Abstract
Lung cells experience hypoxia during development, during travel to high altitude, and in acute and chronic lung diseases. The functional responses evoked by hypoxia are diverse and generally act to protect the cells from hypoxic injury, although some lung cell responses are counterproductive because they degrade normal function of the organ. The cellular O2 sensor responsible for many of these responses involves the mitochondrial electron transport chain. Under hypoxic conditions, increased release of reactive oxygen species from the inner mitochondrial membrane to the intermembrane space leads to the activation of transcription factors, including hypoxia-inducible factor, activation of hypoxic pulmonary vasoconstriction, activation of AMP-dependent protein kinase, and internalization of the membrane Na,K-ATPase from the basolateral membrane of alveolar epithelial cells. Although the specific targets of reactive oxygen species signals are not fully understood, this signaling pathway is critical for development and for normal lung responses in the newborn and the mature lung.
Keywords: oxygen sensing, lung injury, hypoxic pulmonary vasoconstriction, pulmonary circulation, alveolar epithelium
Fetal lung cells experience hypoxia during development, and adult lung cells become hypoxic during travel to high altitude and in lung diseases that disrupt gas exchange function. Cellular hypoxia triggers diverse physiological responses in lung epithelial, endothelial, and smooth muscle cells that are mediated by transcriptional and posttranslational mechanisms. How these cells detect hypoxia and the signaling pathways by which these O2 sensors trigger biological responses are not fully understood. However, a consensus is growing in support of a model whereby mitochondria detect hypoxia and transmit signals in the form of reactive oxygen species (ROS) to the cytosol, where target molecules involved in the functional responses are activated. This article describes a number of these responses and summarizes the evidence in support of a mitochondrial model of cellular oxygen sensing.
It is appropriate to begin by defining tissue hypoxia. Systemic tissues experience a wide range of “normal” in terms of oxygenation. In the myocardium, oxygen tensions of 20 to 25 mm Hg are commonly found at rest, and during maximal exercise the level can decrease to less than 10 mm Hg (1). Cells in the renal cortex normally function at oxygen tensions above 60 mm Hg, whereas those in the renal medulla may be much lower (2). By contrast, cells in the adult lung rarely experience O2 levels less than 40 mm Hg, whereas alveolar epithelial cells normally reside at 100 mm Hg. Hence, it can be argued that the definition of hypoxia in the lung should be different from other tissues. However, pulmonary and nonpulmonary cells demonstrate remarkable similarities in their physiological responses when their levels of oxygenation are decreased from “normal,” even though the definition of “normal” varies widely. Moreover, primary cells dissociated from tissues appear to acclimate quickly to tissue culture conditions and to change their oxygen sensitivity accordingly. These observations suggest that cells have the ability to adjust their definition of “normal” when exposed to chronic changes in their level of oxygenation, possibly by altering the expression genes regulating signaling pathway sensitivity to perturbation. In the context of hypoxia-induced ROS signaling, this could be achieved by altering the expression of antioxidant enzymes, thereby adjusting the effect of oxidant generation on downstream targets. Nevertheless, when challenged with levels of hypoxia that could threaten mitochondrial metabolism, all cells demonstrate protective responses.
Lung Cellular Responses to Hypoxia
Hypoxic Pulmonary Vasoconstriction
Smooth muscle cells from intraparenchymal pulmonary arteries constrict during acute hypoxia in a response termed hypoxic pulmonary vasoconstriction (HPV). The evolutionary pressure driving this response in mammals is probably based on the need to conserve fetal oxygenation during gestation. Because blood retuning to the fetal heart contains relatively high levels of oxygen during placental respiration, sending blood flow through pulmonary capillaries could allow significant oxygen loss to hypoxic amniotic fluid in the lungs. Hypoxia-induced vasoconstriction in the lung therefore tends to limit pulmonary capillary blood flow, whereas the low-resistance pathway through the ductus arteriosis tends to limit the afterload on the right ventricle. By limiting O2 loss from capillary blood to the amniotic fluid, the fetus shifts responsibility for supplying uterine and amniotic cells with oxygen to the mother. In the initial moments after birth, inflation of the newborn lung causes the oxygen tension to increase acutely. Lung cells detect this change and trigger a relaxation of the pulmonary arteries, while cells in the ductus respond to the increase in blood oxygenation by constricting. Thus, the transition from placental to lung respiration requires O2 sensing in lung and vascular cells. The hypoxia-induced constriction response is intrinsic to the smooth muscle cells of the pulmonary artery because it can be demonstrated in cultured cells and in rings of pulmonary artery that have been denuded of endothelium. However, the response is enhanced in the presence of endothelial cells, as evidenced by the more vigorous response in endothelium-intact rings and in precision-cut slices of intact lung. These findings suggest that cell–cell interactions in situ complement the overall functional response to acute decreases in oxygen levels.
