Abstract
AIM
To test the hypothesis that amblyopic neuroretina may have an altered thickness when compared to the normal.
METHODS
Twenty-five amblyopic, young patients between the ages of 7 and 11 years old were studied. The interested neuroretina areas are defined into 10 sub-regions according to superior-inferior, nasal-temoral, and peri-para axis, which cross the fovela structure. The thicknesses of ten, defined macular regions were separately measured by optical coherence tomography (OCT) and analyzed by t-test.
RESULTS
The average thickness of neuroretina in the exact foveola of the amblyopic eyes is larger than that of normal eyes (P<0.05), but the other nine regions have no significant difference. Interestingly, in both the normal and amblyopic eyes, the temporal area looks thinner than other quadrants (P<0.05).
CONCLUSION
Thickness alteration may be associated with amblyopic disorders in young patients. Studying a larger volume of subjects of similar age is required to confirm this observation.
Keywords: amblyopia, macula flava, optical coherence tomography
INTRODUCTION
Amblyopia is defined as reduced best-corrected visual acuity in one or both eyes in response to abnormal visual stimulation during a critical period of development of the visual areas of the brain. The locus of the amblyopic deficit has been sought for the past half century, prompting controversy as to whether the origin is retinal or cortical. Despite some early studies indicating that the retina might be a primary site of pathology in amblyopia, the conclusions reached from a wide range of animal studies and electrophysiological investigations in human subjects are that the retina demonstrates essentially normal physiologic functions in the presence of amblyopia[1].
The current answer is not the final word, however, as many questions still remain. One may ask whether the retina of an amblyope is absolutely normal, or if the abnormality in the visual cortex is the primary and only site of amblyopia. Recent advances in neuroanatomy and neurophysiology have reopened the possibility that there is some retinal dysfunction in amblyopia[1].
Optical coherence tomography (OCT) is an imaging technique that produces high-resolution (within 10-20 µm) cross-sectional images of the retina, allowing noncontact and noninvasive in vivo imaging[2],[3]. The advantages of OCT allow for numerous investigations of the deficits associated with amblyopia in various parts of the retina. However the results of these studies, however, do not agree. Kee and colleagues[4] used OCT to assess and compare the thicknesses of the fovea and the retinal nerve fiber layer (RNFL) of the peripapillary region in 26 children with unilateral amblyopia due to anisometropia or strabismus. They found no significant differences between normal and amblyopic eyes in either the fovea or in the retinal nerve fiber layer. The average thicknesses of the fovea and the retinal nerve fiber layer, however, showed statistically significant differences between the children with anisometropic amblyopia and those with strabismic amblyopia. Altintas[5] compared using OCT, the thickness of the retinal nerve fiber layer (RNFL), the macular volume, and the macular thickness of amblyopic eyes with non-amblyopic eyes in patients with unilateral strabismic amblyopia, showing no significant differences between the two eyes. The retinal neuroepithelial layer in the foveola was not included in their investigation, however.
Yen and associates[6] found RNFL with OCT to be thicker in amblyopic eyes compared with the sound eyes of children with anisometropic amblyopia, but they found no difference in children with strabismic amblyopia. Lempert[7] studied photographs of the optic nerves in human eyes with amblyopia and found an association between reduced disk area and axial length. He noted that the optic disks of hypermetropic eyes with strabismus (with and without amblyopia) were disproportionately smaller compared to those in hypermetropic eyes without amblyopia or esotropia[8]. He speculated that vision impairment in amblyopia is associated with optic nerve hypoplasia and relative microphthalmos.
All of these studies ignored the subtle architecture of the macula, especially the foveola. Because investigations of amblyopic dysfunction have always been related to deficits in the macula, we concentrated our attention on the characteristics of the macula in amblyopic eyes and obtained some pilot morphological data.
MATERIALS AND METHODS
Subjects
This study was approved by the Ethics Committee of Sun Yat-sen University in P.R. China and complied with the tenets of the Declaration of Helsinki for Research Involving Human Tissue. Twenty-five patients between the ages of 7 and 11 were included in this study, which was designed as a prospective observational case series and conducted between November 2004 and March 2005. Written informed consent was obtained from the parents.
