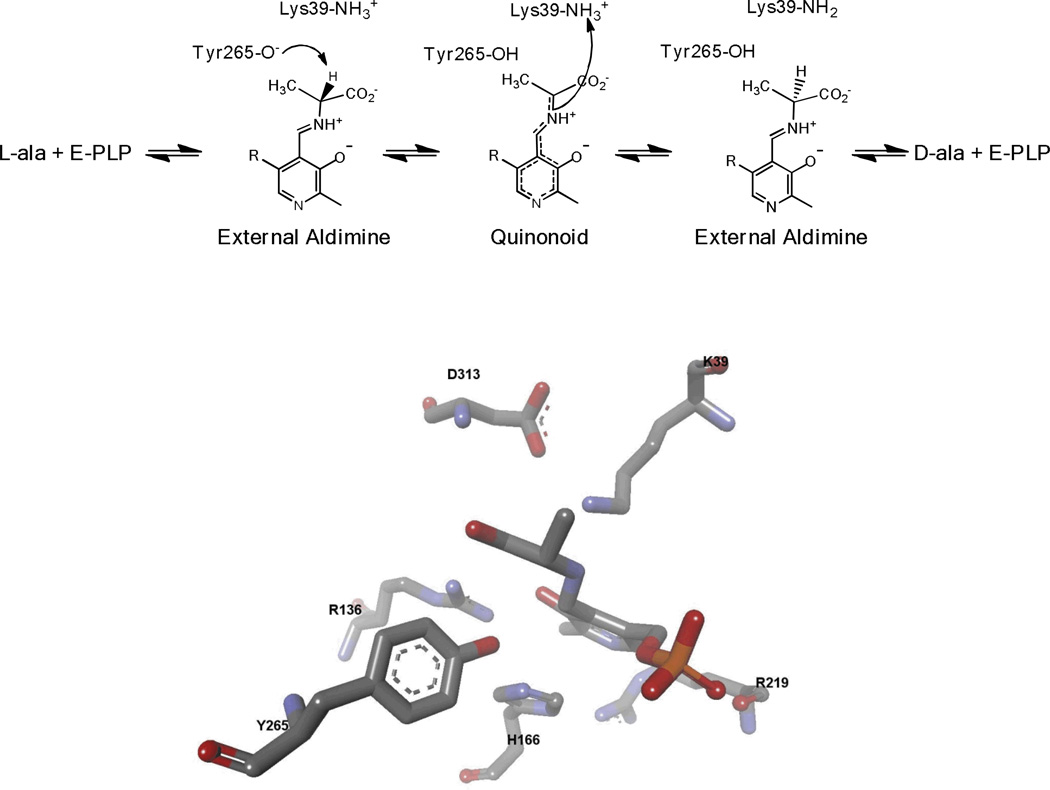
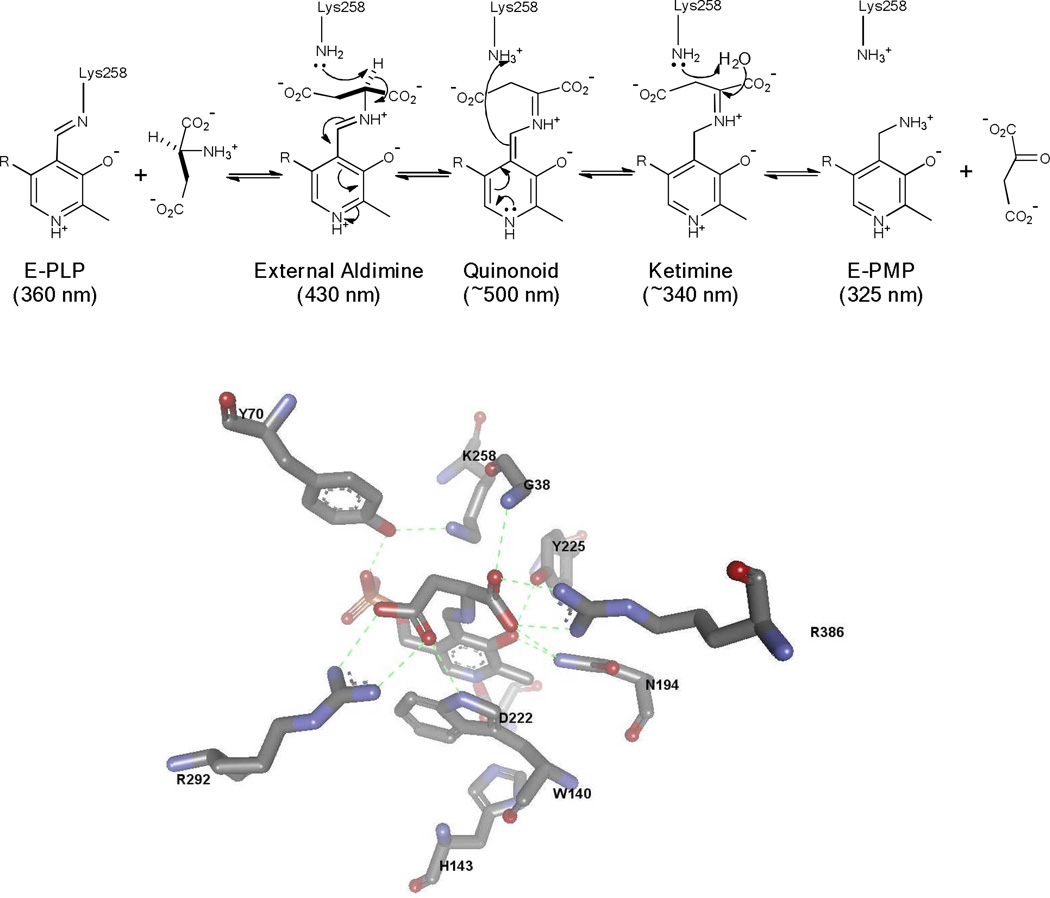
The carbanionic intermediate formed from deprotonation can undergo several different reactions. The simplest productive reaction is reprotonation at Cα on the opposite face from which the initial proton was removed. This is the central step in the reaction catalyzed by amino acid racemases and epimerases (Figure 3). The carbanionic intermediate can also undergo protonation at C4’ of the coenzyme. This leads to a ketimine intermediate that undergoes hydrolysis to complete the first half-reaction of the transamination catalytic cycle (Figure 4). Another possibility is that the carbanionic intermediate can act as a nucleophile. This occurs in aldol and Claisen condensation reactions that occur in amino acid metabolism.[3–7] If there is a good leaving group present at Cβ it can be lost from the carbanionic intermediate to form an aminoacrylate intermediate. This α–β elimination reaction is central to the biosynthesis of tryptophan, cysteine, and other metabolites. [8–12]
Figure 3.
PLP catalyzed racemization mechanism, and the active site structure of alanine racemase with PPL-L-Ala (reduced Schiff base) bound.
Figure 4.
PLP catalyzed transamination mechanism, and the active site structure of AAT with PPL-L-Asp (reduced Schiff base) bound.
Loss of CO2 from the external aldimine intermediate is another common central step for PLP enzymes, most commonly leading to the formation of an amine via protonation of the carbanionic intermediate on the same face from which CO2 was lost. [13] Bioactive amines such as histamine, serotonin, GABA, and others are formed in this way. An unusual decarboxylase, dialkylglycine decarboxylase (DGD), has been studied by our group. [14–18] DGD catalyzes the loss of CO2 from 2,2-dialkylglycines, but the CO2 lost from Cα is not replaced, rather a proton is added to C4’ to yield a ketimine intermediate that is hydrolyzed to yield the pyridoxamine phosphate (PMP) form of the enzyme. The PMP enzyme is converted back to the PLP enzyme by a classical transamination reaction with an α-keto acid, typically pyruvate.
The last possibility for carbanionic intermediate formation is breaking the Cα–Cβ bond in the external aldimine intermediate. This commonly occurs in retro-aldol condensations catalyzed by serine hydroxymethyltransferase and threonine aldolase. [12] In principle, for serine and threonine this can occur either by 1) deprotonation of the Cβ hydroxyl group followed by simultaneous formation of an aldehyde and the carbanionic intermediate, or 2) by attack of a nucleophile (e.g., tetrahydrofolate) on Cβ i n a n Sn2-type reaction with expulsion of the carbanionic intermediate as a leaving group.
One can see that all three bonds to Cα in the external aldimine that do not involve the imino N can be broken heterolytically to form a resonance stabilized, carbanionic intermediate. Consequently, the first problem in controlling reaction specificity arises: how does a given enzyme control which of the three labile bonds to Cα is broken to initiate forward reaction from the external aldimine intermediate given that the carbanion formed from each is approximately equally stabilized? This question was originally addressed by Dunathan who proposed that stereoelectronic effects are the major determinant. [19]
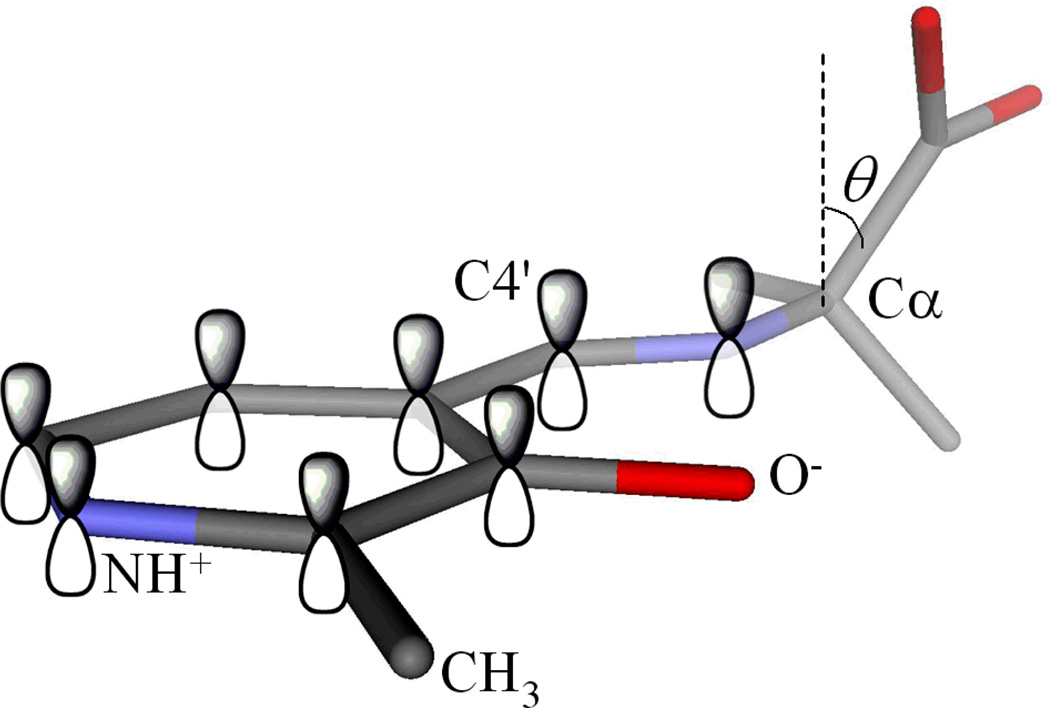
Stereoelectronic effects originate in the geometric dependence of orbital interactions. In the case of PLP, it was proposed that a PLP enzyme is evolved to bind substrates in specific orientations such that the bond to Cα of the external aldimine to be broken is parallel to the conjugated porbitals of the π system constituted by the Schiff base and pyridine ring (Figure 5). For example, if an enzyme requires the C–H bond to be broken to initiate reaction, then it can bind the substrate portion of the external aldimine intermediate such that this bond is aligned parallel to the porbitals. If this occurs, then the C–H bond is much more labile than the other two because as negative charge develops at Cα it will be optimally stabilized by resonance interactions with the conjugated π system. Specifically, as the C–H bond is being broken the carbon is rehybridizing from sp3to sp2and a porbital is forming on Cα. The negative charge forming in the nascent porbital can only be resonance stabilized to the degree that this porbital overlaps with those of the Schiff base and pyridine ring. This overlap and therefore the degree of charge stabilization depend on the cos2 of the angle between the porbitals and the bond being broken (Figure 5). This will be addressed in the first part of this review.
