Abstract
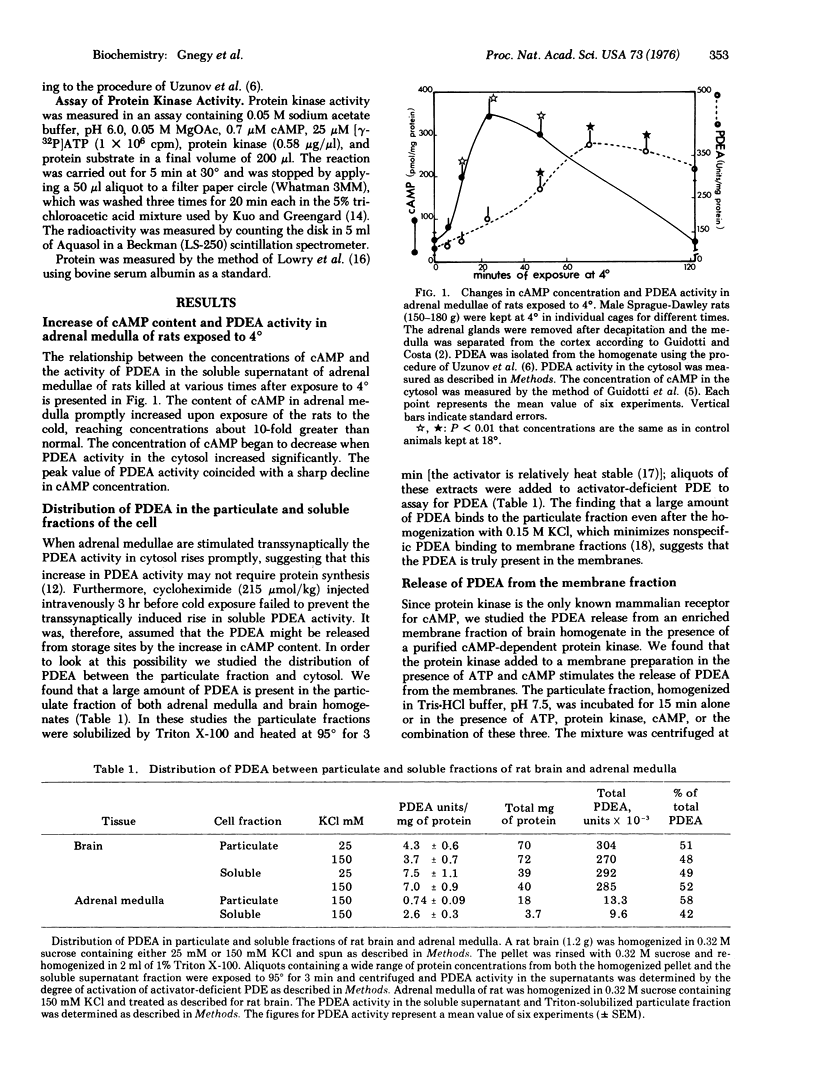
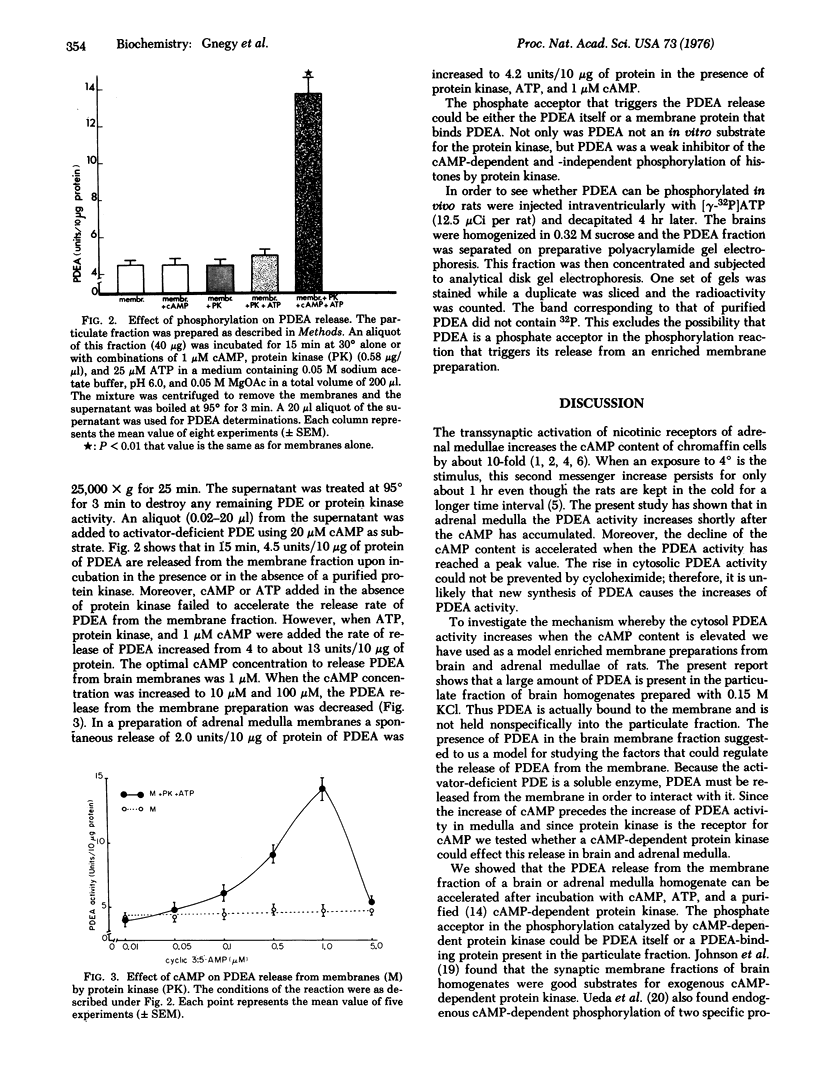
Experiments with cold exposure confirmed previous studies indicating that the endogenous protein acitvator of phosphodiesterase (PDEA) isolated by Cheung participates in the in vivo regulation of 3':5'-cyclic adenosine monophosphate (cAMP) in adrenal medulla. This activator of cAMP phosphodiesterase (PDE) (3':5'-cyclic-AMP 5'-nucleotidohydrolase, EC 3.1.4.17) is present in the particulate as well as the soluble fractions of rat brain. It was found that a purified cAMP-dependent protein kinase (ATP:protein phosphotransferase, EC 2.7.1.37), in the presence of ATP and cAMP, stimulates 3-fold the release of PDEA from the particulate fraction of rat brain and adrenal medulla. The substrate for this phosphorylation could be either a membrane protein that binds PDEA or PDEA itself. In vivo evidence, however, obtained by injecting rats intraventricularly with [gamma-32P]ATP, indicates that the PDEA does not contain radioactive phosphate in its structure. Also, PDEA could not be phosphorylated by protein kinase in vitro. The following mechanism is postulated: when the intracellular content of cAMP increases it activates a protein kinase which phosphorylates a PDEA-binding membrane protein and releases PDEA. In turn this binds to activator-deficient high Km PDE and decreases its Km to facilitate the hydrolysis of the increased concentration of cAMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Donnelly T. E., Jr, Kuo J. F., Miyamoto E., Greengard P. Protein kinase modulator from lobster tail muscle. II. Effects of the modulator on holoenzyme and catalytic subunit of guanosine 3',5'-monophosphate-dependent and adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1973 Jan 10;248(1):199–203. [PubMed] [Google Scholar]
- Filburn C. R., Karn J. An isotopic assay of cyclic 3',5'-nucleotide phosphodiesterase with aluminum oxide columns. Anal Biochem. 1973 Apr;52(2):505–516. doi: 10.1016/0003-2697(73)90055-9. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Costa E. A role for nicotinic receptors in the regulation of the adenylate cyclase of adrenal medulla. J Pharmacol Exp Ther. 1974 Jun;189(3):665–675. [PubMed] [Google Scholar]