The pulmonary pressor response to hypoxia is retained in the adult lung, where it potentially can preserve gas exchange efficiency in lung diseases where poorly ventilated or unventilated hypoxic regions develop. In hypoxic areas, local vasoconstriction tends to decrease blood flow, redistributing perfusion to better ventilated regions of the lung. However, the importance of this in healthy lungs is minimal because few hypoxic alveoli exist in the healthy lung. By contrast, at high altitude or in lung diseases where significant areas of alveolar hypoxia develop, widespread vasoconstriction can cause a sustained increase in pulmonary artery pressure, triggering right ventricular hypertrophy. Sustained increases in pulmonary artery pressure also trigger remodeling of pulmonary arteries in response to the chronic increase in vessel wall stress. Lungs from rodent models of chronic hypoxia demonstrate increases in pulmonary artery wall thickness, increases in pulmonary artery pressure that are sustained during acute return to sea-level atmospheric conditions, and an enhanced response to acute hypoxia. This remodeling is clearly dependent on the oxygen-sensing response in lung cells because the remodeling is reversible if the animal is returned to a chronic normoxic environment.
Alveolar Epithelial Responses to Hypoxia
Many cells in the body respond to hypoxia by activating protective mechanisms that lessen the potential for damage or cell death in the event that oxygen availability becomes critically limiting. One such mechanism involves hypoxic conformance, whereby cells suppress energy-dependent facultative activities to preserve energy supply for obligatory functions required for survival (3). In the lung, alveolar epithelial cells form a barrier separating the alveolar airspace from the interstitium. They also participate in the clearance of edema fluid from the alveolar compartment by transporting sodium from the apical to the basolateralmembrane, using apical amiloride-sensitive sodium channels (ENa) and an ATP-dependent sodium-potassium transporter (Na,K-ATPase) in the basolateral membrane (4). Chloride movement occurs in parallel through a paracellular route, driven by the electrical potential across the epithelial layer. The movement of NaCl across the barrier draws water osmotically from the alveolus to the interstitium, thereby lessening edema. Hypoxia triggers internalization of the Na,K-ATPase, possibly as a cellular protective strategy to limit ATP utilization by this energy-demanding system. Although this might confer protection to the epithelial cell itself, the response lessens the ability to clear alveolar edema and may therefore undermine survival of the whole organism. Because this internalization is triggered by low oxygen conditions, it requires the activation of an O2 sensor to trigger the response. The signaling pathways regulating the internalization of the Na,K-ATPase involves the activation of 5′-AMP–activated protein kinase (AMPK), which phosphorylates PKC zeta on its activation domain (5). The subsequent translocation of PKC zeta to the plasma membrane results in phosphorylation of the Na,K-ATPase, which triggers its internalization. Reactive oxygen species produced during hypoxia are required for this response (6).
Many cells in the lung initiate transcriptional responses to hypoxia by activating hypoxia-inducible factor (HIF). This highly conserved transcription factor was originally discovered in a search to identify the transcription factor responsible for hypoxia-induced activation of the erythropoietin gene (7). Subsequent studies demonstrated that HIF is responsible for activating a wide range of genes in virtually all mammalian cell types in response to hypoxia (8). HIF activates transcription as a heterodimer composed of an oxygen-regulated subunit (HIF-1α) and a constitutively expressed, oxygen-independent component (HIF-1β). A related transcription factor, HIF-2, is regulated in a similar manner, and, although some overlap of regulated genes is apparent, the functions of HIF-1 and HIF-2 vary among cell types and with respect to the genes they control (9). Studies continue to explore the relative roles of HIF-1 and HIF-2 in health and disease, and a full understanding of how these two factors collaborate or conflict with each other has not been achieved. In the case of HIF-1 and HIF-2, the α subunit is continuously expressed, but under normoxic conditions it is degraded by the ubiquitin-proteasome system within minutes after being transcribed (10). The degradation of HIF-1α in normoxia is initiated by hydroxylation of conserved proline residues (p402 and p564 in human HIF-1α) by prolyl hydroxylase domain enzymes (PHDs) (11, 12). Proline hydroxylation facilitates interaction of the subunit with von HippelLindau protein, the E3 ubiquitin ligase that labels it for degradation (13). Four PHDs have been described, although the principal O2–dependent hydroxylase responsible for the stabilization of HIF-1α in hypoxia is PHD2 (14). During hypoxia, the activity of PHD is suppressed, allowing HIF-1α to accumulate, heterodimerize, translocate to the nucleus, and assemble with the transcriptional machinery. Another posttranslational modification site involves an asparagine residue near the carboxy terminal, which is hydroxylated by an asparagine hydroxylase (factor inhibiting HIF) under normoxic conditions (15). Hydroxylation at that site interferes with the assembly of the transcriptional cofactors, thereby derailing transactivation of HIF-dependent genes. The activity of PHDs and factor inhibiting HIF requires 2-oxoglutarate and molecular O2 as substrates and ferric iron as a cofactor (16). Hence, it is suspected that PHD acts as a form of oxygen sensor.