The primary inclusion criterion was a preliminary diagnosis of unilateral amblyopia due to anisometropia. Exclusion criteria included a diagnosis of strabismus or other structural abnormalities of the eye. All patients had foveal fixation. A total of 12 males and 13 females were included in the study, with a mean age±standard deviation (SD) of 8.82±1.47 years (range=7-11 years). Refraction was determined from objective retinoscopic findings under cycloplegia, subtracting a 1.5D working distance correction or the subjective refraction. The visual results were quantified by using the logarithm of the minimal angle of resolution (logMAR). In better eyes, the best-corrected visual acuity ≥0(Snellen 20/20) and refraction error (astigmatism) were converted into the spherical equivalent, which ranged from -1.75 DS to +2.25 DS, with a mean diopter ± standard deviation (SD) of + 0.56±1.11 DS. In the amblyopic eyes (15 right eyes and 10 left eyes), the best-corrected visual acuities ranged from 0.2 to1.0 logMAR with a mean age±standard deviation (SD) of 0.43±0.23 logMAR. Snellen acuities ranged between 20/200 and 20/32, and refraction errors ranged between -5.75DS and +5.50DS, with a mean diopter±standard deviation (SD) of +0.78±3.81DS. The difference in refractive error between the two eyes was at least 1 diopter; the difference in corrected visual acuity between the two eyes was at least 3 logMAR units.
Methods
Equipment
OCT images were obtained using the OCT 2000 (Carl Zeiss Meditec, Inc., Dublin, California, USA), with software version 4.0.
Scanning parameters
Regular macula scanning protocols of the high-density type with a typical 2-mm deep and 6-mm wide image was performed for macular scans. The resolution was 10µm.
Image and data processing
All eyes were scanned by the same trained operators following pupillary dilation to a diameter of at least 5mm. Internal fixation was used for all patients. The macular scan was composed of six linear scans centered at the fovea and equally spaced 30° apart. For macular thickness map analysis, a modified Early Treatment Diabetic Retinopathy Study (ETDRS) grid was generated (Figure 1), with nine sectoral thickness values in three concentric circles with diameters of 1, 3, and 6 mm, obtained from interpolation of the six linear scans. The central foveal thickness was automatically determined by the OCT software and analyzed using data from all six linear scans. The sectoral measurements in the retinal thickness map were calculated from the averaged data from the six individual scans. The thickness of the foveola was defined as the distance between the innermost and outermost foveolar surfaces, and was measured using the manually assisted technique of the program contained within the OCT system software. When the foveal center on the image was difficult to determine, the fixation point was regarded as the foveal center.
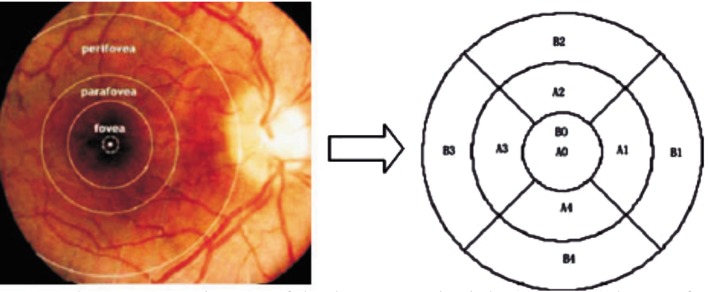
Figure 1. Sectoral macular thickness map.
Diameters of the three concentric circles are 1, 3, and 6 mm from center to outer circle respectively. Areas represented are the foveola (A0), the center of the fovea (B0; diameter = 1000 microns); the nasal, superior, temporal, and inferior quadrants of the parafoveal area (A1-A4; diameter = 3000 microns); and the nasal, superior, temporal, and inferior quadrants of the perifoveal area (B1-B4; diameter = 6000 microns).
Statistical Analysis
Quantitative data was expressed as the mean±SD. The mean values of the amblyopic and better eyes were compared with a paired t test. The average thickness of the neurosensory retinal layer in the nasal, superior, temporal, and inferior quadrants of the parafoveal and perifoveal regions was analyzed by the least significant difference (LSD) test. The correlation between the thickness of the neurosensory retinal layer in the central foveola and the diopter of refractive error in amblyopic eyes was analyzed with Pearson's 2-tailed test. P<0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 12.0 software package (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Main Characteristics of macula on OCT
Measurement using OCT showed that, on the whole, macular thickness in amblyopic eyes is similar to that in better eyes. The macula is thinnest at the foveola (157.96 ± 15.82µm and 151.72±13.95µm respectively); a dark blue echogenic band indicates that only a single layer of cone nuclei is present. The neurosensory retina becomes gradually thicker from the fovea centralis to the parafoveal region, forming the foveal slope. In the perifoveal region, OCT measurement showed that the neurosensory retinal layer in the temporal area is thinner than in other quadrants in both amblyopic and better eyes (Figures 2, 3).
