Abstract
Vascular endothelial growth factor (VEGF) is a potent stimulator of vascular angiogenesis, permeability, and remodeling that also plays important roles in wound healing and tissue cytoprotection. To begin to define the roles of VEGF in diseases like asthma and COPD, we characterized the effects of lung-targeted transgenic VEGF165 and defined the innate immune pathways that regulate VEGF tissue responses. The former studies demonstrated that VEGF plays an important role in Th2 inflammation because, in addition to stimulating angiogenesis and edema, VEGF induced eosinophilic inflammation, mucus metaplasia, subepithelial fibrosis, myocyte hyperplasia, dendritic cell activation, and airways hyperresponsiveness via IL-13–dependent and -independent mechanisms. VEGF was also produced at sites of aeroallergen-induced Th2 inflammation, and VEGF receptor blockade ameliorated adaptive Th2 inflammation and Th2 cytokine elaboration. The latter studies demonstrated that activation of the RIG-like helicase (RLH) innate immune pathway using viral pathogen–associated molecular patterns such as Poly(I:C) or viruses ameliorated VEGF-induced tissue responses. In accord with these findings, Poly(I:C)-induced RLH activation also abrogated aeroallergen-induced Th2 inflammation. When viewed in combination, these studies suggest that VEGF excess can contribute to the pathogenesis of Th2 inflammatory disorders such as asthma and that abrogation of VEGF signaling via RLH activation can contribute to the pathogenesis of viral disorders such as virus-induced COPD exacerbations. They also suggest that RLH activation may be a useful therapeutic strategy in asthma and related disorders.
Keywords: asthma, chronic obstructive pulmonary disease, virus, RIG-like helicase, mitochondrial antiviral signaling molecule
Exaggerated Th2 inflammation and tissue remodeling are characteristic of asthma. A prominent increase in blood vessel number and size and vascular leakage that correlates with disease severity is also characteristic of this disorder. In contrast, type I inflammation and vascular and parenchymal destruction are characteristic features of pulmonary emphysema. Vascular endothelial growth factor (VEGF) was initially defined as vascular permeability factor. It is now known to be a critical regulator of angiogenesis, vascular remodeling, wound healing, and cytoprotection (1–6). Based on this highly variable effector repertoire, it is reasonable to speculate that VEGF dysregulation can contribute to the pathogenesis of asthma and COPD. To test this hypothesis, we characterized the effects of VEGF in the murine lung and defined the innate immune pathways that regulate VEGF tissue responses.
VEGF Excess in Asthma
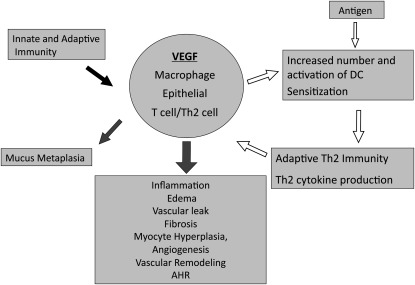
A variety of approaches have been used to define the mediators that are necessary and/or sufficient for asthma-like type II immune and tissue responses in the lung. Overexpression transgenic (Tg) approaches have been particularly useful in this regard. These studies demonstrated that transgenic IL-13, when targeted to the lung under the influence of the CC10 promoter, generates asthma-like eosinophilic inflammation, mucus metaplasia, airway fibrosis, and airways hyperresponsiveness (AHR) after methacholine challenge (7, 8). Interestingly, VEGF was prominently expressed in select airway and alveolar epithelial cells and macrophages in the lungs from these transgenic animals (3). A number of other studies demonstrated that the levels of VEGF were increased in biologic fluids from patients with asthma, where they correlate with disease severity (9, 10). However, the contributions of VEGF in this setting had not been defined. To address this issue, a transgenic approach was used to selectively and inducibly target VEGF165 to the airway epithelium using the CC10 promoter. As previously reported (5), in the wild type (WT) mice and Tg mice in which the transgene was not activated, VEGF levels in bronchoalveolar lavage (BAL) were less than or equal to 15 pg/ml. Increased levels of BAL VEGF were noted within 24 hours and steady-state levels between 0.05 and 12 ng/ml were seen after 1 week of transgene activation. These levels of VEGF are in accord with the levels in biologic fluids from normal subjects and patients with asthma or respiratory syncytial virus (RSV) infection (11–16). In keeping with the known angioregulatory effects of VEGF, increases in angiogenesis and vascular leak were readily appreciated (5). Surprisingly, VEGF also caused impressive eosinophilic inflammation, mucus metaplasia, airway remodeling characterized by subepithelial collagen deposition and airways smooth muscle hyperplasia, and AHR (5). VEGF also enhanced respiratory allergen sensitization and Th2 inflammation and increased the number of activated dendritic cells (DC) in the local microenvironment. In ovalbumin-sensitized and -challenged mice, VEGF was produced by