Mechanisms of O2 Sensing
In the examples of hypoxic responses in lung cells detailed above, it is important to note that the level of hypoxia needed to elicit the response is far less severe than the levels that begin to limit cellular respiration. Mitochondrial cytochrome oxidase, the terminal cytochrome responsible for transferring electrons to O2, exhibits a high apparent affinity for oxygen (17, 18). This characteristic allows isolated cells to sustain their basal respiration rates at a normal level until the extracellular Po2 falls below 5 to 7 mm Hg, as depicted hypothetically in Figure 1 and as published previously (19). The rate of mitochondrial respiration is dictated by the metabolic activity of the cells, as manifested by their rate of ATP hydrolysis (20). Because O2 supply limitation does not limit ATP production until the cellular Po2 reaches a critically low level, it follows that ATP depletion, secondary to mitochondrial limitation, is not likely to be the signal responsible for initiating the responses to hypoxia. Rather, molecular mechanisms must exist that allow the cells to detect even modest decreases in oxygenation and that activate signaling pathways responsible for engaging adaptive responses.
Figure 1.
Hypothesized relationship between extracellular oxygen tension and mitochondrial respiration in dissociated cells. Published studies (26) show that the rate of oxygen consumption does not become limited by O2 until a critically low Po2 is reached, indicating that the apparent Km of the enzyme for O2 is very low.
The ability to detect cellular hypoxia and to activate protective mechanisms in response to small decreases in oxygen tension is an important characteristic of a biological oxygen sensing system. If the response to hypoxia is activated only when the cell approaches anoxia, the opportunity to initiate protective mechanisms to avert a bioenergetic crisis becomes limited. For example, expression of new genes requires the activation of transcription factors such as HIF, translation of the protein, peptide folding, and frequent transport to a particular cell domain. These ATP-dependent functions can be suppressed when the ATP concentration is compromised, undermining the ability of the cell to respond. Other responses, such as the VEGF-dependent regulation of tissue capillary density, evolve over days or weeks, so the homeostatic maintenance of tissue oxygenation requires continuous adjustment of signaling to maintain capillary bed structure at a level consistent with the normal range of oxygen demand by the organ. In terms of oxygen sensing, an early warning system is essential.
A wide range of oxygen sensors have been proposed to exist, and it is likely that several independent systems contribute to the regulation of hypoxic responses in diverse cell types. One model of oxygen sensing implicates a role for the mitochondria, which represent an appealing site for O2 sensing because the terminal cytochrome oxidase binds O2, and these organelles represent the primary site of oxygen utilization in the cell. However, when considering mitochondria as an oxygen-sensing organelle, an important conceptual barrier is encountered. Specifically, if the mitochondria can maintain oxygen consumption and ATP production until the O2 falls to less than 1%, how can this organelle detect a decrease from, say, 6 to 3% O2? Because the ability to generate ATP does not decrease when the cellular O2 level decreases from 6 to 3% O2, it seems unlikely that a decrease in ATP concentration could act as the signal triggering cellular responses. Mills and Jobsis suggested that some hypoxia-sensitive cells, such as the glomus cells of the carotid body, might express an alternative form of cytochrome oxidase with a lower affinity for O2, thereby allowing the cells to limit oxygen consumption at a higher O2 level (21, 22). Experimental evaluation of that hypothesis has not been achieved because the volume of carotid body glomus cells is too small to permit biochemical or proteomic analysis. However, more recent work indicates that virtually all cells in the body respond to hypoxia by activating HIF-1 or HIF-2 (23), so it is unlikely that a unique mechanism in those cells is responsible for their oxygen sensing response.
In the 1980s, Wilson and coworkers measured the relationship between cellular Po2 and cytochrome c reduction in the mitochondria (24–26). Using a novel phosphorescence system capable of measuring low oxygen tensions rapidly and accurately, they found that cytochrome c became progressively more reduced as the extracellular oxygen tension decreased from approximately 60 to approximately 5 mm Hg (25). Yet over the same range of oxygen tensions, the cellular oxygen consumption rate remained unchanged. These findings indicate that cytochrome oxidase behaves as if its affinity for O2 were very high, yet this is not the case. The ability to maintain an O2 consumption rate independent of Po2 arises because the inhibiting effect of lowering the Po2 at the oxidase is balanced by an increase in the reduction state of cytochrome c. Thus, decreasing the Po2 of the cell in the physiological range causes a progressive redox change in the electron transport chain. In this regard, the redox status of the electron transport chain provides a read-out of the Po2 at the oxidase, even though the rate of electron flux (and thus O2 consumption) does not become limited over this range. At the point where the Po2 falls below the critical threshold (∼1% O2), the oxygen consumption begins to decrease because the entire pool of cytochrome c has become populated with electrons (i.e., is fully reduced) and the limit of this compensation is reached. Hence, the redox status of the electron transport chain changes over the physiological range of hypoxia and can potentially act as an O2–sensitive signal.
If the redox status of the electron transport chain is the source of the oxygen sensing by the cell, how is this signal transmitted to activate functional responses to hypoxia? Our model of oxygen sensing implicates reactive oxygen species (ROS) generation by the mitochondria as the critical signal responsible for activating cellular adaptive responses. The evidence for this comes from a wide range of experiments using pharmacological, genetic, and biochemical approaches. The following sections detail the empirical evidence linking mitochondrial ROS production with the cellular response to hypoxia.
