Abstract
AIM
To evaluate the efficacy and safety of corneal collagen crosslinking (CXL) to prevent the progression of post-laser in situ keratomileusis (LASIK) corneal ectasia.
METHODS
In a prospective, nonrandomized, single-centre study, CXL was performed in 20 eyes of 11 patients who had LASIK for myopic astigmatism and subsequently developed keratectasia.The procedure included instillation of 0.1% riboflavin-20% dextrane solution 30 minutes before UVA irradiation and every 5 minutes for an additional 30 minutes during irradiation. The eyes were evaluated preoperatively and at 1-, 3-, 6-, and 12-month intervals. The complete ophthalmologic examination comprised uncorrected visual acuity, best spectacle-corrected visual acuity, endothelial cell count, ultrasound pachymetry, corneal topography, and in vivo confocal microscopy.
RESULTS
CXL appeared to stabilise or partially reverse the progression of post-LASIK corneal ectasia without apparent complication in our cohort. UCVA and BCVA improvements were statistically significant(P<0.05) beyond 12 months after surgery (improvement of 0.07 and 0.13 logMAR at 1 year, respectively). Mean baseline flattest meridian keratometry and mean steepest meridian keratometry reduction (improvement of 2.00 and 1.50 diopters(D), respectively) were statistically significant (P<0.05) at 12 months postoperatively. At 1 year after CXL, mean endothelial cell count did not deteriorate. Mean thinnest cornea pachymetry increased significantly.
CONCLUSION
The results of the study showed a long-term stability of post-LASIK corneal ectasia after crosslinking without relevant side effects. It seems to be a safe and promising procedure to stop the progression of post-LASIK keratectasia, thereby avoiding or delaying keratoplasty.
Keywords: crosslinking, keratoconus, ultraviolet, cornea, ectasia, laser in situ keratomileusis
INTRODUCTION
Post-laser in situ keratomileusis (LASIK) corneal ectasia is one of the most serious side effects of refractive surgery. The disease is characterized by a progressive thinning and steepening of the central and inferior portions of the cornea[1]. Until recently, treatment options were limited. In addition to rigid contact lenses, insertion of intrastromal rings might help mechanically stabilize the cornea[2]. Some cases may need for penetrating keratoplasty[3]. The latter option, however, is far from ideal.
Corneal collagen crosslinking (CXL) using a combination of riboflavin and ultraviolet A (UVA) is a new treatment modality for increasing corneal biomechanical resistance by adding additional polymer bands between collagen fibers[4],[5]. Several in vitro studies reported an increase in the biomechanical parameters of the cornea after CXL[6]–[13]. Over the past 10 years, the use of CXL has demonstrated the potential for retarding or eliminating the progression of keratoconus[5],[14]. Reduction of corneal biomechanical strength seems to be an essential element in the chain of events leading to post-LASIK corneal ectasia[15],[16]. Therefore, CXL may provide an a viable and repeatable alternative for post-LASIK corneal ectasia by stabilizing the biomechanical properties of the cornea and avoiding the need for a penetrating keratoplasty in the majority of cases.
The aim of this prospective, 12-month study was to evaluate the efficacy and safety of CXL in patients who had progressive corneal ectasia after LASIK.
MATERIALS AND METHODS
Patients
Eleven patients (20 eyes) diagnosed with post-LASIK corneal ectasia at the Navy General Hospital from February 2009 to August 2010 were chosen for this study. The cohort included 5 male patients (9 eyes) and 6 female patients (11 eyes) with a mean age of 27.4 years (range: 20 to 36 years). This ongoing study is being conducted according to the principles of the Declaration of Helsinki. It has approval from the Human Research & Ethics Committee of the Navy General Hospital and written informed consent was obtained from all patients prior to their enrollment. All operations were performed by one surgeon (Dr. Li Gang) from February 2009 to February 2011 at Navy General Hospital, Beijing, China.