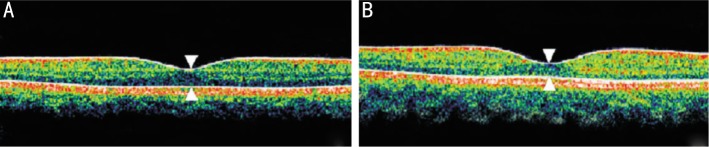
Figure 2. OCT photographs of the neurosensory retina at the center of the fovea.
A: amblyopic eye; B: Better eye; White arrow: Thickness of the neurosensory retina at the center of the fovea.
Figure 3. OCT map of the average thickness of the neurosensory retina in the macula.
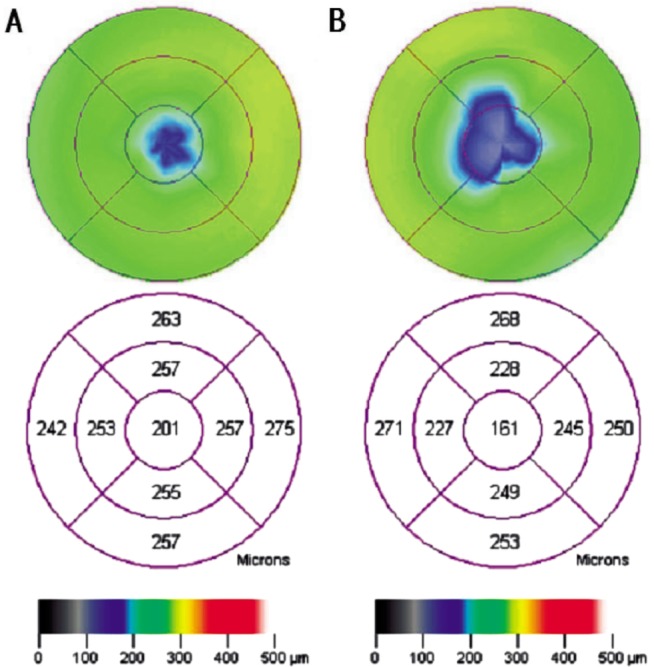
A: Amblyopic eye; B: Better eye.
Quantified measurement by OCT
The mean macular neurosensory retinal thickness of 25 imaged pairs is presented in Table 1. The mean thickness of the neurosensory retinal layer in the center of the foveola (A0) in amblyopic eyes was significantly greater than in better eyes (P<0.05). In the center of the fovea (B0), the mean thickness in amblyopic eyes was 183.56±12.63µm; the mean thickness in better eyes was 180.44±12.18µm (P=0.071). The average difference between the amblyopic eye and the better eye was 3.12±8.26µm. In 18 cases (72%) the neurosensory retinal layer was thicker in the amblyopic eye; in six cases it was thicker in the better eye; in one patient the thickness was the same in both eyes.
Table 1. Thickness of Macular Neurosensory Retina in 25 Imaged Pairs.
| Macular region | Average Thickness of Neurosensory Retina (microns) |
Difference (better eye, amblyopic eye) | P | |
| Better eye (n=25) | Amblyopic eye (n=25) | |||
| A0 | 151.72±13.95 | 157.96±15.82 | 6.24±14.75 | 0.045 |
| B0 | 180.44±12.18 | 183.56±12.63 | 3.12±8.26 | 0.071 |
| A1 | 260.14±14.83 | 261.57±15.44 | 1.64±9.05 | 0.554 |
| A2 | 258.14±14.62 | 260.43±15.74 | 2.28±10.99 | 0.450 |
| A3 | 253.86±10.17 | 255.07±10.22 | 1.21±7.76 | 0.568 |
| A4 | 262.43±11.56 | 261.64±12.87 | -0.79±16.49 | 0.861 |
| B1 | 258.43±15.06 | 262.64±9.43 | 4.21±9.30 | 0.114 |
| B2 | 245.86±14.04 | 253.64±15.01 | 8.64±14.87 | 0.164 |
| B3 | 219.43±10.96 | 219.36±12.73 | -0.71±5.90 | 0.965 |
| B4 | 226.14±12.21 | 228.29±9.02 | 2.29±8.49 | 0.357 |
(Mean±I, µm)
In the parafoveal and perifoveal regions, there was no statistically significant difference between the amblyopic and better eyes in the thickness of the superior, inferior, nasal, and temporal quadrants of the neurosensory retina (P>0.05). The least significant difference (LSD) test was used to analyze the average thickness of the neurosensory retina in the nasal, superior, temporal, and inferior quadrants. The difference was statistically significant (P=0.00) in the perifoveal region. This finding suggests that the temporal area is thinner than other quadrants in the perifoveal region.