ROS Generation Increases during Hypoxia
Although some investigators have claimed that ROS production in the cell decreases during hypoxia (27), other experiments detected increases in the oxidation of redox-sensitive probes such as dichlorfluorecin (DCFH) in live-cell imaging studies (28, 29). Drugs that inhibit electron flux into Complex III, such as myxothiazol, inhibited the hypoxia-induced increase in DCFH oxidation or the oxidation of dihydroethidium (30, 31). Because an increase in ROS generation during hypoxia seems counterintuitive and because multiple reports have identified technical limitations to the use of DCFH, these findings led to a lively debate regarding their validity (32). Accordingly, better tools were needed to detect the ROS generation.
A major target of intracellular H2O2 signaling involves the oxidation of cysteine thiols on proteins. Hence, a fluorescent sensor of protein thiol oxidation was needed. A redox sensor that provides a ratiometric signal would be optimal because the measurement would be independent of the fluorescence intensity, sensor concentration, and other artifacts. We used HSP-FRET, a sensor comprised of cyan fluorescent and yellow fluorescent proteins linked by a thiol containing regulatory domain from the redox-regulated bacterial heat-shock protein HSP-33 (33, 34). When expressed in cells, this sensor provides a ratiometric assessment of protein thiol oxidation. In diverse cell types, including pulmonary arterial smooth muscle cells, HSP-FRET studies revealed an increase in oxidation during hypoxic incubation of the cells, which was inhibited by chemical antioxidants and by overexpression of antioxidant enzymes (35). Cells expressing HSP-FRET that were made completely anoxic failed to exhibit significant oxidation, indicating that O2, which is needed for ROS generation, was required for this response (33). Collectively, those studies recapitulated the responses obtained with the oxidant-sensitive fluorescent dyes and supported the idea that hypoxia augments ROS signaling in diverse cell types.
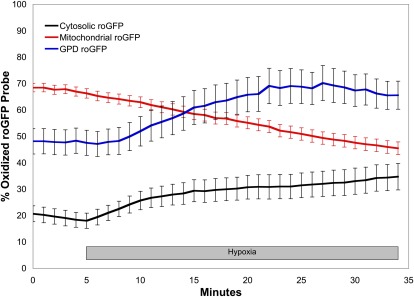
Although the HSP-FRET studies provided independent verification of hypoxia-induced ROS signaling, technical issues with the sensor, such as the inability to successfully target its expression to mitochondria, limited its usefulness. An alternative sensor developed by Remington and colleagues, termed roGFP, is comprised of green fluorescent protein (GFP) containing mutations that insert two cysteine thiol groups into the outer surface of the protein barrel (36–38). When these vicinial thiols are oxidized to form a dithiol linkage, the protein undergoes reciprocal changes in fluorescence emission when excited at two different wavelengths (Figure 2). The redox changes in the sensor are reversible, allowing the redox measurements to be calibrated at the end of an experiment by treatment with a reducing agent followed by a strong oxidizing agent. This feature permits a comparison of the absolute redox status among cell types. In addition, this smaller sensor can be engineered to cause the expressed protein to be trafficked to specific intracellular compartments, permitting the assessment of subcellular redox domains. Using this approach, Waypa and colleagues used roGFP to measure oxidative signaling in the cytosol, mitochondrial intermembrane space (IMS), and the mitochondrial matrix of cultured pulmonary artery smooth muscle cells (PASMCs) (39). Those studies revealed differences in the basal oxidation state among the subcellular compartments, with the matrix showing the highest degree of oxidation, followed by the intermembrane space, and the cytosol showing the lowest level of oxidation (Figure 3). During hypoxia, the cytosol and intermembrane space showed a progressive increase in oxidation, whereas the mitochondrial matrix oxidation status decreased. The increase in the IMS and cytosol is consistent with the idea that the ROS could be generated by the electron transport chain at the outer surface of the inner mitochondrial membrane (39). Superoxide produced there would tend to migrate to the IMS because the strong electrical field across the inner membrane (−180 mV) would drive superoxide anion away from the matrix and toward the IMS (32). There, dismutation to hydrogen peroxide would permit the ROS signaling to pass through the outer membrane to reach the cytosol. Why does hypoxia cause ROS generation in the matrix to decrease? One possibility is that ROS generation in the matrix is unregulated, nonspecific, and Po2 dependent, whereas ROS release to the IMS is a regulated event that is enhanced during hypoxia. Why does the ROS generation in the IMS increase when the availability of O2 for superoxide production is decreased? One possibility is that the increase in cytochrome c reduction during hypoxia causes an increase in the generation of superoxide at Complex III, which can release superoxide near the outer surface of the inner mitochondrial membrane. If the availability of electrons at Complex III is increased, then the superoxide generation could conceivably increase even though the availability of O2 is lessened in hypoxia. The alternative possibility is that Complex III function is O2 dependent, such that decreases in the oxygen level cause an increased release of superoxide to the intermembrane space and a decrease in release to the matrix, despite a decrease in the overall rate of superoxide generation. Presumably this would involve a mechanism whereby O2 dissolved in the inner membrane interacts with Complex III subunits to alter the structure of the Complex in a manner that augments the lifetime of the ubisemiquinone moiety, as detailed below.
Figure 2.