Methods
Inclusion and exclusion criteria
Inclusion criteria were previous LASIK surgery, a ectasia progression indicated with the increase in maximum K readings in several consecutive recordings during a period of up to 6 months. corneal thickness of at least 400 microns at the thinnest point, and being aged between 18 to 50 years. Preoperative ectasia progression was confirmed by serial differential corneal topography and by differential optical pachymetry analysis in all eyes included in the study.
Exclusion criteria were a minimum corneal thickness<400µm at the thinnest point, a history of herpetic keratitis, concurrent corneal infections, corneal opacities, concomitant autoimmune diseases, and any previous non-excimer laser refractive ocular surgery. Pregnant or nursing women, patients with poor compliance, and patients wearing rigid gas permeable lenses for at least four weeks before baseline examination were also excluded.
Protocol
This study comprised 11 consecutive patients who had LASIK at different locations and were referred to ophthalmology of the Navy General Hospital.
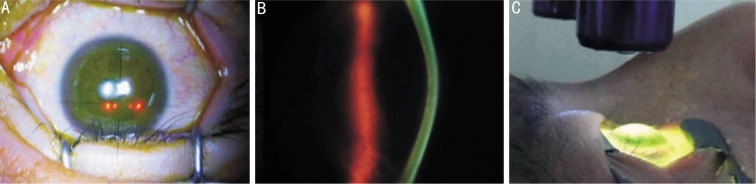
The treatment procedure was conducted under sterile conditions in an operating room. Crosslinking was performed within 2 weeks of the baseline examination using a minor modification of a protocol previously published by Wollensak et al[17]. Briefly, after oxybuprocaine 0.4% (Santen Pharmaceutical Co. Ltd., Osaka, Japan) eye drops were administered, the corneal epithelium was mechanically removed within an 8.0mm diameter exclusively(Figure 1A). Next, 0.1% riboflavin (Medio-Cross, Medio Haus Medizinprodukte GmbH, Germany) was applied every 3 minutes for approximately 30 minutes. Successful penetration of riboflavin through the cornea is ensured by visualization of riboflavin in the anterior chamber by slit-lamp biomicroscopy (Figure 1B). Ultraviolet-A irradiation was accomplished using a commercially available UVA system (XLAMP, Colombia). Prior to treatment, intended 3 mW/cm2 surface irradiance (5.4 J/cm2 surface doses after 30 minutes) was calibrated using a UVA meter (Lasermate Q-Coherent, Santa Clara, CA, USA) at a working distance of 2cm (Figure 1C). During treatment, isotonic riboflavin 0.1% solution and a topical anesthetic agent (oxybuprocaine 0.4%) are administered every 3 minutes to saturate the cornea with riboflavin.
Figure 1. A: The corneal epithelium was mechanically removed within an 8.0 mm diameter; B: The cornea is ensured by visualization of riboflavin in the anterior chamber by slit-lamp biomicroscopy (using blue light); C: Scene of an UV-A delivery device on treatment.
After irradiation, the eye was rinsed with a sterile saline solution, ofloxacin 0.3% (Exocine; Allergan, Irvine, Calif) was applied and a bandage contact lens was inserted and retained for 3 days until re-epithelialization. Fluorometholone eye drops are then applied twice daily for 4 weeks.
Data collection
All patients in the study were scheduled for an initial consultation and follow-up at 1, 3, 6, and 12 months. The following examinations were performed before and after surgery on all patients: uncorrected visual acuity (UCVA) and best spectacle-corrected visual acuity (BSCVA), expressed in logarithm of the minimum angle of resolution (LogMAR),corneal topographic evaluation with the Obscan (Bausch & Lomb Surgical, Salt Lake City, Utah) or Pentacam (Oculus, Wetzlar, Germany), slit-lamp microscopy, direct ophthalmoscopy, noncontact tonometry (CT-80, Topcon Corp, Tokyo, Japan), ultrasound pachymetry (Pachy Meter SP3000; Tomey, Nagoya, Japan), confocal microscopy (HRT II; Heidelberg Engineering, Rostock, Germany). Three consecutive measurements were taken for corneal topography, Scheimpflug analysis, and pachymetry. For corneal topography, the measurement with the highest K value was chosen and for ultrasound pachymetry, the thinnest measurement was chosen. (Each single measurement represents the mean of 3 consecutive measurements.) Patients wearing contact lenses removed their lenses two weeks before their evaluation.