epithelial cells, macrophages, and Th2 cells. In this setting, it was a critical contributor to Th2 inflammation, Th2 cytokine production, and AHR, because these responses were all ameliorated by VEGFR2 blockade (5). When viewed in combination, these studies provide evidence that VEGF production by macrophages, epithelial cells, or T cells may contribute in important ways to the pathogenesis of pulmonary Th2 responses like asthma (Figure 1). Specifically, innate and adaptive immune responses stimulate VEGF production, which induces mucus metaplasia via an IL-13–dependent mechanism and inflammation, edema, vascular leak, tissue fibrosis, myocyte hyperplasia, angiogenesis, vascular remodeling, and AHR via an IL-13–independent pathway(s) (5). In addition, antigen-induced responses increase the number and activation of pulmonary DC leading to heightened adaptive Th2 immunity, heightened Th2 cytokine production, and in turn, heightened VEGF elaboration. This demonstrates how innate and adaptive immunity can work through VEGF-dependent pathways. It also highlights a potential positive feedback loop in which antigen-induced responses lead to VEGF production, which leads to DC activation, enhanced Th2 sensitization, and augmented VEGF elaboration.
Figure 1.
Proposed roles of vascular endothelial growth factor (VEGF) in the lung. During the course of innate and adaptive immune responses, VEGF is produced by a variety of cells, including macrophages, epithelial cells, and Th2 cells. This VEGF can increase the number and state of activation of dendritic cells (DC), thereby augmenting antigen-induced responses, especially Th2 reactions (open arrows). It also induces mucus metaplasia via an IL-13–dependent mechanism and inflammation, edema, vascular leak, fibrosis, myocyte hyperplasia, angiogenesis, vascular remodeling, and airway hyperresponsiveness (AHR) via IL-13–independent mechanisms.
VEGF Deficiency in Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a composite term that is used for patients with emphysema and/or chronic bronchitis. Patients with COPD experience disease exacerbations whose frequency correlates with the rate of disease progression and the health status of the individual. Viruses, particularly rhinovirus, influenza virus, and respiratory syncytial virus (RSV), are important causes of COPD exacerbations (17–22). However, the mechanisms of the inflammatory and remodeling responses that are seen in COPD and the pathways that viruses use in these disorders have not been defined.
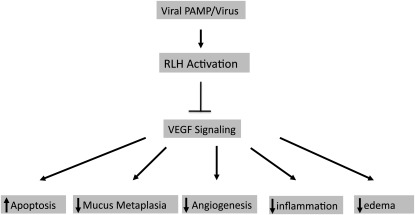
Normal lung function and the ability of the lung to respond to injury appear to be critically dependent on the presence of local functional VEGF (3, 23–26). VEGF deficiency augments oxidant injury and tissue destruction and has been implicated in the pathogenesis of pulmonary emphysema and other disorders (26). However, the processes that regulate VEGF tissue responses in the lung have not been defined. To further define the mechanisms via which cigarette smoke (CS) exposure and viruses interact in the pathogenesis of COPD, we have developed a murine modeling system in which mice are exposed to CS or room air and are then exposed to viral pathogen–associated molecular patterns (PAMPs), such as Poly(I:C), or live viruses. These studies demonstrated that, in contrast to our expectations, CS interacts in a synergistic fashion with viral PAMPs or live viruses to augment tissue inflammation, emphysematous alveolar destruction, epithelial apoptosis, and airway fibrosis (27). Studies of the mechanisms that are involved in these responses have demonstrated a prominent role for the RIG-like helicase (RLH) antiviral innate immune pathway in these animals, because each one of these responses was abrogated in the absence of the mitochondrial antiviral signaling molecule (MAVS), which is the central integrator of this innate immune pathway (27). In keeping with the hypothesis that VEGF deficiency also contributes to the pathogenesis of emphysema, studies were undertaken to determine if viruses and/or viral PAMPs have the ability to abrogate VEGF-induced tissue responses. This hypothesis was tested by treating the VEGF transgenic mice described above with Poly(I:C) or its vehicle control. These studies demonstrated that Poly(I:C) ameliorated the ability of VEGF to induce angiogenesis, edema, inflammation, and mucus accumulation (28). These inhibitory effects were not associated with a decrease in the production of transgenic VEGF and were not altered by null mutations of Toll-like receptor-3. They were, however, abrogated by null mutations of MAVS highlighting the essential role(s) of the RLH innate immune pathway in this response (28). Influenza and RSV had similar inhibitory effects on VEGF-induced angiogenesis (28). When viewed in combination, these studies demonstrate that Poly(I:C) and respiratory viruses inhibit VEGF-induced tissue responses (Figure 2).