Figure 5.
Stereoelectronic effects in the external aldimine intermediate. The alignment of a particular bond with the p orbitals of the conjugated π system selectively labilizes it through GS (hyperconjugative) and TS (resonance) effects.
Another factor in negative charge stabilization that occurs in the transition state is the electrophilic strength of the combined Schiff base and pyridine ring π systems. This is governed by the number of protons present and their placement. PLP Schiff bases have a number of protonation states, and for each protonation states various tautomers exist. [20, 21] At physiological pH, the major protonation state of PLP/α-amino acid Schiff bases in solution has a single proton on the imino N. If enzymes were to use this protonation state then the electrophilic strength of the Schiff base/pyridine ring in the external aldimine intermediate would not be maximal. One way that an enzyme can overcome this is to provide an active site environment that specifically stabilizes the protonation state that is optimal for the reaction being catalyzed. As discussed in the second part of this review, the protonation state that is optimal for a given reaction is not necessarily the most electrophilic one.
Once stereoelectronic and protonation state optimization have occurred, the appropriate bond to Cα can be broken heterolytically to give a reactive carbanionic intermediate. The second major issue for controlling reaction specificity in PLP dependent enzymes is presented here. The carbanionic intermediate should be processed to give the required reaction outcome and this is controlled by the specific placement of side chain functional groups in the active site that direct the bonding changes suitably. This is addressed in the third part of this review.
Stereoelectronic effects
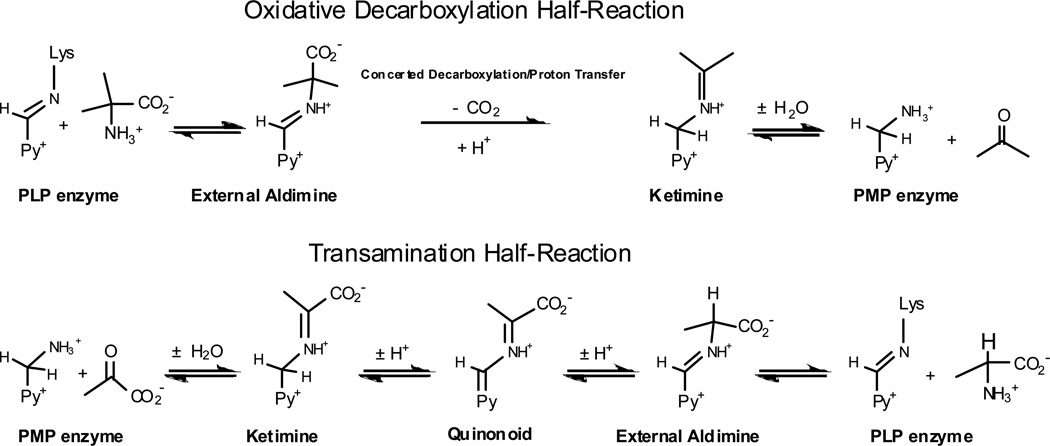
We have used DGD has a vehicle for studying stereoelectronic effects in PLP dependent enzymes.[14, 22–31] This interesting enzyme catalyzes decarboxylation of aminoisobutyric acid in the first half-reaction of a ping-pong kinetic mechanism, while in the second it catalyzes a classical transamination reaction in which the PMP form of the enzyme reacts with pyruvate and regenerates the PLP form of the enzyme and L-alanine. This is illustrated in Figure 6. Thus, DGD catalyzes C-C bond breaking in the first half-reaction, and proton transfer in the second.
Figure 6.
Mechanism for the DGD catalyzed oxidative decarboxylation of 2,2-dialkylglycines and transamination of alanine/pyruvate.
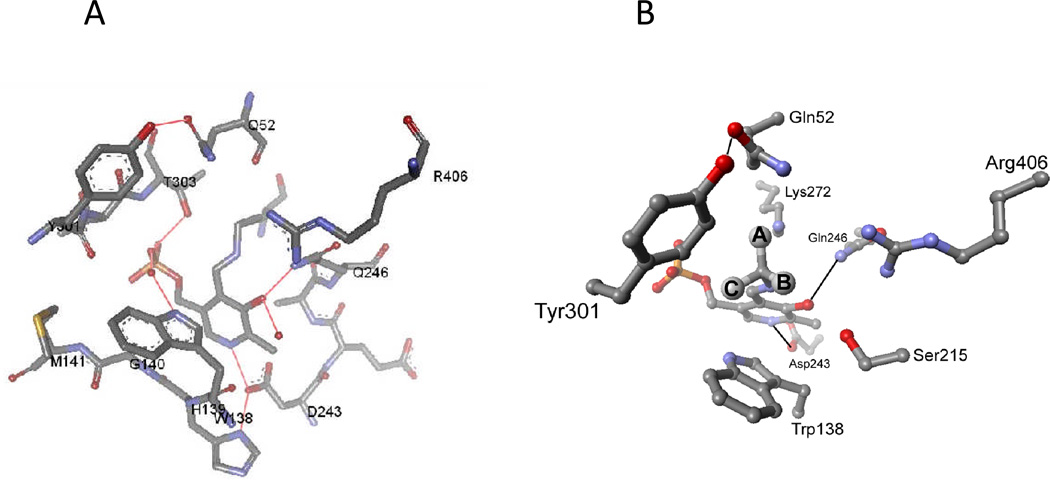
These two reactions have differing requirements for catalysis. For example, the 1,3-proton transfer in transamination requires a general base catalyst. Yet, both reactions form carbanionic intermediates that are resonance stabilized by the coenzyme. This latter commonality requires that both the C-C and the C–H bonds that are labilized occupy the same position in the active site. As discussed above, this position is one in which the bond to be broken is aligned parallel to the porbitals of the conjugated π system formed by PLP and substrate (Figure 5). The active site structure of DGD is shown in Figure 7A. A schematic external aldimine intermediate is shown in Figure 7B. Three binding subsites are labeled in the figure as A, B, and C. The A subsite is the catalytic site since it is only when the bond is in this position that it is aligned parallel to the porbitals of the conjugated π system. Therefore, in the decarboxylation half-reaction the C-C bond must be located at the A subsite while in the transamination half-reaction the C–H bond must be located at the A subsite. These requirements place structural constraints on the active site of DGD. The A and the B subsites must both be able to bind a carboxylate group. This has consequences for the decarboxylation reaction especially, since with simple 2,2-dialkylglycines the catalytic site is capable of accepting a small alkyl group, with the result that the carboxylate can bind in a nonproductive mode interacting with Arg406. This was previously exploited to demonstrate the existence of stereoelectronic effects in DGD using a series of alternative amino acid substrates that alter the equilibrium for binding the carboxylate between the A and B subsites.[18] The larger amino acid substrates promote the carboxylate to the catalytic A site, leading to an increase in kcat.
Figure 7.
(A) Active site structure of DGD. (B) Schematic of the DGD active site showing the locations of the A, B, and C binding subsites.
A series of aminophosphonate inhibitors of DGD was synthesized, and two were found to be slow binding inhibitors.[16] The kinetics of the slow binding inhibition were elucidated as was the structural basis. For the sake of this discussion, the most interesting findings were that the phosphonate group, which is a mimic of the substrate carboxylate group, is bound in a location between the A and B subsites. This was interpreted as evidence for competition between them for binding of the substrate carboxylate. More interesting was the observation that there is a correlation between the degree to which the C–P bond of the inhibitors is aligned parallel to the porbitals of the Schiff base and the rate constant for decarboxylation of the structurally analogous substrates for the inhibitors. The inhibitors for which the C–P bond is more nearly parallel to the porbitals of the π system have corresponding faster reactions for the equivalent substrates, providing additional evidence for the role of stereoelectronic effects in controlling the primary bond breaking step in PLP enzymes.
Studies employing mutants of DGD explored the roles of active site residues in the two halfreactions. Fogle et al.studied three Gln52 mutants, in which this residue was changed to either alanine, isoleucine, or glutamate. [22] The most straightforward mutant, Q52A in which the amide functional group is removed, also has the least impact on the rate of decarboxylation. The kcat for this mutant is 0.12 s−1, compared 10 s−1 for the wild type enzyme. The simplest interpretation of this result is that the loss of the amide functional group at position 52 results in the carboxylate group of AIB being favored at the B subsite 100-fold more than it is in the wild type enzyme, or in other words that Gln52 provides approximately 2.7 kcal per mole of binding energy for the substrate carboxylate at the A subsite. Thus, Gln52 can be seen as a competitor in the tug-of-war between the A and B subsites for the substrate carboxylate group. Based on the structure, Gln52 is the main protagonist at the A subsite while Arg406 is the main one at the B subsite.
The results with the Q52I mutant were more complicated.[22] It was made because Ile occupies the analogous position in the structurally very similar enzyme GABA aminotransferase. This mutation had a drastic deleterious effect on the rate constant for the formation of the external aldimine intermediate as well as decarboxylation. It was proposed that the Ile side chain sterically inhibits substrate binding and dispromotes the reactive confirmation in which the substrate carboxylate is in the A subsite.
The Q52E mutant was constructed in order to prevent substrate carboxylate binding in the A subsite via electrostatic repulsion between the carboxylate groups.[22] The rate constant for decarboxylation of AIB is 104-fold reduced in Q52E, while that for transamination is much less affected. Therefore, the effect of the mutation is rather specific to the decarboxylation half-reaction. This allowed the estimation of the magnitude of stereoelectronic effects in DGD. The rate constant for decarboxylation of AIB in Q52E was taken as the rate constant for decarboxylation from the B subsite, while the value for decarboxylation from the activated A subsite was calculated from the rate constant for AIB decarboxylation by wild type DGD and the effect of alternate substrates on it. Under these assumptions, it was inferred that the free energy of activation for loss of CO2 from the A subsite is 7.3 kcal/mole less than loss of CO2 from the B subsite. This value is considered a lower limit on the true value. Nevertheless, it would account for a 105-fold preference for breaking the bond at the A versus the B subsite, which is highly significant physiologically.
Recently, work with mutants at positions 138 and 406 in the active site of DGD was reported.[14] In Figure 7, it can be seen that are Arg406 is located in the B subsite and interacts with the carboxylate group of amino acid substrates. It was hypothesized that changing Arg406 to methionine would results in the carboxylate group of AIB binding only at the stereoelectronically activated A subsite, thereby increasing kcat by removing nonproductive binding in the external aldimine intermediate. This prediction was not borne out, rather the R406M mutant has a kcat for AIB decarboxylation that is decreased approximately 100-fold from that of wild type DGD. This surprising result inspired construction of the R406K mutant in which the positively charged side chain of Arg is replaced with the similarly charged Lys side chain. R406K has a 10-fold decreased rate constant for AIB decarboxylation. Similarly, there was a 100-fold and 10-fold increase in the Michaelis constants for AIB with R406M and R406K, respectively. The latter result is consistent with Arg406 being directly involved in the binding of substrate carboxylate, but the decrease in the kcat value for decarboxylation is not easily reconciled with the simple functional active site model constructed for DGD. It appears that positive charge in the B subsite is required for efficient decarboxylation to occur, although the reason for this is not clear.