Statistical Analysis
Statistical calculations were performed using SPSS 13.0 (SPSS Inc., Chicago, USA). One-way analysis of variance (ANOVA) test and correlation analysis were used to analyze the data. Statistical significance was set at P <0.05.
RESULTS
Visual Acuity
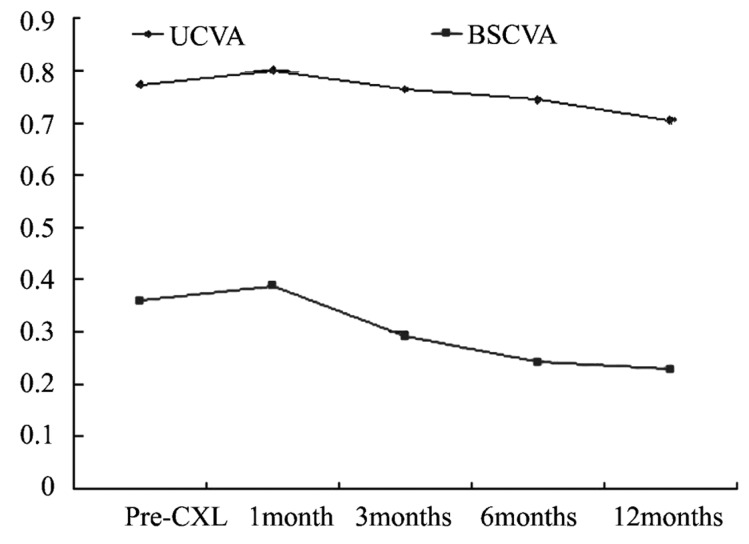
Figure 2 summarizes the UCVA and BSCVA data, expressed in logMAR and covering 12 months follow-up period. Mean baseline UCVA was 0.77±0.32. At 1 month post CXL, mean UCVA was 0.80±0.28; at 3 months, 0.76±0.30; at 6 months, 0.74±0.30; and at 12 months, 0.70±0.33. Mean baseline BSCVA was 0.36±0.30. At 1 month after the procedure, mean BSCVA was 0.39±0.30; at 3 months, 0.29±0.25; at 6 months, 0.24±0.23; and at 12 months, 0.23±0.23. Both UCVA and BSCVA slowly improved between 3 and 12 months postoperatively. The improvements in UCVA and BSCVA were statistically significant (0.07±0.07, t=4.189, P<0.01 and 0.13±0.17, t=3.470, P<0.01, respectively) at 12 months after CXL when compared with preoperative levels.
Figure 2. UCVA and BSCVA in the 20 treated eyes before and after crosslinking.
Topographic Results
Topographic results are shown in Table 1.Mean baseline flattest meridian keratometry, steepest meridian keratometry and astigmatic keratometry were 43.01±5.52D, 45.37±5.64D, and 2.36±1.47D, respectively. At 12 months, these readings were 41.57±5.02D, 43.23±5.37D, and 1.66±0.70D, respectively, a difference that was statistically significant for the flattest and steepest meridian keratometry (1.45±1.72D, t=3.765, P<0.01, and 2.14±1.23D, t=7.803, P<0.01, respectively), but for astigmatic keratometry the difference was not statistically significant (0.58±1.04D, t=1.588, P=0.133).
Table 1. Clinical characteristics of the post-LASIK corneal ectasia patients.
| Mean standard deviation |
|||||
| Before CXL | 1 Month | 3 Months | 6 Months | 12 Months | |
| K-max (D) | 45.37±5.64 | 45.77±5.42 | 45.49±5.55 | 44.42±5.69 | 43.23±5.37 |
| K-min (D) | 43.01±5.52 | 43.55±5.01 | 43.69±5.19 | 42.55±5.13 | 41.57±5.02 |
| Astigmatism (D) | 2.36±1.47 | 2.22±1.20 | 1.65±1.25 | 1.86±1.18 | 1.66±0.70 |
| CT(µm) | 436.10±32.15 | 420.60±37.14 | 426.65±32.13 | 439.35±27.68 | 448.50±22.61 |
| EC (cells/mm2) | 2538±525 | 2611±416 | 2511±494 | 2461±453 | 2610±438 |
| IOP(mmHg) | 10.45±2.37 | 11.70±2.22 | 11.45±1.73 | 12.60±2.62 | 13.30±2.41 |
CXL: Cross-linking; K-max: Steepest meridian keratometry; K-min: Flattest meridian keratometry; CT=Corneal thinnest pachymetry; EC: Endothelial cell count; IOP: Intraocular pressure.