Figure 2.
Effects of RLH activation on vascular endothelial growth factor (VEGF) tissue responses. The activation of the RIG-like helicase (RLH) pathway inhibits VEGF effector responses, resulting in decreased mucus metaplasia, angiogenesis, inflammation, and edema. It also abrogates VEGF-mediated cytoprotection by increasing cellular apoptosis.
Poly(I:C) Regulation of Aeroallergen-Induced Th2 Inflammation
The studies noted above highlight the importance of VEGF excess in the pathogenesis of Th2 inflammation and the ability of RLH innate immune activation to abrogate the tissue effects of pulmonary VEGF. When viewed in combination, these studies allow for the hypothesis that RLH immune activation can also diminish Th2 inflammatory events in the lung. To test this hypothesis, mice were sensitized and challenged with the aeroallergen ovalbumin (OVA) in the presence of alum and challenged with OVA after treatment with Poly(I:C) or its vehicle control. OVA-sensitized and -challenged mice treated with the vehicle control manifest prominent increases in BAL and tissue total cell, macrophage, eosinophil, and lymphocyte recovery. In all cases these inductive effects were abrogated by treatment with Poly(I:C). This inhibition was mediated by a MAVS-dependent pathway because the inhibitory effects of Poly(I:C) were not seen in MAVS-null animals. Thus, in accord with the critical role that VEGF plays in Th2 inflammation and the ability of RLH activation to abrogate the tissue effects of VEGF, Poly(I:C) was a potent inhibitor of adaptive Th2 inflammation.
Conclusions
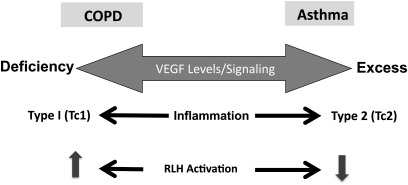
The studies noted above suggest that VEGF has a variety of roles in the lung and in lung disorders. They allow for the interesting speculation that there is a VEGF axis in the lung extending from deficiency on one end to excess at the other (Figure 3). They allow for the interesting hypothesis that VEGF excess contributes to the pathogenesis of Th2 inflammation and, in turn, asthma-like responses. It will be interesting to see if VEGF excess plays a role in the recently described Th2-low patients with asthma (29). They also allow for the speculation that emphysema is mediated, in part, by events that decrease the levels of VEGF and/or its ability to signal. By demonstrating that RLH innate immune activation can abrogate VEGF signaling, they also provide a pathogenetic mechanism via which viral PAMPs and/or viruses can contribute to the pathogenesis of COPD. They also highlight the potential use of RLH agonists such as Poly(I:C) in attempts to control Th2 inflammation and ameliorate the effects of VEGF excess in the lung.
Figure 3.
Proposed vascular endothelial growth factor (VEGF) axis in the lung. Based on these and other studies, it is reasonable to propose that VEGF excess contributes to the pathogenesis of Th2 responses in diseases such as asthma, and that a deficiency of VEGF or VEGF signaling contributes to the pathogenesis of the emphysema in chronic obstructive pulmonary disease (COPD) and the type 1 responses that are seen in these patients. They also allow for the hypothesis that RIG-like helicase (RLH) activation will inhibit type 2 (asthma-like) and shift toward type 1 (emphysema-like) responses.
Footnotes
Supported by National Institutes of Health grant HL-079328 (J.A.E.).