The use of the alternative substrates 1-aminocyclopentane-1-carboxylate and 1-aminocyclohexane-1-carboxylate provided additional evidence for the requirements of a positive charge at position 406 for efficient decarboxylation catalysis. [14] These cyclic substrates force the carboxylate group in the A subsite. The rate constants for decarboxylation of the external aldimine intermediates formed with these substrates are decreased approximately 1000-fold for the Arg406 mutants. The requirements for a positive charge at residue 406 is counterintuitive since positive charge would be expected to stabilize the negatively charged ground state of the substrate carboxylate, thereby making loss of CO2 more difficult. This mystery is still unresolved.
The C subsite in the active site of DGD is thought to be formed by the indole ring of Trp138 and the side chain of Met141. Mutations at these two positions were employed to test the active site functional model used to analyze stereoelectronic effects.[14] The W138F mutant should enlarge the C subsite. Indeed, the W138F mutant is much more tolerant of larger substrates in the transamination half-reaction, which requires the side chain of the substrates to be in the C subsite. The M141R mutant was combined with the W138F mutant in an effort to bring about specificity for a carboxylate group at the C subsite. Reactions with the D and L isomers of glutamate show the new C subsite will was indeed capable of binding a carboxylate group but not that on Cγ of glutamate but on Cα of D-glutamate. The glutamate side chain binds in the B subsite. The result was that this mutant was 1000-fold more active in the decarboxylation of L-glutamate than was the wild type enzyme, while it bound D-glutamate with much higher affinity than wild type enzyme. The double mutant has a specificity ratio for L-glutamate over AIB that is 108-fold higher than that for WT. These results provide further support for the functional active site model of DGD which is based on stereoelectronic activation of the bond to be broken at the A subsite.
For comparative purposes, an interesting relative of DGD is GABA aminotransferase. The X-ray structure of GABA aminotransferase was determined using the structure of DGD as a molecular replacement search model. [32] It shows remarkable similarity to that of DGD but with important differences that correlate with the lack of decarboxylase activity. In an ambitious attempt to introduce decarboxylation activity into GABA aminotransferase, 12 active site variants with combinations of mutations were constructed, with the intention of introducing a binding site that would position the carboxylate of glutamate such that the C-C bond is parallel to the p orbitals of the π system.[33] These experiments had varied success. A moderate 10-fold increase in decarboxylation of L-glutamate was achieved with the E211S/I50H/V80D triple mutant. On the other hand, this mutant will also showed one of the largest decreases in transamination activity, giving a formal change of reaction specificity. The X-ray structures of mutants provide an explanation for these disappointing results. Although DGD and GABA aminotransferase are very similar in structure, there are important differences in the positions of active site residues. It was hypothesized that these Cα structural differences prevent appropriate placement of the introduced side chains for decarboxylation catalysis. This highlights an important lesson that has been learned time and again in protein engineering of enzymes: that the exact positioning of active site residues is critical to catalysis and this positioning is achieved by many subtle interactions between residues including those that lie outside of the active site. Our laboratory used the results with GABA aminotransferase as an impetus to develop methods for identifying these second sphere interactions that position active site residues. In unpublished work, we have developed a program that analyzes multiple sequence alignments for both structural conservation and covariation, and applied this to the interconversion of enzymes. Our success with this method will be reported in a forthcoming publication.
Another enzyme under investigation in our laboratory is alanine racemace. [34–38] It provides D-alanine that is required by bacteria for cell wall biosynthesis. L-alanine reacts with the enzyme to form an external aldimine that is deprotonated by Tyr265 to give a high energy quinonoid intermediate, which is subsequently reprotonated on the opposite face by Lys39 to give the D-alanine external aldimine intermediate (Figure 3).[36, 37] The enzyme from Geobacillus stearothermophilus is highly active, with kcat values of approximately 1000 s−1. Multiple kinetic isotope effects were employed to demonstrate the existence of the carbanionic intermediate, since it does not accumulate to spectroscopically detectable levels on the enzyme. [37] With a defined mechanism in hand, a series of racemization progress curves was globally fitted to this mechanism, allowing all eight of the rate constants in the mechanism to be defined. [36] In separate, independent work, Major and Gao performed mixed quantum mechanical/molecular mechanical simulations of the alanine racemase reaction and confirmed our conclusions drawn from global kinetic analysis.[39, 40]
Our global analysis work has been criticized by Johnson in two papers that describe a kinetic analysis program that his company has developed.[41, 42] A careful reading of our published work shows that narrow but reasonable limits on the eight rate constants were carefully spelled out. [36] This restriction on the values of the rate constants was key to the success of our methodology, but was ignored by Johnson. The original work has been repeated in our laboratory by multiple workers since publication, using different data analysis programs and yielding essentially identical results. In unpublished work, we have explored the constraints required in global analysis of enzyme kinetic data to obtain values of microscopic rate constants. This provides a general framework for defining free energy profiles using information from kinetic experiments that are commonly employed in the laboratory.
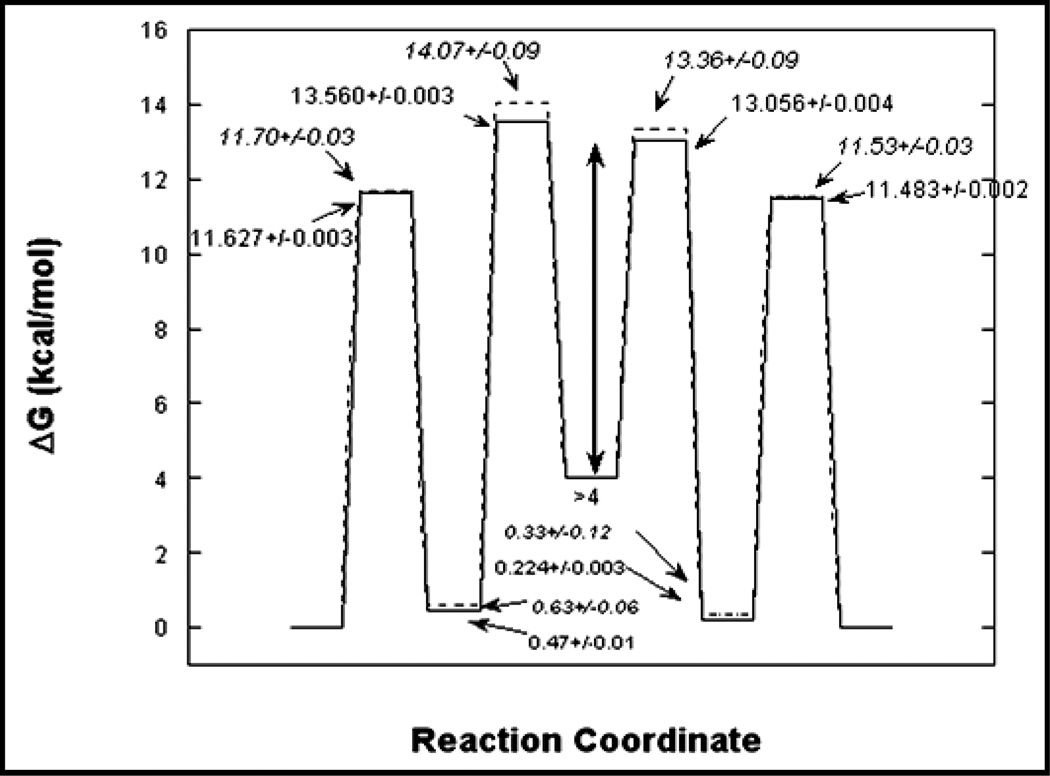
Global analysis of racemization progress curves was extended to the determination of isotopic free energy profiles for this enzyme. [34] In this work, three different types of racemization progress curves were employed for global analysis. The first was the usual racemization of protiated substrates in H2O, the second was racemization of deuterated substrates in H2O, and a third was equilibrium perturbation type experiments in which D- and L-alanine are initially at equal concentrations with one stereoisomer being deuterated. The crux of this analysis was the diversity of racemization progress curves employed and the similarity of the rate constants for the protiated and deuterated reactions. Satisfyingly, using a completely new and independent set of data from slightly different conditions, the free energy profile obtained is very similar to that from our original work (Figure 8).
Figure 8.
Isotopic free energy profiles for alanine racemase. The solid line is for protiated alanine and the dashed is for deuterated alanine.
The isotopic free energy profiles allow one to calculate intrinsic isotope effects for each step of the reaction.[34] The intrinsic kinetic isotope effects can be directly related to transition state structure, unlike observed kinetic isotope effects which are often compromised in magnitude by kinetic complexity. Based on the chemical similarity of the reaction from both directions, one expects to observe kinetic isotope effects that are similar for both stereoisomers. Indeed, this was observed: the primary kinetic isotope effects for deprotonation of either stereoisomer is approximately 1.6. This value is small compared to the theoretical limit of approximately 7. These isotope effects were interpreted within the context of the Westheimer model for kinetic isotope effects as indicating an early transition state for the deprotonation of D-alanine by Lys39 and a late transition state for the deprotonation of the L-alanine by Tyr265. Additionally, it allowed us to estimate the pKa of Cα in the external aldimine intermediate to be between those of Lys39 and Tyr265, with a value of approximately 9. This is in reasonable agreement with the calculations by Major and Gao which estimate the pKa to be approximately 12.
Interestingly, isotope effects were clearly observed on the rate constants for formation and decomposition of the external aldimine intermediate for both stereoisomers.[34] A normal kinetic isotope effect was observed for formation while an inverse kinetic isotope effect was observed for decomposition. This can be used to calculate an equilibrium isotope effect on formation of the external aldimine intermediate. The values for the equilibrium isotope effects are normal and approximately 1.25 for both stereoisomers, indicating that external aldimine formation is favored with the protiated substrate.
The origin of this isotope effects is in hyperconjugation. Alignment of the Cα–H bond in the external aldimine intermediate parallel to the p orbitals of the π system allows removal of electron density from the Cα–H bond into the electrophilic π system. This weakens the bond compared to that in solution, lowering its vibrational frequency and giving an isotope effect. This provides direct support for Dunathan's hypothesis that stereoelectronic effects are an important means by which PLP enzymes control the primary events in determining reaction specificity. It also suggests that the catalytic effects of PLP are felt not only in the transition state but also in the ground state. That is, there is significant ground state electronic destabilization in play in catalysis by PLP enzymes. Ground state electronic destabilization in the enzyme catalyzed reactions has recently been reviewed in the literature.
PLP protonation state
Over the last decade or so, evidence has been accumulating suggesting that not all PLP enzymes protonate the pyridine N as a prerequisite to catalysis. The first solid piece of evidence was the determination of the x-ray structure of tryptophan synthase.[43] It showed that, unlike AAT, the pyridine N does not interact with the carboxylate group of an aspartate or glutamate in the active site, rather it interacts with the hydroxyl group of a serine, which would ostensibly prohibit protonation of the pyridine N. Structures determined subsequently showed that all fold type II PLP enzymes employee either a serine, threonine, or cysteine residue for interaction with the pyridine N of bound PLP.[44] The X-ray structure of alanine racemase, which is a member of fold type III, shows Arg219 interacting with the pyridine N. [45–47] This latter interaction certainly precludes protonation of the pyridine N since the intrinsic basicity of the guanidino group (~13) is much higher than that of the pyridine N (~5), and the positive charge on Arg219 is not compatible with a protonated pyridine N.