Pachymetry Results
Pachymetry results are shown in Table 1.There was a significant decrease in thinnest pachymetry between baseline and 1 month (mean change -15.50±21.47µm; t=-3.23, P<0.01). This was followed by a slowly increase from 3 months to 12 months. The mean increase in thinnest pachymetry from baseline to 12months was statistically significant(mean change 12.40±18.49µm; t=3.00, P<0.01 ).
Endothelial cell count results
Mean baseline endothelial cell count was 2538±525 cell/mm2. One month after the procedure, it was 2611±416 cell/mm2, at 3 months 2511±494 cell/mm2, at 6 months 2461±453 cell/mm2, and at 12 months endothelial cell count was 2610±438 cell/mm2. The difference between baseline and 12 months was not significant (73±209, t=1.55, P=0.138), indicating that CXL did not induce endothelial damage in the 1-year follow-up period(Table 1).
Intraocular pressure(IOP) results
Mean baseline IOP was 10.45±2.37mmHg (Table 1). One month after the procedure, it was 11.70±2.22mmHg, at 3 months 11.45±1.73mmHg, at 6 months 12.60±2.62mmHg, and at 12 months IOP was 13.30±2.41mmHg. The difference between baseline and 12 months was significant (2.85±2.25, t=5.654, P<0.001).
Biomicroscopy Results
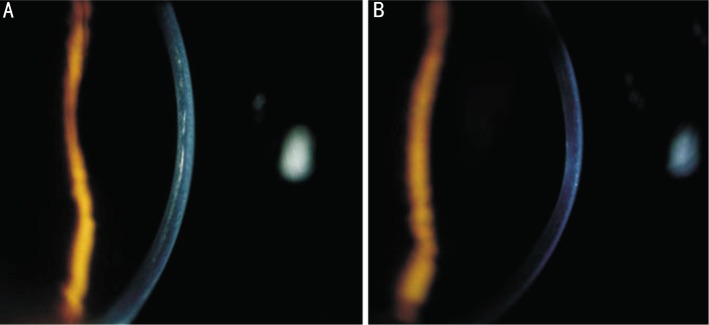
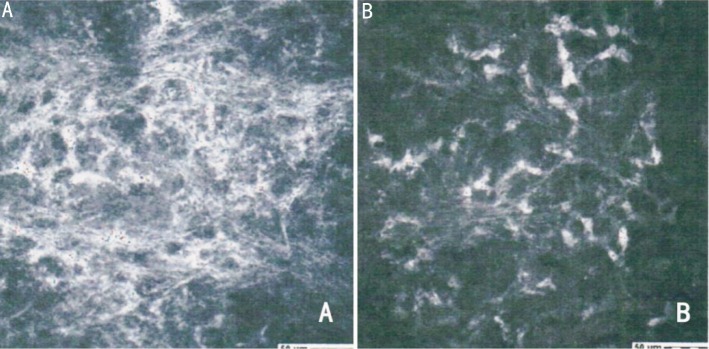
In the early postoperative period, 8 eyes had corneal opacity comparable to the subepithelial haze after photorefractive keratectomy. The haze was not subepithelial, however. Rather, it was visible in the middle stroma of stromal thickness (Figure 3). Twelve months after crosslinking, all corneas were clear. The postoperative in vivo confocal microscopy examinations of the treated corneas showed at a depth of 150µm the extracellular maxtrix grew denser as the keratocytes population increased (Figure 4). This increase seemed compatible with intermediated stromal haze. The postoperative best correct visual acuity data suggest that this haze did not seem to impair patient vision.