Changes in fluorescence properties of roGFP, a ratiometric redox sensor, in response to changes in the oxidation-reduction state of the cysteine thiols. GSH = reduced glutatione; S = oxidized cytsteine thiol; SH = reduced cysteine thiol.
Figure 3.
The redox status of the cytosol, mitochondrial intermembrane space (IMS), and mitochondrial matrix differ under normoxic conditions in pulmonary artery smooth muscle cells (39). During acute hypoxia, the cytosol and IMS become more oxidized, whereas the matrix compartment thiol oxidation decreases. Data extracted from Waypa and colleagues (39).
Role of Complex III in Hypoxia-Induced ROS Generation
In the mitochondrial electron transport chain, electron pairs removed from NADH (by Complex I) and from succinate (by Complex II) are transferred to ubiquinone, an organic electron carrier (coenzyme Q10) (Figure 4). When populated with two electrons, the ubiquinol diffuses to Complex III, where it binds to a pocket near the outer surface of the inner membrane (the Qo site). The first electron is transferred to the Rieske iron-sulfur protein (RISP), leaving a semiquinone radical in that pocket. The second electron is then transferred from the semiquinone to the b cytochromes, thereby returning the oxidized molecule to the membrane ubiquninone pool. The semiquinone radical is a likely source of superoxide production, and the lifetime of that moiety is therefore a potential regulator of superoxide production during hypoxia. In the absence of RISP, oxidation of ubiquinol cannot proceed, and superoxide generation at the Qo site is halted.
Figure 4.
Mitochondrial reactive oxygen species generation at Complex III in the mitochondrial electron transport chain. IMS = intermembrane space; SOD = superoxide dismutase.
To test this idea experimentally, Guzy and colleagues generated a shRNA knockdown of the RISP gene in a tumor cell line and measured the consequences for HSP-FRET oxidation during hypoxia (34). As predicted, knock down of RISP expression caused a significant attenuation of the oxidant signal generated during hypoxia, indicating that a functional Complex III is required for hypoxia-induced ROS generation by mitochondria. In a related study, Bell and colleagues used siRNA to knock down RISP in cultured cells and found that this attenuated hypoxia-induced DCFH oxidation (40). Similarly, Mansfield and colleagues studied cells carrying a genetic deletion of cytochrome c (41). In the absence of cytochrome c, cellular respiration is halted, as is the transfer of electrons from Complex III. Because the RISP center stays fully reduced, the oxidation of ubiquinol and the formation of ubisemiquinone is abolished. Accordingly, they found that cytochrome c deletion abrogated the increase in ROS generation during hypoxia. Subsequent studies by Korde and Wang used siRNA to knock down RISP in PASMCs, and their results confirmed previous studies indicating that hypoxia-induced ROS generation is abolished in the absence of a functional Complex III (42).
Role of Mitochondrial O2 Sensing in Regulating Cell Responses to Hypoxia
Based on redox measurements with HSP-FRET and roGFP, studies in cultured cells indicated that hypoxia triggers an increase in ROS generation in PASMCs. Are these increases in ROS signals involved in triggering the contractile response during acute HPV? The emerging answer to this question is yes, although the details regarding the specific targets of ROS signals are not fully clear (43). In this regard, low concentrations of exogenous H2O2 are sufficient to activate vasoconstriction in the intact lung, antioxidants abrogate the contractile response to hypoxia without blocking the response to a receptor-mediated vasoconstrictor such as U46619, and mitochondrial Complex III inhibition selectively blocks the constrictor response to hypoxia (44–46). A large number of reviews have weighed the evidence concerning the significance of ROS generation in HPV (43, 46–49). However, in vivo studies where the ability to generate mitochondrial ROS is inhibited, or where the critical targets of ROS signaling are genetically deleted, are required to provide a definitive test of this hypothesis.
The mechanisms regulating the stabilization of HIF-1α in hypoxia have been the focus of extensive study. Because PHDs require O2 as an enzymatic substrate for the reactions with HIF-1α, it is natural to expect that the lesser O2 availability in hypoxia would slow the hydroxylation and subsequent degradation of the α subunit by the proteasome. In that regard, PHD can theoretically function as an O2 sensor (16). However, a growing body of evidence from multiple laboratories indicates that mitochondria-derived ROS signals play an important role in regulating HIF-α stabilization by controlling the activity of PHD. The original description of this mechanism came from studies in ρ0 cells that lack mitochondrial DNA and a functional electron transport chain (30). Those cells can stabilize HIF-1α in response to PHD inhibitors but are unable to stabilize HIF-1α in hypoxia, indicating that the O2–sensing response requires functional mitochondria. Also, mitochondrial electron transport chain inhibitors acting upstream from Complex III were able to abolish hypoxic induction of HIF and expression of HIF-dependent genes. Subsequent studies showed that exogenous H2O2 was sufficient to induce HIF-1α stabilization in normoxic cells (50). Using genetic knock down of RISP, subsequent studies demonstrated that the loss of hypoxia-stimulated ROS generation was associated with a loss of HIF-α stabilization, as was the deletion of cytochrome c (34, 41).