Evidence that pyridine N protonation is not required for PLP catalysis comes from model studies in solution as well. For example, PLP catalyzes nonenzymatic transamination and decarboxylation at pH values where the pyridine N is unprotonated, and its protonation increases the rates of these reactions by a small factor.[48, 49] It has also been documented that salicylaldehyde can catalyze racemization of amino acids in solution, although transamination with this catalyst has not been observed. [50–56] Recent work from the group of Richard has quantitated the catalytic contribution to deprotonation from the various functional groups on PLP. [57–62] A priori, there is no reason to expect that PLP enzymes employ a single protonation state of the external aldimine intermediate, and different protonation states may be one means by which PLP enzymes can control reaction specificity. We previously proposed that the unprotonated pyridine N in alanine racemase causes the high energy of the carbanionic intermediate, and that this high energy serves a productive role in maintaining specificity for racemization versus transamination.[36, 37]
In addition to experimental studies, theoretical studies have also questioned the requirements for protonation of the pyridine N in PLP catalysis. Liao et al. performed ab initio calculations on transamination reactions.[63, 64] One of their conclusions was that protonation of the pyridine N by Asp222 does not lower the activation barrier to a great extent, in agreement with solution studies. Casasnovas et al. performed calculations on the 3-hydroxypyridine-4-aldehyde/alanine Schiff base, which more explicitly examined the effect of protonation state of the pyridine and imine nitrogens on the stabilibility of the carbanionic intermediate. [65] Their results suggest that protonation of the pyridine N has a strong effect on the stability of the enolimine versus ketoenamine tautomers of the Schiff bases, which has been confirmed experimentally.
Additionally, their calculations support the commonsense conclusion that the importance of pyridine N protonation to stabilizing carbanionic intermediates is dependent on the protonation states of the Schiff base N. [65] When the Schiff base N is protonated, protonation of the pyridine N has a small effect on the stability of the carbanionic intermediate. When the Schiff base N is unprotonated, protonation of the pyridine N has a much larger effect on stability of the carbanionic intermediate. Similar conclusions were drawn from previous calculations. [66, 67]
Casasnovas et al. performed additional calculations to arrive at atomic charges for the various protonation states of the carbanionic intermediate. [65] These allow one to draw an additional, important conclusion: protonation of the pyridine N promotes proton transfer to C4’ of PLP as is required in transamination. This is also in agreement with studies of nonenzymatic reactions using salicylaldehyde as a catalyst, where only racemization, not transamination, is observed. Therefore, enzymes for which protonation at C4’ is detrimental may have evolved to avoid protonation of the pyridine N as a way to control reaction specificity.
One can imagine that certain reaction types catalyzed by PLP enzymes require the formation of a distinct carbanionic intermediate. For example, amino acid decarboxylases in generally replace stereospecifically the α-carboxylate group with a proton to give an amine product with retention of configuration. This could not easily be achieved by a concerted decarboxylation/proton transfer mechanism, and a distinct carbanionic intermediate is expected. One can apply similar reasoning to aminotransferases and the central 1,3-prototropic shift. Indeed, recent N-15 NMR studies of AAT have confirmed that the pyridine N in this enzyme is protonated and exists as an ion pair with Asp222. [68] On the other hand, the group of enzymes that catalyze α–β elimination reactions can potentially catalyzed these without the formation of distinct carbanionic intermediates through an E2-type elimination reaction rather than an E1cb-type reaction. Cook and coworkers have studied the enzyme O-acetylserine sulfhydrylase, which appears to employ an E2-type mechanism. [11, 69–74]
O-Acetylserine sulfhydrylase catalyzes the final step in cysteine biosynthesis, in which acetic acid is eliminated from O-acetylserine to form an aminoacrylate intermediate that subsequently reactions with bisulfide to yield cysteine. The acetate leaving group is a good one and does not require general acid catalytic assistance. Thus, it is reasonable that it departs from Cβ at the same time that the proton is being removed from the Cα without formation of a distinct carbanionic intermediate. Abundant evidence in support of this hypothesis has been ushered by Cook. [11]
Tryptophan synthase is a PLP enzyme that catalyzes the final step in tryptophan biosynthesis, and is structurally very similar O-acetylserine sulfhydrylase. [8, 9] Tryptophan synthase has the chemically more difficult task of eliminating water from serine to form an aminoacrylate intermediate that subsequently undergoes reaction with indole to form tryptophan. Dunn and coworkers have studied extensively the pre-steady-state kinetics of tryptophan synthase and concluded that the wild type enzyme indeed forms a carbanionic intermediate prior to elimination of hydroxide from Cβ, in an E1cb-type mechanism. [75–83] In work from Miles and coworkers, it was shown that mutation of Ser377, which hydrogen bonds to the pyridine N, to aspartate (S377D) causes a dramatic accumulation of a carbanionic quinonoid intermediate that absorbs at 510 nm. [84] The longer wavelength absorption maximum for the carbanionic intermediate in the mutant versus the wild type enzyme (~460 nm) is good evidence that the pyridine N is indeed protonated in the S377D mutant, yet protonation of the pyridine N reduces the overall activity of the enzyme by more than 100-fold. This suggests that the greater negative charge density at Cα of the carbanionic intermediate when the pyridine N is unprotonated facilitates the elimination of the poor leaving group at Cβ of serine. By contrast, similar mutations in O-acetylserine sulfhydrylase had essentially no effect on the activity of the enzyme or on the observation of a carbanionic intermediate, in line with the ease of elimination of acetate as a leaving group. [85]
In experiments with AAT in which the opposite switch was made by removing the carboxylate that interacts with the pyridine N (D222A mutant), the consequences were severe: a 104 reduction in kcat/KM and a 103 reduction in kcat. [86–88] In additional work with this mutant, Kagamiyama and coworkers found that the coenzyme analogue N-methyl PLP does not restore full activity to the enzyme, increasing the rate constant for the reaction with aspartate only 10-fold. [87] In aminolevulinic acid synthase, an enzyme related in structure to AAT but catalyzing a Claisen condensation as the first step in heme biosynthesis, the D279A mutant has no detectable activity and reconstitution of it with N-methyl PLP does not increase it to detectable levels, although it did allow spectroscopic detection of quinonoid accumulation in the reverse reaction. [89] Similar results were obtained with ornithine decarboxylase. [90]
These results might be explained by less interesting effects such as altered coenzyme binding and orientation, but a real possibility that must be considered is that even in PLP enzymes in which the pyridine N interacts with the carboxylate group there are steps in the reaction mechanism that require the pyridine N to be uncharged (unprotonated), which is not possible with N-methyl PLP. For AAT, it would be interesting to know by how much the rate constant for deprotonation of Cα in the external aldimine intermediate is reduced (as measured by exchange with solvent) in the D222A mutant compared to the rate of transamination which requires protonation at C4’. This would give an indication of how important protonation of the pyridine N in the carbanionic intermediate is to leveling the reactivity of Cα and C4’ for protonation.
We recently published the synthesis of 1-deazaPLP and presented evidence that it binds tightly to AAT. [91] We are interested in this coenzyme analogue because it is isosteric with PLP and completely removes the ring nitrogen. No positive charge can develop on the ring and, additionally, the role of the greater electronegativity of N versus C can be deciphered. In work that has been submitted for publication, we showed that this coenzyme analogue has a very large effect on AAT, a much smaller effect on alanine racemase, and the smallest effect on O-acetylserine sulfhydrylase. These results are in agreement with those discussed above, and suggest an important role for modulation of the coenzyme electrophilicity in controlling reaction specificity.
Active site interactions
Once the carbanionic intermediate is generated from the external aldimine intermediate in PLP enzymes, its fate is partially controlled by the protonation states of the intermediate as discussed above, and probably largely controlled by its interaction with active site residues. One of the more remarkable demonstrations of the importance of active site residues in determining reaction specificity was reported by Seebeck and Hilvert in experiments on alanine racemase.[92, 93] An examination of the active site structure led them to postulate that removing Tyr265, which is the base catalyst that deprotonates the L-alanine external aldimine intermediate, would allow His166 to act as a general base in a retro-aldol reaction. The Y265A mutant indeed shows a 105-fold increase in activity for conversion of β–phenylserine to benzaldehyde and glycine over the wild type enzyme, although the kcat value for this reaction is 104-fold less than that for alanine racemization by the wild type enzyme.
A medically very important enzyme that has been characterized in the past decade is mammalian serine racemase. [35, 94–108] In mammals, D-serine is a coagonist of the N-methyl-D-aspartate receptor in the brain, and prevention of D-serine formation has potentially valuable medical consequences. An interesting and important property of this enzyme is that the racemization of L-serine to D-serine is accompanied by the elimination of water from serine, which occur at approximately equal rates. The biological significance of this coexistence of racemization and elimination activities is not clear, but is this subject of active research. In the context of this review, the existence of these two activities at equal levels in one enzyme is a remarkable example of the lack of substrate specificity in a PLP enzyme. The mechanism of both reactions is initiated by proton abstraction from Cα in the external aldimine intermediate to give a carbanionic intermediate that partitions between reprotonation on the opposite face of Cα and loss of hydroxide from Cβ. This highlights the importance of specific interactions between reaction intermediates and active site side chains in determining the pathway that a common intermediate takes in a given reaction. It is not yet clear either from the structure of the enzyme or other studies how this is achieved by serine racemase and certainly warrants further attention given the medical importance of this enzyme.
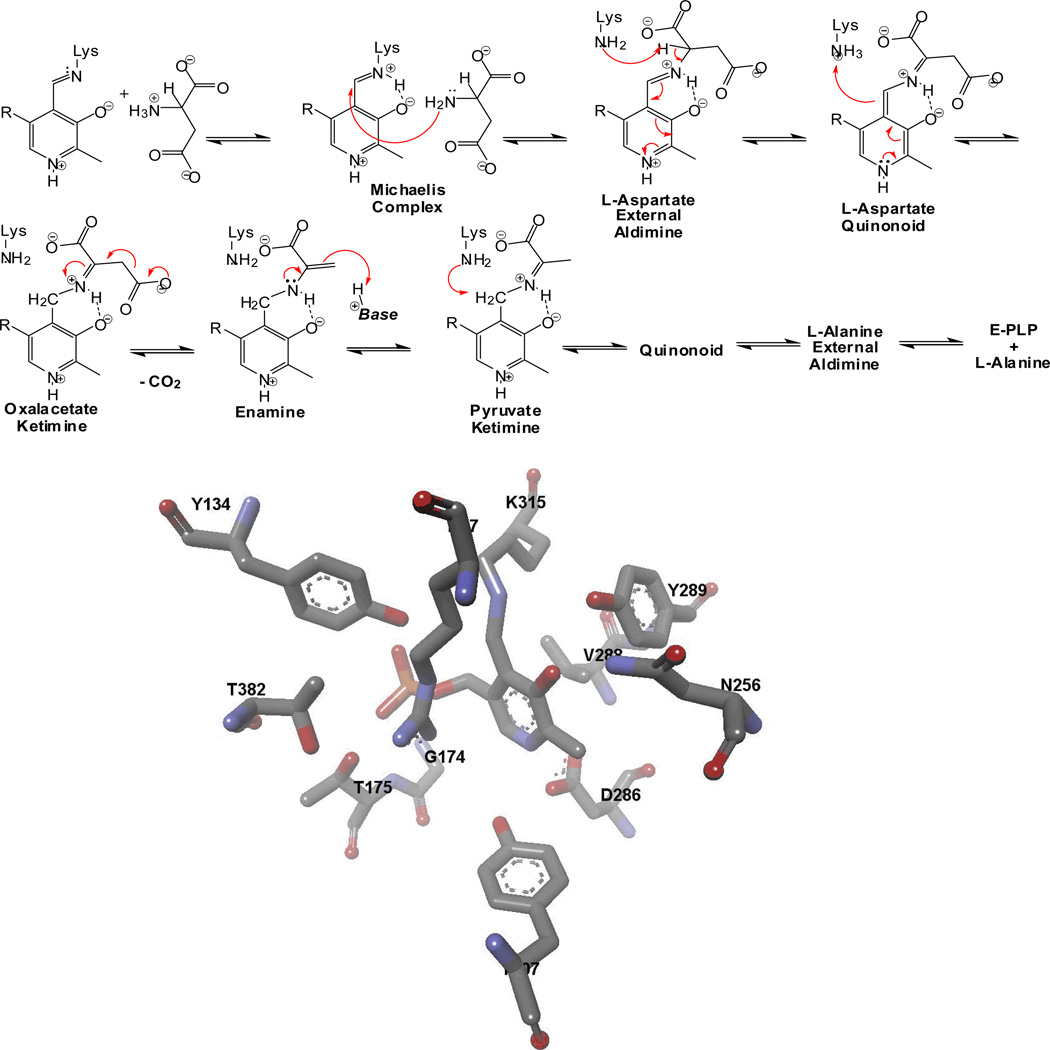
The complex mechanism of aspartate β-decarboxylase provides many opportunities for mistakes during the course of its reaction. The accepted reaction mechanism for this enzyme is shown in Figure 9. It involves initial 1,3-proton transfer from Cα of L-aspartate in the external aldimine intermediate to the coenzyme C4’ (the central step in transamination) followed by β-decarboxylation from the ketimine, and subsequent reverse 1,3-proton transfer and release of L-alanine. Past studies showed that it forms pyruvate ~1/1000 turnovers [109], which is the highest known error frequency for a PLP enzyme (unless one considers the elimination of water from serine as a mistake in the serine racemase reaction). The basis for this low reaction specificity is important to decipher as a counter example to the basis of the high reaction specificity of other PLP enzymes.