Relationship between the thickness of the neurosensory retina in central foveola and diopter of refractive error
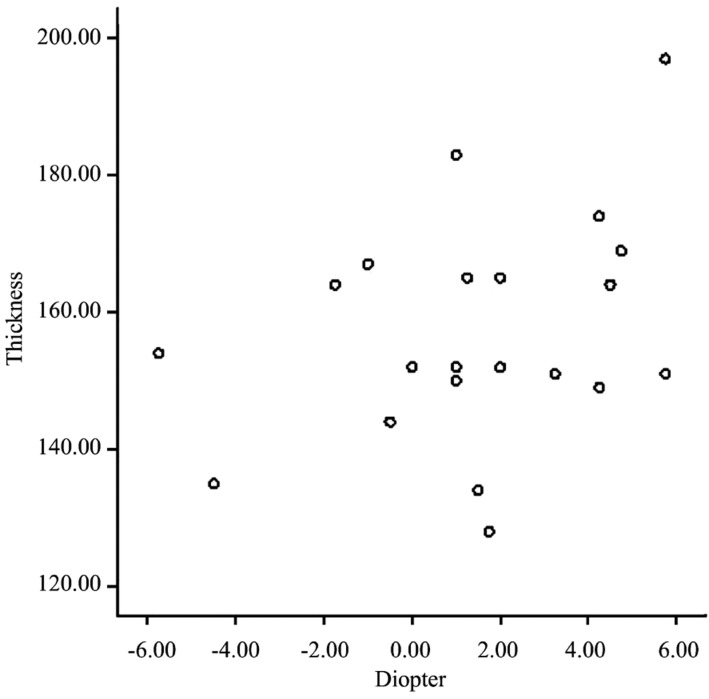
When analyzed with Pearson's 2-tailed test, the thickness of the neurosensory retina in the central foveola was not correlated with the diopter of refractive error (r =0.378; P=0.062) in amblyopic eyes (Figure 4).
Figure 4. Scatterplot of the correlation between the diopter of visual refractive error and thickness of the central foveola.
The correlation is nonsignificant (r=0.378; P=0.062).
DISCUSSION
In this study the macular thickness of the neurosensory retina in 25 subjects was measured by OCT. All subjects were first-visit patients with unilateral amblyopia due to anisometropia. Patients with strabismus were excluded to ensure foveal fixation. The age range of the subjects was restricted to 7 to 11 years to reduce the risk of errors in measuring retinal thickness because of age. Patients with a refraction error greater than ±6.0 diopters were excluded as well, making the reliability of this study excellent.
Measured by OCT, the general characteristics of the macula in amblyopic eyes are similar to those of better eyes in the parafoveal and perifoveal regions. However, OCT measurement showed increased thickness of the neurosensory layer in the center of the foveola in amblyopic eyes that was not correlated with the diopter of refractive error. This change can be attributed to the effect of amblyopia. The mean thickness of the neurosensory layer in the center of the fovea in the amblyopic eye was greater than that in the better eye but the difference was not statistically significant (P=0.071).
The results of this study partially corroborate the findings of other recent functional studies of retinal abnormalities in amblyopia. It is well-known that amblyopia is a developmental disorder that degrades spatial vision and stereopsis, and is almost always related to deficits in foveal vision. Liu and Wu[9] studied the characteristics of macular light sensitivity (MLS) in amblyopic children with an automated perimeter. They found impairment only in the 6°area of the center macular. Similarly, recent multifocal ERG studies in amblyopic eyes have reported central retinal dysfunction in amblyopia. Ju et al[10] compared the amplitude densities and latencies of multifocal electroretinograms (mfERG) in amblyopic and normal eyes. They found that the N1-wave and P1-wave amplitude densities of the mfERG first-order kernel and the P1-wave amplitude densities of the second-order kernel in amblyopic eyes were significantly attenuated in the central region of the visual field. There was no significant difference in the latencies of mfERG between the amblyopic and control eyes. They concluded that the X ganglion cells in the central fovea of amblyopic eyes are abnormal but that the visual information transmission time in the retina is normal in amblyopic eyes. All these functional studies raise the possibility that such visual abnormalities are related to selective deficits in foveal vision. This possibility corresponds with our finding of morphological change in the macula in amblyopic eyes.