Not all mitochondrial inhibition inhibits HIF stabilization; some modifications augment HIF. In humans, genetic mutations in Complex II are associated with paragangliomas and pheochromocytomas. Complex II is a four-subunit (A, B, C, and D) complex that functions as succinate dehydrogenase (SDH) and thereby links the Krebs cycle to the electron transport chain (51). Most frequently, these tumors carry mutations that result in somatic loss of the remaining allele in subjects who are heterozygous for the B, C, or D subunits of SDH (52). Mutations in the A subunit do not cause these responses and instead result in a bioenergetic crisis that resembles the effects caused by the mutation of other Krebs cycle enzymes. In cells with mutations in the B or D subunits, HIF-1α becomes constitutively stabilized under normoxia (53, 54), which can explain the tumorigenic behavior of these cells. Two opposing theories have emerged to explain why HIF is activated. According to one theory, the loss of succinate dehydrogenase activity causes succinate to accumulate in the cell. High levels of succinate can inhibit prolyl hydroxylases because of the structural similarity to 2-oxoglutarate, the normal substrate. The inhibition of PHD by succinate could then induce HIF-α stabilization in the cell (53). That model fails to provide a satisfactory explanation for why SDHA mutations fail to produce the same effect in terms of HIF and tumor formation because they should result in the same increase in succinate seen for mutations in the other subunits. Another problem is that all of the Krebs cycle enzymes are equilibrium systems and are reversible. Therefore, the blockade at a single enzyme in the cycle produces only a small accumulation of its substrates because the earlier enzymes also become inhibited when their products accumulate. Hence, one would predict that earlier enzymes should become inhibited as 2-oxoglutarate, isocitrate, and citrate concentrations rise, rather than creating a large accumulation of succinate just upstream from the site of inhibition.
A preferred explanation suggests that ROS production from the SDHA subunit is responsible for the HIF-α stabilization and tumor phenotype in these cells (54). In SDH, electrons removed from succinate by the catalytic A subunit are transferred to ubiquinone through a pathway involving the B, C, and D subunits. Hence, disruption of the B, C, or D subunits would strand electrons on the flavin group in the A subunit, leaving transfer to O2 (generating superoxide) as the only escape pathway. Indeed, disruption of the B (but not the A) subunit causes an increase in cellular ROS signaling, and the resulting normoxic stabilization of HIF-1α is abolished by chemical antioxidants (54). Thus, mitochondrial disruptions that augment ROS signaling can provide a gain-of-function in terms of the activation of hypoxia responses.
Chemical mitochondrial inhibitors that bind at the Qo site prevent ubiquinol binding, prevent hypoxia-induced ROS generation, and inhibit the hypoxic stabilization of HIF. They also block oxygen consumption and oxidative phosphorylation, which is lethal to some cells. In an effort to discover biologically active small molecules that alter tumor angiogenesis, Kwon and coworkers performed a screen of crude extracts from a microbial library using cultured endothelial cells. Under hypoxic conditions, endothelial cells rearrange to form tube-like structures, analogous to capillaries in hypoxic tissue. Their screen sought to identify small molecules that would block this hypoxic response without killing the cells. They identified terpestacin, a small molecule synthesized by a fungal species, as an inhibitor of the hypoxic response (55). Administration of the purified compound to the endothelial cultures blocked the hypoxic response, and administration of the compound to mice with subcutaneous tumor xenografts suppressed tumor growth, tumor VEGF expression, and tumor angiogenesis. Next, they performed a phage display analysis to identify the cellular protein(s) that interact with terpestacin. That analysis determined that the specific target of the molecule is a subunit of mitochondrial Complex III. Subsequent studies revealed that terpestacin blocks the hypoxic responses in cells by inhibiting the hypoxia-dependent increase in ROS generation, thereby inhibiting hypoxia-dependent HIF-1α stabilization in the cell. However, terpestacin does not appear to inhibit the stabilization of HIF-1α induced by direct PHD inhibitors, indicating that the response is specific to hypoxia (55). These findings provide independent verification, through an unbiased screen, that Complex III is involved in the signaling of hypoxia and the activation of adaptive responses. More recent work suggests that other novel small molecules may have a similar effect (56), although it is not clear whether these act at mitochondrial Complex III. A small molecule that blinds a cell to the fact that it is hypoxic could prove useful as a chemotherapeutic agent or adjuvant. Hypoxic conditions in tumors drive angiogenesis, and HIF-dependent genes play important roles in the survival, proliferation, and metastatic behaviors of many tumor types. Pharmacological suppression of the oxygen sensing system in tumor cells could therefore undermine the adaptive mechanisms that allow tumor cells to survive and proliferate in the hypoxic tumor microenvironment while rendering them more vulnerable to conventional chemotherapeutic agents.