Figure 9.
Mechanism of aspartate β-decarboxylase, and active site structure.
Aspartate β–decarboxylase has been well studied kinetically and its X-ray structure was recently solved.[109–126] It was reported to have kcat value of approximately 400 s−1. [127] Recently, Phillips et al. reported stopped-flow kinetic studies that show the enzyme catalyzes the initial 1,3-proton tranfer with a rate constant greater than 1000 s−1. [128] They additionally found that one or more ketimine intermediates is/are the dominant species in the steady-state. Combined with small C-13 kinetic isotope effects measured by O'Leary, one can say with some certainty that steps subsequent to loss of CO2 are largely rate determining for aspartate β-decarboxylase. [129] The possible steps include the reverse 1,3-prototropic shift from the pyruvate ketimine to the L-alanine external aldimine, and the decomposition of this external aldimine into products. This makes chemical sense given that hydrolysis of the pyruvate ketimine leads to the major side product observed.
The groups of Wang and Lee have characterized a series of site directed mutants of this enzyme. [121, 122, 127] One series of mutants examined residues responsible for the dodecameric quaternary structure, while another series was based on differences between aspartate β-decarboxylase and AAT. From the mechanistic point of view, the most interesting mutation reported is F204W, in which the phenylalanine residue in front of PLP is changed to a tryptophan as is found in AATs. This single change does not significantly alter the decarboxylation activity, but it increases the tranamination activity 3-fold.
Amazingly, the reverse, conversion of AAT into aspartate β-decarboxylase, was achieved by the group of Christen by only three active site mutations. [130] The triple mutant Y225R/R292K/R386A shows a kcat value of 0.08 s−1 for β-decarboxylation of aspartate and 0.01 s−1 for transamination. The authors did not perform detailed mechanistic experiments that would provide insight into just how these mutations alter the reaction specificity of the enzyme, therefore, one can only speculate on how this occurs. Aspartate β-decarboxylase and AAT have steps leading to the oxaloacetate ketimine intermediate in common. AAT normally hydrolyzes this intermediate to complete the first half-reaction. To achieve a decrease in transamination activity with a simultaneous increase in β-decarboxylation activity, ketimine hydrolysis would have to be disfavored and loss of CO2 from the ketimine intermediate would have to be promoted. It is clear neither from the existing kinetic studies nor from the X-ray structure of the mutant enzyme how this is achieved, and additional work on this interesting finding would be valuable.
A similarly complex reaction mechanism is responsible for the conversion of O-phospho-L-homoserine into threonine. [131–139] In recent work from Hayashi's lab, strong evidence in support of the participation of the first product of the reaction, inorganic phosphate, as an acid-base catalyst of subsequent steps in the reaction was presented. [131] They showed that when L-vinylglycine (which is an intermediate in the proposed mechanism for O-phospho-L-homoserine) was used as a substrate, threonine was formed as a product only when inorganic phosphate was present in the buffer. Sulfate was not capable of substituting for phosphate, and in the absence of phosphate only α-ketobutyrate was formed. This illustrates a remarkable strategy for controlling reaction specificity, product-assisted catalysis, in which the first product of the reaction is a required catalyst for subsequent steps.
In summary, stereoelectronic effects are an important part of the process of controlling reaction specificity in PLP enzymes. The role that PLP protonation states play in reaction specificity is likely to be very important, but is not well clarified at the moment. It is probable that there is a strong interplay between stereoelectronic effects and the protonation state of PLP. Once the carbanionic intermediate is formed from the external aldimine intermediate, the interaction of this intermediate with active site residues is a major factor in determining the route that it takes. The details of these interactions and how they determine reaction outcome are likely to be specific to each reaction type catalyzed by PLP enzymes, and general principles are less likely to be gleaned from their understanding.
References
- 1.Christen P, Metzler DE. Transaminases. New York: Wiley; 1985. [Google Scholar]
- 2.John RA. Pyridoxal phosphate-dependent enzymes. Biochim Biophys Acta. 1995;1248:81–96. doi: 10.1016/0167-4838(95)00025-p. [DOI] [PubMed] [Google Scholar]
- 3.Toth K, Amyes TL, Richard JP, Malthouse JP, NiBeilliu ME. Claisen-type addition of glycine to pyridoxal in water. J Am Chem Soc. 2004;126:10538–10539. doi: 10.1021/ja047501v. [DOI] [PubMed] [Google Scholar]
- 4.Kerbarh O, Campopiano DJ, Baxter RL. Mechanism of alpha-oxoamine synthases: identification of the intermediate Claisen product in the 8-amino-7-oxononanoate synthase reaction. Chem Commun (Camb) 2006:60–62. doi: 10.1039/b511837a. [DOI] [PubMed] [Google Scholar]
- 5.Kubota T, Shimono J, Kanameda C, Izumi Y. The first thermophilic alpha-oxoamine synthase family enzyme that has activities of 2-amino-3-ketobutyrate CoA ligase and 7-keto-8-aminopelargonic acid synthase: cloning and overexpression of the gene from an extreme thermophile, Thermus thermophilus, and characterization of its gene product. Biosci Biotechnol Biochem. 2007;71:3033–3040. doi: 10.1271/bbb.70438. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt A, Sivaraman J, Li Y, Larocque R, Barbosa JA, Smith C, Matte A, Schrag JD, Cygler M. Three-dimensional structure of 2-amino-3-ketobutyrate CoA ligase from Escherichia coli complexed with a PLP-substrate intermediate: inferred reaction mechanism. Biochemistry. 2001;40:5151–5160. doi: 10.1021/bi002204y. [DOI] [PubMed] [Google Scholar]
- 7.Edgar AJ, Polak JM. Molecular cloning of the human and murine 2-amino-3-ketobutyrate coenzyme A ligase cDNAs. Eur J Biochem. 2000;267:1805–1812. doi: 10.1046/j.1432-1327.2000.01175.x. [DOI] [PubMed] [Google Scholar]
- 8.Raboni S, Bettati S, Mozzarelli A. Tryptophan synthase: a mine for enzymologists. Cell Mol Life Sci. 2009;66:2391–2403. doi: 10.1007/s00018-009-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn MF, Niks D, Ngo H, Barends TR, Schlichting I. Tryptophan synthase: the workings of a channeling nanomachine. Trends Biochem Sci. 2008;33:254–264. doi: 10.1016/j.tibs.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Rabeh WM, Cook PF. Structure and mechanism of O-acetylserine sulfhydrylase. J Biol Chem. 2004;279:26803–26806. doi: 10.1074/jbc.R400001200. [DOI] [PubMed] [Google Scholar]
- 11.Cook PF. Alpha, beta-elimination reaction of O-acetylserine sulfhydrylase. Is the pyridine ring required? Biochim Biophys Acta. 2003;1647:66–69. doi: 10.1016/s1570-9639(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 12.Schirch V, Szebenyi DM. Serine hydroxymethyltransferase revisited. Curr Opin Chem Biol. 2005;9:482–487. doi: 10.1016/j.cbpa.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Smith DM, Thomas NR, Gani D. A comparison of pyridoxal 5'-phosphate dependent decarboxylase and transaminase enzymes at a molecular level. Experientia. 1991;47:1104–1118. doi: 10.1007/BF01918374. [DOI] [PubMed] [Google Scholar]
- 14.Fogle EJ, Toney MD. Mutational analysis of substrate interactions with the active site of dialkylglycine decarboxylase. Biochemistry. 2010;49:6485–6493. doi: 10.1021/bi100648w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogle EJ, Liu W, Woon ST, Keller JW, Toney MD. Role of Q52 in catalysis of decarboxylation and transamination in dialkylglycine decarboxylase. Biochemistry. 2005;44:16392–16404. doi: 10.1021/bi051475b. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Rogers CJ, Fisher AJ, Toney MD. Aminophosphonate inhibitors of dialkylglycine decarboxylase: structural basis for slow binding inhibition. Biochemistry. 2002;41:12320–12328. doi: 10.1021/bi026318g. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Jin X, Medhekar R, Chen X, Dieckmann T, Toney MD. Rapid kinetic and isotopic studies on dialkylglycine decarboxylase. Biochemistry. 2001;40:1367–1377. doi: 10.1021/bi001237a. [DOI] [PubMed] [Google Scholar]
- 18.Sun S, Zabinski RF, Toney MD. Reactions of alternate substrates demonstrate stereoelectronic control of reactivity in dialkylglycine decarboxylase. Biochemistry. 1998;37:3865–3875. doi: 10.1021/bi972055s. [DOI] [PubMed] [Google Scholar]
- 19.Dunathan HC. Conformation and reaction specificity in pyridoxal phosphate enzymes. Proc Natl Acad Sci U S A. 1966;55:712–716. doi: 10.1073/pnas.55.4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan-Huot M, Sharif S, Tolstoy PM, Toney MD, Limbach HH. NMR Studies of the Stability, Protonation States, and Tautomerism of C-13- and N-15-Labeled Aldimines of the Coenzyme Pyridoxal 5'-Phosphate in Water. Biochemistry. 2010;49:10818–10830. doi: 10.1021/bi101061m. [DOI] [PubMed] [Google Scholar]
- 21.Chan-Huot M, Niether C, Sharif S, Tolstoy PM, Toney MD, Limbach HH. NMR studies of the protonation states of pyridoxal-5 '-phosphate in water. J Mol Struct. 2010;976:282–289. [Google Scholar]
- 22.Fogle EJ, Liu WS, Woon ST, Keller JW, Toney MD. Role of Q52 in catalysis of decarboxylation and transamination in dialkylglycine decarboxylase. Biochemistry. 2005;44:16392–16404. doi: 10.1021/bi051475b. [DOI] [PubMed] [Google Scholar]
- 23.Liu WS, Toney MD. Kinetic and thermodynamic analysis of the interaction of cations with dialkylglycine decarboxylase. Biochemistry. 2004;43:4998–5010. doi: 10.1021/bi035854l. [DOI] [PubMed] [Google Scholar]