It is well-known that the macula flava has a distinctive developmental trajectory during the formation of the human retina. First, the developmental course of the macula flava is protracted; it begins at 10 to 11 weeks of gestation, and, in humans, is fully mature at two or four years of age. It is the last region of the macula to reach maturity, which it does in two stages: differentiation during gestation and full development after birth. The cones in the foveola and on the foveal slope develop entirely in the postnatal period[11]. Second, some cell types are excluded or at least reduced in number from the foveal region during development of the macula flava, including rods, short-wavelength-sensitive (SWS) cones and blue-cone bipolar cells[12]-[17]. Third, non-neuronal cells (such as microglial cells) that are initially spread evenly throughout the incipient fovea migrate out of the developing foveal region. At later stages of development, both endothelial cells and astrocytes are prevented from migrating into the developing fovea[16]. Finally, cone nuclei form a single layer along the external limiting membrane, making the peak density of cones in the foveola approximately three times the density of cones at the base of the foveal slope[18]. These features of foveal development and architecture make this region well adapted for high acuity vision, but are also vulnerable. Any abnormality, whether in the course of gestation or postnatal development, impairs the foveola both in function and in structure. The stages at which impairments occur and whether these impairments are primary or secondary from the visual cortex have yet to be studied and researched.
Footnotes
Foundation items: Supported by Key Projects in the Medicine Health Science and Technology from Guangzhou, China (No.201102A211005); Fundamental Research Funds of State Key Laboratory of Ophthalmology, China
REFERENCES
- 1.Hess RF. Amblyopia: site unseen. Clin Exp Optom. 2001;84:321–336. doi: 10.1111/j.1444-0938.2001.tb06604.x. [DOI] [PubMed] [Google Scholar]
- 2.Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, Puliafito CA, Fujimoto JG. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–332. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 3.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kee SY, Lee SY, Lee YC. Thicknesses of the fovea and retinal nerve fiber layer in amblyopic and normal eyes in children. Korean J Ophthalmol. 2006;20:177–181. doi: 10.3341/kjo.2006.20.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altintas O, Yuksel N, Ozkan B. Thickness of the retinal nerve fiber layer, macular thickness, and macular volume in patients with strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2005;42:216–221. doi: 10.3928/01913913-20050701-03. [DOI] [PubMed] [Google Scholar]
- 6.Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004;45:2224–2230. doi: 10.1167/iovs.03-0297. [DOI] [PubMed] [Google Scholar]
- 7.Lempert P. The axial length/disc area ratio in anisometropic hyperopic amblyopia: a hypothesis for decreased unilateral vision associated with hyperopic anisometropia. Ophthalmology. 2004;111:304–308. doi: 10.1016/j.ophtha.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Lempert P. Axial length-disc area ratio in esotropic amblyopia. Arch Ophthalmol. 2003;121:821–824. doi: 10.1001/archopht.121.6.821. [DOI] [PubMed] [Google Scholar]
- 9.Liu SZ, Wu XY. Macular light sensitivity in amblyopic children. Zhongguo Xieshi Yu Xiaoer Yanke Zazhi. :112–114. [Google Scholar]
- 10.Ju H, Zhao KX, Zhou N, Zhang W. Investigation of multifocal electroretinograms in amblyopia. Zhonghua Yanke Zazhi. 2004;40:655–662. [PubMed] [Google Scholar]
- 11.Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- 12.Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986;26:847–855. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- 13.Provis JM, van Driel D, Billson FA, Russell P. Development of the human retina: patterns of cell distribution and redistribution in the ganglion cell layer. J Comp Neurol. 1985;233:429–451. doi: 10.1002/cne.902330403. [DOI] [PubMed] [Google Scholar]
- 14.Bumsted K, Hendrickson A. Distribution and development of short-wavelength cones differ between Macaca monkey and human fovea. J Comp Neurol. 1999;403:502–516. [PubMed] [Google Scholar]
- 15.Diaz-Araya C, Provis JM. Evidence of photoreceptor migration during early foveal development: a quantitative analysis of human fetal retinae. Vis Neurosci. 1992;8:505–514. doi: 10.1017/s0952523800005605. [DOI] [PubMed] [Google Scholar]
- 16.Dorn EM, Hendrickson L, Hendrickson AE. The appearance of rod opsin during monkey retinal development. Invest Ophthalmol Vis Sci. 1995;36:2634–2651. [PubMed] [Google Scholar]
- 17.Provis JM, Sandercoe T, Hendrickson AE. Astrocytes and blood vessels define the foveal rim during primate retinal development. Invest Ophthalmol Vis Sci. 2000;41:2827–2836. [PubMed] [Google Scholar]
- 18.Curcio CA, Allen KA, Sloan KR, et al. et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]