Figure 3. Corneal stromal haze was seen in the middle stroma of stromal thickness 1 month after surgery(A), but the same cornea became clear 12 months post-CXL(B).
Figure 4. Stromal haze was detectable by in vivo HRT II confocal microscopy(A), which compared with the stroma detected before CXL (B) (400×400µm).
DISCUSSION
Since1998, Seiler firstly descripted post-refractive-surgery keratectasia, which was recognized as a rare but major sight threatening complication of corneal refractive laser surgery[18],[19]. Now,more and more such complications were reported. The treatment of post-refractive-surgery ectasia is still frustrating. Recently, several studies reported post-refractive-surgery ectasia was successfully treated by CXL[20]-[22], which indicate that CXL maybe a choice for post-refractive-surgery ectasia. To the best of our knowledge, this is the first CXL study for post-LASIK corneal ectasia in China, in which preoperative and postoperative refractive, topographic, pachymetry, biomicroscopy and confocal microscopy outcomes have been analyzed in eyes with post-LASIK corneal ectasia and followed up for one-year.
Visual acuity is the most important parameter that may reflect the efficacy of CXL treatment. In mild keratoconus, there was a trend toward an increase in BSCVA after crosslinking[23]. The same result was also found in our study, which showed UCVA and BSCVA was statistically significant improvement beyond twelve months after CLX. However, there had some fluctuation of visual acuity in early stage, especially in the first month, that even appeared a bit of decrease, which may be caused by the epithelial debridement or other reactivity, for example, edema in the cornea patients may complain some blur. In the following months, there was a slow but continuous improvement of the UCVA and BSCVA, which increased about 0.07 and 0.13 respectively, reached preoperative levels at 12 months after CXL. This phenomenon may be related with the flatting of the cornea. Improvement in visual acuity was in line with improvement in topographic parameters. After an initial worsening of topographic indices, there was a slow but continuous improvement during a period of up to 12 months.
As Table 1 shows, despite a dramatic change observed in corneal power with an increase in flattest meridian keratometry, steepest meridian keratometry and astigmatic keratometry values at 1 month after CXL, from the 3rd to the 12th postoperative month instantaneous topography maps showed that the procedure had a significant effect in flattening and regularizing corneal curvature. The observed reduction in flattest and steepest K values was probably the result of the increased biomechanical stability of the cornea after crosslinking and was in line with findings in primary keratoconus patients treated similarly.
One of the pathologic characters of keratoconus is the progressive corneal thinning, thus we think the corneal thick may be an important efficacy parameters of stabilization of the keratoconus. In our study, we found a significant decrease in central corneal thickness in the initial 3 months. The largest decrease was observed 1 month after surgery, with a significant decrease of 15.5µm.At the end of our study the corneal thickness return to the pre-CXL level. In Doors[24] study, the AS-OCT pachymetry maps also showed a decrease in CCT after keratoconus surgery. This decrease was significant only at 3 months after corneal crosslinking, with decreases 11.0µm. Vinciguerra and associates also found a significant decrease in CCT from 427µm before surgery to 389µm at 3 months and 422µm at 3 months after surgery using Pentacam measurements[20].
There are several factors related this phenomenan. Firstly, several methods were adopted to measure the corneal thickness, such as ultrasonic, AS-OCT, Obscan and Pentacam imaging, which use different optical principles to construct the image of the anterior segment. A study by Seiler and Hafezi implied that the refractive index of the cornea changed, especially at the site of the demarcation line, because of structure modifications after corneal crosslinking[25]. So it is partly refractive index and partly other factors that introduce these pachymetry measurement differences. Additionally, the ultrasonic speed correlates with the elastic modulus in the cornea and increasing the elastic modulus produces a higher speed. Therefore, this would suggest that corneas after treatment are often thinner than other thickness measurements because of that effect. Another reason of cornea thinner is associated with the keratocytes. At the initial stage, there are many keratocytes apoptosis which may reduce the synthesizing of cornea collagen[26]. During the follow-up months, there were more and more activated keratocytes and there were also more new collagen synthesized. Transmission and scanning electron microscopy show clearly that the collagen fiber is thicker by 13% in the crosslinked eye[27], which may explain it eventually returns to its original thickness.