- 24.Liu WS, Rogers CJ, Fisher AJ, Toney MD. Arninophosphonate inhibitors of dialkylglycine decarboxylase: Structural basis for slow binding inhibition. Biochemistry. 2002;41:12320–12328. doi: 10.1021/bi026318g. [DOI] [PubMed] [Google Scholar]
- 25.Zhou XZ, Jin XG, Medhekar R, Chen XY, Dieckmann T, Toney MD. Rapid kinetic and isotopic studies on dialkylglycine decarboxylase. Biochemistry. 2001;40:1367–1377. doi: 10.1021/bi001237a. [DOI] [PubMed] [Google Scholar]
- 26.Malashkevich VN, Strop P, Keller JW, Jansonius JN, Toney MD. Crystal structures of dialkylglycine decarboxylase inhibitor complexes. J Mol Biol. 1999;294:193–200. doi: 10.1006/jmbi.1999.3254. [DOI] [PubMed] [Google Scholar]
- 27.Zhou XZ, Toney MD. pH studies on the mechanism of the pyridoxal phosphate-dependent dialkylglycine decarboxylase. Biochemistry. 1999;38:311–320. doi: 10.1021/bi981455s. [DOI] [PubMed] [Google Scholar]
- 28.Zhou XZ, Kay S, Toney MD. Coexisting kinetically distinguishable forms of dialkylglycine decarboxylase engendered by alkali metal ions. Biochemistry. 1998;37:5761–5769. doi: 10.1021/bi973010u. [DOI] [PubMed] [Google Scholar]
- 29.Sun SX, Zabinski RF, Toney MD. Reactions of alternate substrates demonstrate stereoelectronic control of reactivity in dialkylglycine decarboxylase. Biochemistry. 1998;37:3865–3875. doi: 10.1021/bi972055s. [DOI] [PubMed] [Google Scholar]
- 30.Sun SX, Bagdassarian CK, Toney MD. Pre-steady-state kinetic analysis of the reactions of alternate substrates with dialkylglycine decarboxylase. Biochemistry. 1998;37:3876–3885. doi: 10.1021/bi972056k. [DOI] [PubMed] [Google Scholar]
- 31.Toney MD, Hohenester E, Cowan SW, Jansonius JN. Dialkylglycine Decarboxylase Structure - Bifunctional Active-Site and Alkali-Metal Sites. Science. 1993;261:756–759. doi: 10.1126/science.8342040. [DOI] [PubMed] [Google Scholar]
- 32.Liu WS, Peterson PE, Carter RJ, Zhou XZ, Langston JA, Fisher AJ, Toney MD. Crystal structures of unbound and aminooxyacetate-bound Escherichia coli gamma-aminobutyrate aminotransferase. Biochemistry. 2004;43:10896–10905. doi: 10.1021/bi049218e. [DOI] [PubMed] [Google Scholar]
- 33.Liu WS, Peterson PE, Langston JA, Jin XG, Zhou XZ, Fisher AJ, Toney MD. Kinetic and crystallographic analysis of active site mutants of Escherichia coli gamma-aminobutyrate aminotransferase. Biochemistry. 2005;44:2982–2992. doi: 10.1021/bi048657a. [DOI] [PubMed] [Google Scholar]
- 34.Spies MA, Toney MD. Intrinsic primary and secondary hydrogen kinetic isotope effects for alanine racemase from global analysis of progress curves. J Am Chem Soc. 2007;129:10678–10685. doi: 10.1021/ja067643k. [DOI] [PubMed] [Google Scholar]
- 35.Dixon SM, Li P, Liu R, Wolosker H, Lam KS, Kurth MJ, Toney MD. Slow-binding human serine racemase inhibitors from high-throughput screening of combinatorial libraries. J Med Chem. 2006;49:2388–2397. doi: 10.1021/jm050701c. [DOI] [PubMed] [Google Scholar]
- 36.Spies MA, Woodward JJ, Watnik MR, Toney MD. Alanine racemase free energy profiles from global analyses of progress curves. J Am Chem Soc. 2004;126:7464–7475. doi: 10.1021/ja049579h. [DOI] [PubMed] [Google Scholar]
- 37.Spies MA, Toney MD. Multiple hydrogen kinetic isotope effects for enzymes catalyzing exchange with solvent: application to alanine racemase. Biochemistry. 2003;42:5099–5107. doi: 10.1021/bi0274064. [DOI] [PubMed] [Google Scholar]
- 38.Sun S, Toney MD. Evidence for a two-base mechanism involving tyrosine-265 from arginine-219 mutants of alanine racemase. Biochemistry. 1999;38:4058–4065. doi: 10.1021/bi982924t. [DOI] [PubMed] [Google Scholar]
- 39.Major DT, Gao J. A combined quantum mechanical and molecular mechanical study of the reaction mechanism and alpha-amino acidity in alanine racemase. J Am Chem Soc. 2006;128:16345–16357. doi: 10.1021/ja066334r. [DOI] [PubMed] [Google Scholar]
- 40.Major DT, Nam K, Gao J. Transition state stabilization and alpha-amino carbon acidity in alanine racemase. J Am Chem Soc. 2006;128:8114–8115. doi: 10.1021/ja062272t. [DOI] [PubMed] [Google Scholar]
- 41.Johnson KA, Simpson ZB, Blom T. Global kinetic explorer: a new computer program for dynamic simulation and fitting of kinetic data. Anal Biochem. 2009;387:20–29. doi: 10.1016/j.ab.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 42.Johnson KA, Simpson ZB, Blom T. FitSpace explorer: an algorithm to evaluate multidimensional parameter space in fitting kinetic data. Anal Biochem. 2009;387:30–41. doi: 10.1016/j.ab.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Hyde CC, Ahmed SA, Padlan EA, Miles EW, Davies DR. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988;263:17857–17871. [PubMed] [Google Scholar]
- 44.Jansonius JN. Structure, evolution and action of vitamin B6-dependent enzymes. Curr Opin Struct Biol. 1998;8:759–769. doi: 10.1016/s0959-440x(98)80096-1. [DOI] [PubMed] [Google Scholar]
- 45.Shaw JP, Petsko GA, Ringe D. Determination of the structure of alanine racemase from Bacillus stearothermophilus at 1.9-A resolution. Biochemistry. 1997;36:1329–1342. doi: 10.1021/bi961856c. [DOI] [PubMed] [Google Scholar]
- 46.Stamper GF, Morollo AA, Ringe D. Reaction of alanine racemase with 1-aminoethylphosphonic acid forms a stable external aldimine. Biochemistry. 1999;38:6714. doi: 10.1021/bi995075y. [DOI] [PubMed] [Google Scholar]
- 47.Morollo AA, Petsko GA, Ringe D. Structure of a Michaelis complex analogue: propionate binds in the substrate carboxylate site of alanine racemase. Biochemistry. 1999;38:3293–3301. doi: 10.1021/bi9822729. [DOI] [PubMed] [Google Scholar]
- 48.Zabinski RF, Toney MD. Metal ion inhibition of nonenzymatic pyridoxal phosphate catalyzed decarboxylation and transamination. Journal of the American Chemical Society. 2001;V123:193–198. doi: 10.1021/ja0026354. [DOI] [PubMed] [Google Scholar]
- 49.Maley JR, Bruice TC. Catalytic reactions involving azomethines. XII. Transamination of 1-methyl-3-hydroxy-4-formylpyridinium chloride. Arch Biochem Biophys. 1970;136:187–192. doi: 10.1016/0003-9861(70)90340-1. [DOI] [PubMed] [Google Scholar]
- 50.Fischer JR, Fischer RJ, Abbott EH. Models of Vitamin-B6 Enzymes .3. Steric and Electronic Effects in Carbon Hydrogen-Bond Breaking Reactions of Bis(Salicylideneglycinato)Cobaltate(Iii) Anions. Inorg Chem. 1990;29:1682–1687. [Google Scholar]
- 51.Fischer JR, Abbott EH. Differential Reactivity of the Alpha-Methylene Protons of Bis(Pyridoxylideneglycinato)Cobalt(Iii) - Inferences for Vitamin-B6 Catalyzed Reactions. Journal of the American Chemical Society. 1979;101:2781–2782. [Google Scholar]
- 52.Ando M, Emoto S. Catalytic Activities of Salicylaldehyde Derivatives .8. Kinetic Studies of Catalytic Racemization of L-Glutamic Acid at 25-Degrees-C. B Chem Soc Jpn. 1978;51:2366–2368. [Google Scholar]
- 53.Ando M, Emoto S. Catalytic Activities of Salicylaldehyde Derivatives .5. Syntheses and Catalytic Activities of Some Trimethylammonio Derivatives of Salicylaldehyde in Racemization of L-Glutamic Acid. B Chem Soc Jpn. 1978;51:2433–2434. [Google Scholar]
- 54.Ando M, Emoto S. Catalytic Activities of Salicylaldehyde Derivatives .7. Synthesis and Catalytic Activity of (2-Formyl-3-Hydroxyphenyl)Dimethylsulfonium Salt in Racemization of L-Glutamic Acid. B Chem Soc Jpn. 1978;51:2437–2438. [Google Scholar]
- 55.Ando M, Emoto S. Catalytic Activities of Salicylaldehyde Derivatives .I. Catalytic Effects of 4-Formyl-3-Hydroxyphenyltrimethylammonium Bromide on Racemization of L-Amino Acid. B Chem Soc Jpn. 1969;42 2624-&. [Google Scholar]
- 56.Ando M, Emoto S. Catalytic Activities of Salicylaldehyde Derivatives .2. Kinetic Studies of Racemization of Amino Acid. B Chem Soc Jpn. 1969;42 2628-&. [Google Scholar]
- 57.Crugeiras J, Rios A, Riveiros E, Richard JP. Substituent Effects on the Thermodynamic Stability of Imines Formed from Glycine and Aromatic Aldehydes: Implications for the Catalytic Activity of Pyridoxal-5 '-phosphate. Journal of the American Chemical Society. 2009;131:15815–15824. doi: 10.1021/ja906230n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richard JP, Amyes TL, Crugeiras J, Rios A. Pyridoxal 5 '-phosphate: electrophilic catalyst extraordinaire. Current Opinion in Chemical Biology. 2009;13:475–483. doi: 10.1016/j.cbpa.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Go MK, Richard JP. Alanine-dependent reactions of 5 '-deoxypyridoxal in water. Bioorg Chem. 2008;36:295–298. doi: 10.1016/j.bioorg.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crugeiras J, Rios A, Riveiros E, Amyes TL, Richard JP. Glycine enolates: The effect of formation of iminium ions to simple ketones on alpha-amino carbon acidity and a comparison with pyridoxal iminium ions. Journal of the American Chemical Society. 2008;130:2041–2050. doi: 10.1021/ja078006c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toth K, Richard JP. Covalent catalysis by pyridoxal: Evaluation of the effect of the cofactor on the carbon acidity of glycine. Journal of the American Chemical Society. 2007;129:3013–3021. doi: 10.1021/ja0679228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rios A, Crugeiras J, Amyes TL, Richard JP. Glycine enolates: The large effect of iminium ion formation on alpha-amino carbon acidity. Journal of the American Chemical Society. 2001;123:7949–7950. doi: 10.1021/ja016250c. [DOI] [PubMed] [Google Scholar]
- 63.Liao RZ, Ding WJ, Yu JG, Fang WH, Liu RZ. Theoretical studies on pyridoxal 5 '-phosphate-dependent transamination of alpha-amino acids. J Comput Chem. 2008;29:1919–1929. doi: 10.1002/jcc.20958. [DOI] [PubMed] [Google Scholar]