Author Disclosure: C.G.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.H.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.E. received consultancy fees from Promedior Inc., served on the Advisory Board of Intermune Inc., and owns stock in Intermune Inc. and Merck, Inc. He also received patents with Yale University for chitinases in lung inflammation, mir-1 in VEGF tissue responses, IL-18 in COPD, and VEGF in asthma.
References
- 1.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005;438:932–936 [DOI] [PubMed] [Google Scholar]
- 3.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005;438:967–974 [DOI] [PubMed] [Google Scholar]
- 5.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances Th2-mediated sensitization and inflammation in the lung. Nat Med 2004;10:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel C, Bauer A, Wiesnet M, Preissner KT, Schaper W, Marti HH, Fischer S. Flt-1, but not Flk-1 mediates hyperpermeability through activation of the PI3-k/Akt pathway. J Cell Physiol 2007;212:236–243 [DOI] [PubMed] [Google Scholar]
- 7.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Jr, Chapman HA, Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol 2001;107:1034–1038 [DOI] [PubMed] [Google Scholar]
- 10.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol 2001;107:295–301 [DOI] [PubMed] [Google Scholar]
- 11.Ohta Y, Ohta N, Tamura M, Wu J, Tsunezuka Y, Oda M, Watanabe G. Vascular endothelial growth factor expression in airways of patients with lung cancer: a possible diagnostic tool of responsive angiogenic status on the host side. Chest 2002;121:1624–1627 [DOI] [PubMed] [Google Scholar]
- 12.Meyer KC, Cardoni A, Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med 2000;135:332–338 [DOI] [PubMed] [Google Scholar]
- 13.Nishigaki Y, Fujiuchi S, Yamazaki Y, Matsumoto H, Takeda A, Fujita Y, Okamoto K, Fujikane T, Shimizu T, Kikuchi K. Increased vascular endothelial growth factor in acute eosinophilic pneumonia. Eur Respir J 2003;21:774–778 [DOI] [PubMed] [Google Scholar]
- 14.Kanazawa H, Hirata K, Yoshikawa J. Involvement of vascular endothelial growth factor in exercise induced bronchoconstriction in asthmatic patients. Thorax 2002;57:885–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asai K, Kanazawa H, Otani K, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol 2002;110:571–575 [DOI] [PubMed] [Google Scholar]
- 16.Lee CG, Yoon HJ, Zhu Z, Link H, Wang Z, Gwaltney JM, Landry M, Elias JA. Respiratory syncytial virus stimulation of vascular endothelial cell growth factor/vascular permeability factor. Am J Respir Cell Mol Biol 2000;23:662–669 [DOI] [PubMed] [Google Scholar]
- 17.Ko FW, Ip M, Chan PK, Fok JP, Chan MC, Ngai JC, Chan DP, Hui DS. A 1-year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest 2007;131:44–52 [DOI] [PubMed] [Google Scholar]
- 18.Mallia P, Contoli M, Caramori G, Pandit A, Johnston SL, Papi A. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr Pharm Des 2007;13:73–97 [DOI] [PubMed] [Google Scholar]
- 19.Mallia P, Johnston SL. How viral infections cause exacerbation of airway diseases. Chest 2006;130:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Gorman C, McHenry E, Coyle PV. Human metapneumovirus in adults: a short case series. Eur J Clin Microbiol Infect Dis 2006;25:190–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sajjan US, Jia Y, Newcomb DC, Bentley JK, Lukacs NW, LiPuma JJ, Hershenson MB. H. Influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J 2006;20:2121–2123 [DOI] [PubMed] [Google Scholar]
- 22.Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax 2006;61:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 2009;119:1298–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtagic E, Jedrychowski MP, Nugent MA. Neutrophil elastase cleaves VEGF to generate a VEGF fragment with altered activity. Am J Physiol Lung Cell Mol Physiol 2009;296:L534–L546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medford AR, Ibrahim NB, Millar AB. Vascular endothelial cell growth factor receptor and coreceptor expression in human acute respiratory distress syndrome. J Crit Care 2009;24:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuder RM, Yun JH. Vascular endothelial growth factor of the lung: friend or foe. Curr Opin Pharmacol 2008;8:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 2008;118:2771–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma B, De la Cruz CS, Hartl D, Kang M-J, Takyar S, Homer S, Lee CG, Elias JA. Rig-like helicase innate immunity inhibits VEGF tissue responses via a type I interferon-dependent mechanism. Am J Respir Crit Care Med 2011;183:1322–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]