Spoerl et al[28] demonstrated that the UVA intensity used for crosslinking is far below the damage threshold for the corneal endothelium, iris, lens, and retina. During our treatment of CXL in post-LASIK corneal ectasia, under the epithelium off method, we did not observe any complications, such as epithelial ingrowth, diffuse lamellar keratitis (sands of the Sahara), or elevations of the flap margin[29]. We also didn't observe the change of the endothelium.But the danger of procedure for corneal flap should be noticed. Maybe the epithelium-on method is an alternative way.
In the early postoperative period, some eyes had corneal stromal opacity as other reports[30].As we known, corneal scarring is a deposit of amorphous collagen of keratocytes that has transformed into myofibroblasts, and so called haze is the product of disruption and death of keratocytes and as soon as the keratocytes repopulate that area, the cornea becomes homogeneously clear. Therefore we think this kind of corneal stromal opacity was termed haze because it was transient. However, this kind of haze was in the deep stromal, which was different with the PRK, the latter within a depth of 60µm under the corneal epithelium. Also, the postoperative best correct visual acuity data suggest that this haze did not seem to impair patient vision.
The evidence of this small series suggests that CXL appears to stabilise or partially reverse the progression of LASIK-induced keratectasia without apparent complication. We believe crosslinking should be performed in patients with progressive iatrogenic keratectasia as early as possible to prevent a further deterioration. As an non-random, non-comparative, single-centre study, the present preliminary study is subject to limitation. However, the results are promising and suggest that it would be worthwhile to further investigate this procedure in iatrogenic ectasia after corneal refractive surgery. If CXL effect turns out to be stable over a longer period, the procedure could be combined with intracorneal ring or customized corneal surface refractive ablation to partially correct the refractive error of patients with keratoconus[31].
REFERENCES
- 1.Comaish IF, Lawless MA. Progressive post-LASIK keratectasia: biomechanical instability or chronic disease process? J Cataract Refract Surg. 2002;28:2206–2213. doi: 10.1016/s0886-3350(02)01698-x. [DOI] [PubMed] [Google Scholar]
- 2.Kymionis GD, Siganos CS, Kounis G, Astyrakakis N, Kalyvianaki MI, Pallikaris IG. Management of post-LASIK corneal ectasia with Intacs inserts:one-year results. Arch Ophthalmol. 2003;121:322–326. doi: 10.1001/archopht.121.3.322. [DOI] [PubMed] [Google Scholar]
- 3.Seitz B, Rozsı ´val P, Feuermannova A, Langenbucher A, Naumann GO. Penetrating keratoplasty for iatrogenic keratoconus after repeat myopic laser in situ keratomileusis: histological findings and literature review. J Cataract Refract Surg. 2003;29:2217–2224. doi: 10.1016/s0886-3350(03)00406-1. [DOI] [PubMed] [Google Scholar]
- 4.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 7.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 8.Dupps WJ, Jr, Netto MV, Herekar S, Krueger RR. Surface wave elastometry of the cornea in porcine and human donor eyes. J Refract Surg. 2007;23:66–75. doi: 10.3928/1081-597x-20070101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha KM, Ramos-Esteban JC, Qian Y, Herekar S, Krueger RR. Comparative study of riboflavin-UVA cross-linking and “flashlinking” using surface wave elastometry. J Refract Surg. 2008;24:S748–S751. doi: 10.3928/1081597X-20080901-20. [DOI] [PubMed] [Google Scholar]
- 10.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 11.Mazzotta C, Balestrazzi A, Traversi C, Baiocchi S, Caporossi T, Tommasi C, Baiocchi S, Caporossi T, Tommasi C, Caporossi A. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen; ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26:390–397. doi: 10.1097/ICO.0b013e318030df5a. [DOI] [PubMed] [Google Scholar]