- 64.Liao RZ, Ding WJ, Yu JG, Fang WH, Liu RZ. Water-assisted transamination of glycine and formaldehyde. J Phys Chem A. 2007;111:3184–3190. doi: 10.1021/jp070130v. [DOI] [PubMed] [Google Scholar]
- 65.Casasnovas R, Salva A, Frau J, Donoso J, Munoz F. Theoretical study on the distribution of atomic charges in the Schiff bases of 3-hydroxypyridine-4-aldehyde and alanine. The effect of the protonation state of the pyridine and imine nitrogen atoms. Chem Phys. 2009;355:149–156. [Google Scholar]
- 66.Toney MD. Computational studies on nonenzymatic and enzymatic pyridoxal phosphate catalyzed decarboxylations of 2-aminoisobutyrate. Biochemistry. 2001;40:1378–1384. doi: 10.1021/bi0012383. [DOI] [PubMed] [Google Scholar]
- 67.Bach RD, Canepa C, Glukhovtsev MN. Influence of electrostatic effects on activation barriers in enzymatic reactions: Pyridoxal 5 '-phosphate-dependent decarboxylation of alpha-amino acids. Journal of the American Chemical Society. 1999;121:6542–6555. [Google Scholar]
- 68.Sharif S, Fogle E, Toney MD, Denisov GS, Shenderovich IG, Buntkowsky G, Tolstoy PM, Huot MC, Limbach HH. NMR localization of protons in critical enzyme hydrogen bonds. Journal of the American Chemical Society. 2007;129 doi: 10.1021/ja0728223. 9558-+. [DOI] [PubMed] [Google Scholar]
- 69.Tai CH, Cook PF. O-acetylserine sulfhydrylase. Adv Enzymol Relat Areas Mol Biol. 2000;74:185–234. doi: 10.1002/9780470123201.ch5. [DOI] [PubMed] [Google Scholar]
- 70.Hui TA, Rong GA, Salsi E, Campanini B, Bettati S, Kumar VP, Karsten WE, Mozzarelli A, Cook PF. Identification of the Structural Determinants for the Stability of Substrate and Aminoacrylate External Schiff Bases in O-Acetylserine Sulfhydrylase-A. Biochemistry. 2010;49:6093–6103. doi: 10.1021/bi100473v. [DOI] [PubMed] [Google Scholar]
- 71.Chattopadhyay A, Meier M, Ivaninskii S, Burkhard P, Speroni F, Campanini B, Bettati S, Mozzarelli A, Rabeh WM, Li L, Cook PF. Structure, mechanism, and conformational dynamics of O-acetylserine sulfhydrylase from Salmonella typhimurium: Comparison of a and b isozymes. Biochemistry. 2007;46:8315–8330. doi: 10.1021/bi602603c. [DOI] [PubMed] [Google Scholar]
- 72.Rabeh WM, Alguindigue SS, Cook PF. Mechanism of the addition half of the O-acetylserine sulfhydrylase-A reaction. Biochemistry. 2005;44:5541–5550. doi: 10.1021/bi047479i. [DOI] [PubMed] [Google Scholar]
- 73.Rabeh WM, Cook PF. Structure and mechanism of O-acetylserine sulfhydrylase. Journal of Biological Chemistry. 2004;279:26803–26806. doi: 10.1074/jbc.R400001200. [DOI] [PubMed] [Google Scholar]
- 74.Daum S, Tai CH, Cook PF. Characterization of the S272A, D site-directed mutations of O-acetylserine sulfhydrylase: Involvement of the pyridine ring in the alpha, beta-elimination reaction. Biochemistry. 2003;42:106–113. doi: 10.1021/bi0268044. [DOI] [PubMed] [Google Scholar]
- 75.Lai J, Niks D, Wang Y, Domratcheva T, Barends TR, Schwarz F, Olsen RA, Elliott DW, Fatmi MQ, Chang CE, Schlichting I, Dunn MF, Mueller LJ. X-ray and NMR crystallography in an enzyme active site: the indoline quinonoid intermediate in tryptophan synthase. J Am Chem Soc. 2011;133:4–7. doi: 10.1021/ja106555c. [DOI] [PubMed] [Google Scholar]
- 76.Barends TR, Domratcheva T, Kulik V, Blumenstein L, Niks D, Dunn MF, Schlichting I. Structure and mechanistic implications of a tryptophan synthase quinonoid intermediate. Chembiochem. 2008;9:1024–1028. doi: 10.1002/cbic.200700703. [DOI] [PubMed] [Google Scholar]
- 77.Hur O, Niks D, Casino P, Dunn MF. Proton transfers in the beta-reaction catalyzed by tryptophan synthase. Biochemistry. 2002;41:9991–10001. doi: 10.1021/bi025568u. [DOI] [PubMed] [Google Scholar]
- 78.Woehl EU, Dunn MF. Monovalent metal ions play an essential role in catalysis and intersubunit communication in the tryptophan synthase bienzyme complex. Biochemistry. 1995;34:9466–9476. doi: 10.1021/bi00029a023. [DOI] [PubMed] [Google Scholar]
- 79.Brzovic PS, Hyde CC, Miles EW, Dunn MF. Characterization of the functional role of a flexible loop in the alpha-subunit of tryptophan synthase from Salmonella typhimurium by rapid-scanning, stopped-flow spectroscopy and site-directed mutagenesis. Biochemistry. 1993;32:10404–10413. doi: 10.1021/bi00090a016. [DOI] [PubMed] [Google Scholar]
- 80.Drewe WF, Jr, Koerber SC, Dunn MF. Application of rapid-scanning, stopped-flow spectroscopy to the characterization of intermediates formed in the reactions of L- and D-tryptophan and beta-mercaptoethanol with Escherichia coli tryptophan synthase. Biochimie. 1989;71:509–519. doi: 10.1016/0300-9084(89)90182-x. [DOI] [PubMed] [Google Scholar]
- 81.Roy M, Miles EW, Phillips RS, Dunn MF. Detection and identification of transient intermediates in the reactions of tryptophan synthase with oxindolyl-L-alanine and 2,3-dihydro-L-tryptophan. Evidence for a tetrahedral (gem-diamine) intermediate. Biochemistry. 1988;27:8661–8669. doi: 10.1021/bi00423a023. [DOI] [PubMed] [Google Scholar]
- 82.Drewe WF, Jr, Dunn MF. Characterization of the reaction of L-serine and indole with Escherichia coli tryptophan synthase via rapid-scanning ultraviolet-visible spectroscopy. Biochemistry. 1986;25:2494–2501. doi: 10.1021/bi00357a032. [DOI] [PubMed] [Google Scholar]
- 83.Drewe WF, Jr, Dunn MF. Detection and identification of intermediates in the reaction of L-serine with Escherichia coli tryptophan synthase via rapid-scanning ultraviolet-visible spectroscopy. Biochemistry. 1985;24:3977–3987. doi: 10.1021/bi00336a027. [DOI] [PubMed] [Google Scholar]
- 84.Jhee KH, Yang LH, Ahmed SA, McPhie P, Rowlett R, Miles EW. Mutation of an active site residue of tryptophan synthase (beta-serine 377) alters cofactor chemistry. J Biol Chem. 1998;273:11417–11422. doi: 10.1074/jbc.273.19.11417. [DOI] [PubMed] [Google Scholar]
- 85.Campanini B, Raboni S, Vaccari S, Zhang L, Cook PF, Hazlett TL, Mozzarelli A, Bettati S. Surface-exposed tryptophan residues are essential for O-acetylserine sulfhydrylase structure, function, and stability. Journal of Biological Chemistry. 2003;278:37511–37519. doi: 10.1074/jbc.M305138200. [DOI] [PubMed] [Google Scholar]
- 86.Onuffer JJ, Kirsch JF. Characterization of the apparent negative co-operativity induced in Escherichia coli aspartate aminotransferase by the replacement of Asp222 with alanine. Evidence for an extremely slow conformational change. Protein Eng. 1994;7:413–424. doi: 10.1093/protein/7.3.413. [DOI] [PubMed] [Google Scholar]
- 87.Yano T, Hinoue Y, Chen VJ, Metzler DE, Miyahara I, Hirotsu K, Kagamiyama H. Role of an active site residue analyzed by combination of mutagenesis and coenzyme analog. J Mol Biol. 1993;234:1218–1229. doi: 10.1006/jmbi.1993.1672. [DOI] [PubMed] [Google Scholar]
- 88.Yano T, Kuramitsu S, Tanase S, Morino Y, Kagamiyama H. Role of Asp222 in the catalytic mechanism of Escherichia coli aspartate aminotransferase: the amino acid residue which enhances the function of the enzyme-bound coenzyme pyridoxal 5'-phosphate. Biochemistry. 1992;31:5878–5887. doi: 10.1021/bi00140a025. [DOI] [PubMed] [Google Scholar]
- 89.Gong J, Hunter GA, Ferreira GC. Aspartate-279 in aminolevulinate synthase affects enzyme catalysis through enhancing the function of the pyridoxal 5'-phosphate cofactor. Biochemistry. 1998;37:3509–3517. doi: 10.1021/bi9719298. [DOI] [PubMed] [Google Scholar]
- 90.Osterman AL, Kinch LN, Grishin NV, Phillips MA. Acidic residues important for substrate binding and cofactor reactivity in eukaryotic ornithine decarboxylase identified by alanine scanning mutagenesis. J Biol Chem. 1995;270:11797–11802. doi: 10.1074/jbc.270.20.11797. [DOI] [PubMed] [Google Scholar]
- 91.Griswold WR, Toney MD. Chemoenzymatic synthesis of 1-deaza-pyridoxal 5'-phosphate. Bioorg Med Chem Lett. 2010;20:1352–1354. doi: 10.1016/j.bmcl.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seebeck FP, Guainazzi A, Amoreira C, Baldridge KK, Hilvert D. Stereoselectivity and expanded substrate scope of an engineered PLP-dependent aldolase. Angew Chem Int Ed Engl. 2006;45:6824–6826. doi: 10.1002/anie.200602529. [DOI] [PubMed] [Google Scholar]
- 93.Seebeck FP, Hilvert D. Conversion of a PLP-dependent racemase into an aldolase by a single active site mutation. J Am Chem Soc. 2003;125:10158–10159. doi: 10.1021/ja036707d. [DOI] [PubMed] [Google Scholar]
- 94.Jiraskova-Vanickova J, Ettrich R, Vorlova B, Hoffman HE, Lepsik M, Jansa P, Konvalinka J. Inhibition of Human Serine Racemase, an Emerging Target for Medicinal Chemistry. Curr Drug Targets. 2011 doi: 10.2174/138945011795677755. [DOI] [PubMed] [Google Scholar]
- 95.Gogami Y, Kobayashi A, Ikeuchi T, Oikawa T. Site-directed mutagenesis of rice serine racemase: evidence that Glu219 and Asp225 mediate the effects of Mg2+ on the activity. Chem Biodivers. 2010;7:1579–1590. doi: 10.1002/cbdv.200900257. [DOI] [PubMed] [Google Scholar]
- 96.Mori H, Inoue R. Serine racemase knockout mice. Chem Biodivers. 2010;7:1573–1578. doi: 10.1002/cbdv.200900293. [DOI] [PubMed] [Google Scholar]