Other Sources of ROS Generation during Hypoxia
Although mitochondria appear to be an important source of ROS signaling during hypoxia, other sources may also be important. The NAD(P)H oxidase family of ROS-generating enzymes is abundantly expressed in lung cells (57, 58), and chronic hypoxia leads to an up-regulation of Nox4 expression in the lung and in the media of small pulmonary arteries (58), probably through a HIF-mediated mechanism. Moreover, suppression of Nox4 expression in pulmonary artery smooth muscle cells in culture attenuated their proliferation in hypoxia and the generation of reactive oxygen species (58). Increased Nox4 expression is observed in thickened pulmonary arteries of patients with idiopathic pulmonary fibrosis (59) and idiopathic pulmonary hypertension (58). Emerging evidence suggests that amplification of endogenous ROS signaling by NADPH oxidase family members, particularly NOX4, may also regulate the hypoxic response (57, 58, 60). Some evidence suggests that NADPH oxidase activation may be triggered by mitochondria-derived ROS signals during acute hypoxia (61). However, studies of whether NOX4 knockout can prevent the remodeling of the pulmonary arterial system during chronic hypoxia in the mouse model have not been reported, so the requirement for Nox4 in the remodeling response is not established.
Alveolar Epithelial Cells
The internalization of Na,K-ATPase subunits from the basolateral membrane of alveolar epithelial cells in response to hypoxia, as described above, may represent an attempt of the cells to suppress ATP utilization. However, this response limits alveolar fluid clearance and may sustain pulmonary edema in acute lung injury or could promote alveolar edema in normal lungs at high altitude. In either case, what O2 sensor triggers this response? Studies by Comellas and colleagues (62) indicate that antioxidants prevented the internalization and that hypoxic alveolar epithelial cells deficient in mitochondrial function (ρ0 cells) failed to exhibit hypoxia-induced ROS signaling and failed to internalize the ATPase. PKC-zeta was also required for the response because treatment with an antagonizing peptide inhibited the internalization. In alveolar epithelial cells, hypoxia also leads to the phosphorylation of AMPK in a ROS-dependent manner (6); this response was required for the Na,K-ATPase internalization. In other studies, Emerling and colleagues found that the hypoxic activation of AMPK occurs at levels of hypoxia that are mild enough to avoid an increase in the AMP/ATP ratio (63). Thus, the functional responses to hypoxia in alveolar epithelium appear to be regulated by increases in ROS signaling through a pathway that includes PKC-ζ and AMPK.
Summary
Physiological hypoxia triggers diverse responses involving transcriptional and posttranslational mechanisms in lung cells through the activation of cellular oxygen sensors. Mitochondrial redox changes track with cellular Po2, and increases in the generation of superoxide at Complex III are required for triggering these functional responses. The requirement for functional Complex III is clear. Although the precise mechanism linking the oxygen concentration to the increase in ROS is not fully established, the release of ROS to the intermembrane space, rather than the matrix, seems to be involved. Although genetic disassembly of the electron transport chain can block these responses, newly discovered small molecules that interact with Complex III can also block the hypoxic response without interfering with oxidative phosphorylation or oxygen consumption. Treatment of lung diseases, such as hypoxia-mediated vasoconstriction, lung cancer, pulmonary hypertension, and high-altitude pulmonary edema, may benefit from these drugs that blind the cells to the presence of hypoxia and blunt their ability to respond.
Footnotes
Supported by NIH grants HL35440 and RR025355.
Author Disclosure: P.T.S. received honorarium, support for travel, and payment for writing or reviewing the manuscript from Boehringer Ingelheim.
References
- 1.Gayeski TE, Honig CR. Intracellular PO2 in individual cardiac myocytes in dogs, cats, rabbits, ferrets, and rats. Am J Physiol 1991;260:H522–H531 [DOI] [PubMed] [Google Scholar]
- 2.Schumacker PT, Cain SM. The concept of a critical oxygen delivery. Intensive Care Med 1987;13:223–229 [DOI] [PubMed] [Google Scholar]
- 3.Budinger GRS, Duranteau J, Chandel NS, Schumacker PT. Hibernation during hypoxia in cardiomyocytes: role of mitochondria as the O2 sensor. J Biol Chem 1998;273:3320–3326 [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA. The acute respiratory distress syndrome. N Engl J Med 1996;334:1469–1470 [DOI] [PubMed] [Google Scholar]
- 5.Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest 2008;118:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol 2009;29:3455–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA 1991;88:5680–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med 2010;2:336–361 [DOI] [PubMed] [Google Scholar]
- 9.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood 2009;114:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA 1998;95:7987–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468–472 [DOI] [PubMed] [Google Scholar]
- 12.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001;292:464–468 [DOI] [PubMed] [Google Scholar]
- 13.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999;399:271–275 [DOI] [PubMed] [Google Scholar]
- 14.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 2004;279:38458–38465 [DOI] [PubMed] [Google Scholar]
- 15.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev 2002;16:1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 2004;5:343–354 [DOI] [PubMed] [Google Scholar]