- Guidotti A., Costa E. Involvement of adenosine 3',5'-monophosphate in the activation of tyrosine hydroxylase elicited by drugs. Science. 1973 Mar 2;179(4076):902–904. doi: 10.1126/science.179.4076.902. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Zivkovic B., Pfeiffer R., Costa E. Involvement of 3',5'-cyclic adenosine monophosphate in the increase of tyrosine hydroxylase activity elicited by cold exposure. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(2):195–206. doi: 10.1007/BF00500650. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Maeno H., Greengard P. Phosphorylation of endogenous protein of rat brain by cyclic adenosine 3',5'-monophosphate-dependent protein kinase. J Biol Chem. 1971 Dec 25;246(24):7731–7739. [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y. Cyclic 3',5'-nucleotide phosphodiesterase, IV. Two enzymes with different properties from brain. Biochem Biophys Res Commun. 1971 Mar 5;42(5):968–974. doi: 10.1016/0006-291x(71)90525-0. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y., Uenishi K. Regulation of nucleoside cyclic 3':5'-monophosphate phosphodiesterase activity from rat brain by a modulator and Ca2+. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3526–3530. doi: 10.1073/pnas.70.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S. L., Jr, Corbin J. D., Park C. R. On the question of translocation of heart cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1501–1504. doi: 10.1073/pnas.72.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. An assay method for cyclic AMP and cyclic GMP based upon their abilities to activate cyclic AMP-dependent and cyclic GMP-dependent protein kinases. Adv Cyclic Nucleotide Res. 1972;2:41–50. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin Y. M., Liu Y. P., Cheung W. Y. Cyclic 3':5'-nucleotide phosphodiesterase. Purification, characterization, and active form of the protein activator from bovine brain. J Biol Chem. 1974 Aug 10;249(15):4943–4954. [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]
- Uzunov P., Weiss B. Separation of multiple molecular forms of cyclic adenosine-3',5'-monophosphate phosphodiesterase in rat cerebellum by polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1972 Sep 19;284(1):220–226. doi: 10.1016/0005-2744(72)90060-5. [DOI] [PubMed] [Google Scholar]
- Wickson R. D., Boudreau R. J., Drummond G. I. Activation of 3',5'-cyclic adenosine monophosphate phosphodiesterase by calcium ion and a protein activator. Biochemistry. 1975 Feb 25;14(4):669–675. doi: 10.1021/bi00675a004. [DOI] [PubMed] [Google Scholar]