- 12.Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23:503–507. doi: 10.1097/01.ico.0000105827.85025.7f. [DOI] [PubMed] [Google Scholar]
- 13.Spoerl E, Wollensak G, Dittert D-D, Seiler T. Thermomechanical behaviour of collagen-cross-linked porcine cornea. Ophthalmologica. 2004;218:136–140. doi: 10.1159/000076150. [DOI] [PubMed] [Google Scholar]
- 14.Wittig-Silva G, Whiting M, Lamoureux E, Lindsay RG, Sullivan LG, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus:preliminary results. J Refract Surg. 2008;24:S720–S725. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 15.Dupps WJ, Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–720. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krueger RR, Dupps WJ., Jr Biomechanical effects of femtosecond and microkeratome-based flap creation: prospective contralateral examination of two patients. J Refract Surg. 2007;23:800–807. doi: 10.3928/1081-597X-20071001-10. [DOI] [PubMed] [Google Scholar]
- 17.Wollensak G, Sporl E, Seiler T. Treatment of keratoconus by collagen crosslinking. Ophthalmologe. 2003;100:44–49. doi: 10.1007/s00347-002-0700-3. [DOI] [PubMed] [Google Scholar]
- 18.Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. 1998;24:1007–1009. doi: 10.1016/s0886-3350(98)80057-6. [DOI] [PubMed] [Google Scholar]
- 19.Seiler T, Koufala K, Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg. 1998;14:312–317. doi: 10.3928/1081-597X-19980501-15. [DOI] [PubMed] [Google Scholar]
- 20.Vinciguerra P, Camesasca FI, Albè E, Trazza T. Corneal collagen cross-linking for ectasia after excimer laser refractive surgery:1-year results. J Refract Surg. 2010;26:486–497. doi: 10.3928/1081597X-20090910-02. [DOI] [PubMed] [Google Scholar]
- 21.Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with ribofl avin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:2035–2040. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Salgado J P, Khoramnia R, Lohmann C P, Mohrenfels CW. Corneal collagen crosslinking in post-LASIK keratectasia. Br J Ophthalmol. 2011;95:493–497. doi: 10.1136/bjo.2010.179424. [DOI] [PubMed] [Google Scholar]
- 23.Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 24.Doors M, Tahzib NG, Eggink FA, Berednschot TTJM, Webers CAB, Nuij RMMA. Use of Anterior Segment Optical Coherence Tomography to Study Corneal Changes After Collagen Cross-linking. Am J Ophthalmol. 2009;148:844–851. doi: 10.1016/j.ajo.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Seiler T, Hafezi F. Corneal cross-linking-inducedstromal demarcation line. Cornea. 2006;25:1057–1059. doi: 10.1097/01.ico.0000225720.38748.58. [DOI] [PubMed] [Google Scholar]
- 26.Mencucci R, Marini M, Paladini I, Sarchielli E, Sgambati E, Menchini U, Vannelli GB. Effects of riboflavin/UVA corneal cross-linking on keratocytes and collagen fibres in human cornea. Clinical and Experimental Ophthalmology. 2010;38:49–56. doi: 10.1111/j.1442-9071.2010.02207.x. [DOI] [PubMed] [Google Scholar]
- 27.Wollensack G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23:503–507. doi: 10.1097/01.ico.0000105827.85025.7f. [DOI] [PubMed] [Google Scholar]
- 28.Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 29.Kymionis GD, Bouzoukis DI, Diakonis VF, Portaliou DM, Pallikaris AI, Yoo SH. Diffuse lamellar keratitis after corneal crosslinking in a patient with post-laser in situ keratomileusis corneal ectasia. J Cataract Refract Surg. 2007;33:2135–2137. doi: 10.1016/j.jcrs.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 30.Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Experiment Ophthalmol. 2007;35:580–582. doi: 10.1111/j.1442-9071.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 31.Kanellopoulos AJ, Binder PS. Management of corneal ectasia after LASIK with combined, same-day, topography-guided partial transepithelial PRK and collagen cross-linking:The Athens Protocol. J Refract Surg. 2010;5:1–9. doi: 10.3928/1081597X-20101105-01. [DOI] [PubMed] [Google Scholar]