- 97.Foltyn VN, Zehl M, Dikopoltsev E, Jensen ON, Wolosker H. Phosphorylation of mouse serine racemase regulates D-serine synthesis. FEBS Lett. 2010;584:2937–2941. doi: 10.1016/j.febslet.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 98.Mustafa AK, Ahmad AS, Zeynalov E, Gazi SK, Sikka G, Ehmsen JT, Barrow RK, Coyle JT, Snyder SH, Dore S. Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J Neurosci. 2010;30:1413–1416. doi: 10.1523/JNEUROSCI.4297-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith MA, Mack V, Ebneth A, Moraes I, Felicetti B, Wood M, Schonfeld D, Mather O, Cesura A, Barker J. The structure of mammalian serine racemase: evidence for conformational changes upon inhibitor binding. J Biol Chem. 2010;285:12873–12881. doi: 10.1074/jbc.M109.050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goto M, Yamauchi T, Kamiya N, Miyahara I, Yoshimura T, Mihara H, Kurihara T, Hirotsu K, Esaki N. Crystal structure of a homolog of mammalian serine racemase from Schizosaccharomyces pombe. J Biol Chem. 2009;284:25944–25952. doi: 10.1074/jbc.M109.010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AH, Roder JC. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet. 2009;18:3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoffman HE, Jiraskova J, Ingr M, Zvelebil M, Konvalinka J. Recombinant human serine racemase: enzymologic characterization and comparison with its mouse ortholog. Protein Expr Purif. 2009;63:62–67. doi: 10.1016/j.pep.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Strisovsky K, Jiraskova J, Mikulova A, Rulisek L, Konvalinka J. Dual substrate and reaction specificity in mouse serine racemase: identification of high-affinity dicarboxylate substrate and inhibitors and analysis of the beta-eliminase activity. Biochemistry. 2005;44:13091–13100. doi: 10.1021/bi051201o. [DOI] [PubMed] [Google Scholar]
- 104.Strisovsky K, Jiraskova J, Barinka C, Majer P, Rojas C, Slusher BS, Konvalinka J. Mouse brain serine racemase catalyzes specific elimination of L-serine to pyruvate. FEBS Lett. 2003;535:44–48. doi: 10.1016/s0014-5793(02)03855-3. [DOI] [PubMed] [Google Scholar]
- 105.Neidle A, Dunlop DS. Allosteric regulation of mouse brain serine racemase. Neurochem Res. 2002;27:1719–1724. doi: 10.1023/a:1021607715824. [DOI] [PubMed] [Google Scholar]
- 106.De Miranda J, Santoro A, Engelender S, Wolosker H. Human serine racemase: moleular cloning, genomic organization and functional analysis. Gene. 2000;256:183–188. doi: 10.1016/s0378-1119(00)00356-5. [DOI] [PubMed] [Google Scholar]
- 107.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr, Ferris CD, Snyder SH. Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci U S A. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tate SS, Meister A. Regulation and subunit structure of aspartate beta-decarboxylase. Studies on the enzymes from Alcaligenes faecalis and Pseudomonas dacunhae. Biochemistry. 1970;9:2626–2632. doi: 10.1021/bi00815a010. [DOI] [PubMed] [Google Scholar]
- 110.Tate SS, Meister A. L-aspartate-beta-decarboxylase: structure, catalytic activities, and allosteric regulation. Adv Enzymol Relat Areas Mol Biol. 1971;35:503–543. doi: 10.1002/9780470122808.ch9. [DOI] [PubMed] [Google Scholar]
- 111.Kakimoto T, Kato J, Shibatani T, Nishimura N, Chibata I. Crystalline L-aspartate beta-decarboxylase of Pseudomonas dacunhae. II. Subunit and amino acid composition. J Biol Chem. 1970;245:3369–3374. [PubMed] [Google Scholar]
- 112.Bowers WF, Czubaroff VB, Haschemeyer RH. Subunit structure of L-aspartate beta-decarboxylase from Alcaligenes faecalis. Biochemistry. 1970;9:2620–2625. doi: 10.1021/bi00815a009. [DOI] [PubMed] [Google Scholar]
- 113.Palekar AG, Tate SS, Meister A. Inhibition of aspartate beta-decarboxylase by aminomalonate. Stereospecific decarboxylation of aminomalonate to glycine. Biochemistry. 1970;9:2310–2315. doi: 10.1021/bi00813a014. [DOI] [PubMed] [Google Scholar]
- 114.Tate SS, Relyea NM, Meister A. Interaction of L-aspartate beta-decarboxylase with beta-chloro-L-alanine. Beta-elimination reaction and active-site labeling. Biochemistry. 1969;8:5016–5021. doi: 10.1021/bi00840a051. [DOI] [PubMed] [Google Scholar]
- 115.Tate SS, Meister A. Regulation of the activity of L-aspartate beta-decarboxylase by a novel allosteric mechanism. Biochemistry. 1969;8:1660–1668. doi: 10.1021/bi00832a048. [DOI] [PubMed] [Google Scholar]
- 116.Tate SS, Meister A. The effects of various vitamin B6 5'-phosphate derivatives on the structure and activities of L-aspartate beta-decarboxylase. Biochemistry. 1969;8:1056–1065. doi: 10.1021/bi00831a037. [DOI] [PubMed] [Google Scholar]
- 117.Kakimoto T, Kato J, Shibatani T, Nishimura N, Chibata I. Crystalline L-aspartate beta-decarboxylase of Pseudomonas dacunhae. I. Crystallization and some physiocochemical properties. J Biol Chem. 1969;244:353–358. [PubMed] [Google Scholar]
- 118.Chibata I, Kakimoto T, Kato J, Shibatani T, Nishimura N. On the activation mechanism of L-aspartate beta-decarboxylase from Pseudomonas dacunhae by alpha-ketoglutarate. Biochem Biophys Res Commun. 1968;32:375–379. doi: 10.1016/0006-291x(68)90670-0. [DOI] [PubMed] [Google Scholar]
- 119.Miles EW, Meister A. The mechanism of the reaction of beta-hydroxyaspartate with L-aspartate beta-decarboxylase. A new type of pyridoxal 5'-phosphate-enzyme inhibition. Biochemistry. 1967;6:1734–1743. doi: 10.1021/bi00858a023. [DOI] [PubMed] [Google Scholar]
- 120.Novogrodsky A, Meister A. Control of Aspartate Beta-Decarboxylase Activity by Transamination. J Biol Chem. 1964;239:879–888. [PubMed] [Google Scholar]
- 121.Wang NC, Ko TP, Lee CY. Inactive S298R disassembles the dodecameric L-aspartate 4-decarboxylase into dimers. Biochem Biophys Res Commun. 2008;374:134–137. doi: 10.1016/j.bbrc.2008.06.110. [DOI] [PubMed] [Google Scholar]
- 122.Wang NC, Lee CY. Enhanced transaminase activity of a bifunctional L-aspartate 4-decarboxylase. Biochem Biophys Res Commun. 2007;356:368–373. doi: 10.1016/j.bbrc.2007.02.141. [DOI] [PubMed] [Google Scholar]
- 123.Wang NC, Lee CY. Molecular cloning of the aspartate 4-decarboxylase gene from Pseudomonas sp. ATCC 19121 and characterization of the bifunctional recombinant enzyme. Appl Microbiol Biotechnol. 2006;73:339–348. doi: 10.1007/s00253-006-0475-6. [DOI] [PubMed] [Google Scholar]
- 124.Rathod PK, Fellman JH. Identification of mammalian aspartate-4-decarboxylase. Arch Biochem Biophys. 1985;238:435–446. doi: 10.1016/0003-9861(85)90184-5. [DOI] [PubMed] [Google Scholar]
- 125.Rathod PK, Fellman JH. Regulation of mammalian aspartate-4-decarboxylase: its possible role in oxaloacetate and energy metabolism. Arch Biochem Biophys. 1985;238:447–451. doi: 10.1016/0003-9861(85)90185-7. [DOI] [PubMed] [Google Scholar]
- 126.Lima S, Sundararaju B, Huang C, Khristoforov R, Momany C, Phillips RS. The crystal structure of the Pseudomonas dacunhae aspartate-beta-decarboxylase dodecamer reveals an unknown oligomeric assembly for a pyridoxal-5'-phosphate-dependent enzyme. J Mol Biol. 2009;388:98–108. doi: 10.1016/j.jmb.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 127.Chen HJ, Ko TP, Lee CY, Wang NC, Wang AH. Structure, assembly, and mechanism of a PLP-dependent dodecameric L-aspartate beta-decarboxylase. Structure. 2009;17:517–529. doi: 10.1016/j.str.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 128.Phillips RS, Lima S, Khristoforov R, Sudararaju B. Insights into the mechanism of Pseudomonas dacunhae aspartate beta-decarboxylase from rapid-scanning stopped-flow kinetics. Biochemistry. 2010;49:5066–5073. doi: 10.1021/bi100272g. [DOI] [PubMed] [Google Scholar]
- 129.Rosenberg RM, O'Leary MH. Aspartate beta-decarboxylase from Alcaligenes faecalis: carbon-13 kinetic isotope effect and deuterium exchange experiments. Biochemistry. 1985;24:1598–1603. doi: 10.1021/bi00328a004. [DOI] [PubMed] [Google Scholar]
- 130.Graber R, Kasper P, Malashkevich VN, Strop P, Gehring H, Jansonius JN, Christen P. Conversion of aspartate aminotransferase into an L-aspartate beta-decarboxylase by a triple active-site mutation. J Biol Chem. 1999;274:31203–31208. doi: 10.1074/jbc.274.44.31203. [DOI] [PubMed] [Google Scholar]
- 131.Murakawa T, Machida Y, Hayashi H. Product-assisted catalysis as the basis of the reaction specificity of threonine synthase. J Biol Chem. 2011;286:2774–2784. doi: 10.1074/jbc.M110.186205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Covarrubias AS, Hogbom M, Bergfors T, Carroll P, Mannerstedt K, Oscarson S, Parish T, Jones TA, Mowbray SL. Structural, biochemical, and in vivo investigations of the threonine synthase from Mycobacterium tuberculosis. J Mol Biol. 2008;381:622–633. doi: 10.1016/j.jmb.2008.05.086. [DOI] [PubMed] [Google Scholar]
- 133.Mas-Droux C, Biou V, Dumas R. Allosteric threonine synthase. Reorganization of the pyridoxal phosphate site upon asymmetric activation through S-adenosylmethionine binding to a novel site. J Biol Chem. 2006;281:5188–5196. doi: 10.1074/jbc.M509798200. [DOI] [PubMed] [Google Scholar]
- 134.Omi R, Goto M, Miyahara I, Mizuguchi H, Hayashi H, Kagamiyama H, Hirotsu K. Crystal structures of threonine synthase from Thermus thermophilus HB8: conformational change, substrate recognition, and mechanism. J Biol Chem. 2003;278:46035–46045. doi: 10.1074/jbc.M308065200. [DOI] [PubMed] [Google Scholar]
- 135.Garrido-Franco M, Ehlert S, Messerschmidt A, Marinkovic S, Huber R, Laber B, Bourenkov GP, Clausen T. Structure and function of threonine synthase from yeast. J Biol Chem. 2002;277:12396–12405. doi: 10.1074/jbc.M108734200. [DOI] [PubMed] [Google Scholar]
- 136.Thomazeau K, Curien G, Dumas R, Biou V. Crystal structure of threonine synthase from Arabidopsis thaliana. Protein Sci. 2001;10:638–648. doi: 10.1110/ps.44301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Laber B, Maurer W, Hanke C, Grafe S, Ehlert S, Messerschmidt A, Clausen T. Characterization of recombinant Arabidopsis thaliana threonine synthase. Eur J Biochem. 1999;263:212–221. doi: 10.1046/j.1432-1327.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- 138.Curien G, Job D, Douce R, Dumas R. Allosteric activation of Arabidopsis threonine synthase by S-adenosylmethionine. Biochemistry. 1998;37:13212–13221. doi: 10.1021/bi980068f. [DOI] [PubMed] [Google Scholar]
- 139.Laber B, Gerbling KP, Harde C, Neff KH, Nordhoff E, Pohlenz HD. Mechanisms of interaction of Escherichia coli threonine synthase with substrates and inhibitors. Biochemistry. 1994;33:3413–3423. doi: 10.1021/bi00177a035. [DOI] [PubMed] [Google Scholar]