- 17.Chandel NS, Budinger GRS, Schumacker PT. Molecular oxygen modulates cytochrome c oxidase function. J Biol Chem 1996;271:18672–18677 [DOI] [PubMed] [Google Scholar]
- 18.Chandel NS, Budinger GRS, Choe SH, Schumacker PT. Cellular respiration during hypoxia: role of cytochrome oxidase as the oxygen sensor in hepatocytes. J Biol Chem 1997;272:111–112 [DOI] [PubMed] [Google Scholar]
- 19.Fisher AB, Dodia C. Lung as a model for evaluation of critical intracellular PO2 and PCO. Am J Physiol 1981;241:E47–E50 [DOI] [PubMed] [Google Scholar]
- 20.Schumacker PT, Chandel N, Agusti AGN. Oxygen conformance of cellular respiration in hepatocytes. Am J Physiol 1993;265:L395–L402 [DOI] [PubMed] [Google Scholar]
- 21.Mills E, Jobsis FF. Simultaneous measurement of cytochrome a3 reduction and chemoreceptor afferent activity in the carotid body. Nature 1970;225:1147–1149 [DOI] [PubMed] [Google Scholar]
- 22.Mills E, Jobsis FF. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J Neurophysiol 1972;35:405–428 [DOI] [PubMed] [Google Scholar]
- 23.Maxwell PH, Pugh CW, Ratcliffe PJ. Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci USA 1993;90:2423–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson DF, Erecinska M. Effect of oxygen concentration on cellular metabolism. Chest 1985;88:229S–232S [DOI] [PubMed] [Google Scholar]
- 25.Wilson DF, Rumsey WL, Green TJ, Vanderkooi JM. The oxygen dependence of mitochondrial oxidative phosphorylation measured by a new optical method for measuring oxygen concentration. J Biol Chem 1988;263:2712–2718 [PubMed] [Google Scholar]
- 26.Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem 1990;265:15392–15399 [PubMed] [Google Scholar]
- 27.Archer S, Michelakis E. The mechanism(s) of hypoxic pulmonary vasoconstriction: potassium channels, redox O(2) sensors, and controversies. News Physiol Sci 2002;17:131–137 [DOI] [PubMed] [Google Scholar]
- 28.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 1998;273:11619–11624 [DOI] [PubMed] [Google Scholar]
- 29.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 1998;273:18092–18098 [DOI] [PubMed] [Google Scholar]
- 30.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 1998;95:11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol 1999;277:H2240–H2246 [DOI] [PubMed] [Google Scholar]
- 32.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 2006;91:807–819 [DOI] [PubMed] [Google Scholar]
- 33.Robin E, Guzy RD, Loor G, Iwase H, Waypa GB, Marks JD, Vanden Hoek TL, Schumacker PT. Oxidant stress during simulated ischemia primes cardiomyocytes for cell death during reperfusion. J Biol Chem 2007;282:19133–19143 [DOI] [PubMed] [Google Scholar]
- 34.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 2005;1:401–408 [DOI] [PubMed] [Google Scholar]
- 35.Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 2006;99:970–978 [DOI] [PubMed] [Google Scholar]
- 36.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem 2004;279:13044–13053 [DOI] [PubMed] [Google Scholar]
- 37.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem 2004;279:22284–22293 [DOI] [PubMed] [Google Scholar]
- 38.Remington SJ. Fluorescent proteins: maturation, photochemistry and photophysics. Curr Opin Struct Biol 2006;16:714–721 [DOI] [PubMed] [Google Scholar]
- 39.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 2009;106:526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 2005;1:409–414 [DOI] [PubMed] [Google Scholar]
- 41.Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab 2005;1:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korde AS, Yadav VR, Zheng YM, Wang YX. Primary role of mitochondrial Rieske iron-sulfur protein in hypoxic ROS production in pulmonary artery myocytes. Free Radic Biol Med 2011;50:945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connolly MJ, Aaronson PI. Cell redox state and hypoxic pulmonary vasoconstriction: recent evidence and possible mechanisms. Respir Physiol Neurobiol 2010;174:165–174 [DOI] [PubMed] [Google Scholar]
- 44.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 2001;88:1259–1266 [DOI] [PubMed] [Google Scholar]
- 45.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 2002;91:719–726 [DOI] [PubMed] [Google Scholar]
- 46.Waypa GB, Schumacker PT. Hypoxia-induced changes in pulmonary and systemic vascular resistance: where is the O2 sensor? Respir Physiol Neurobiol 2010;174:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol 2010;174:182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuchs B, Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Grimminger F, Seeger W, Gudermann T, Weissmann N. Redox signaling and reactive oxygen species in hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 2010;174:282–291 [DOI] [PubMed] [Google Scholar]
- 49.Schumacker PT, Ward JP. Physiological redox signaling in lung, cardiovascular and neural cells: progress, controversy and potential. Respir Physiol Neurobiol 2010;174:163–164 [DOI] [PubMed] [Google Scholar]
- 50.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial Complex III stabilize HIF-1-alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 2000;275:25130–25138 [DOI] [PubMed] [Google Scholar]
- 51.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000;287:848–851 [DOI] [PubMed] [Google Scholar]
- 52.McWhinney SR, Pilarski RT, Forrester SR, Schneider MC, Sarquis MM, Dias EP, Eng C. Large germline deletions of mitochondrial complex II subunits SDHB and SDHD in hereditary paraganglioma. J Clin Endocrinol Metab 2004;89:5694–5699 [DOI] [PubMed] [Google Scholar]
- 53.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005;7:77–85 [DOI] [PubMed] [Google Scholar]
- 54.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of SdhB, but not SdhA, subunit of Complex II triggers ROS-dependent HIF activation and tumorigenesis. Mol Cell Biol 2007;28:718–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung HJ, Shim JS, Lee J, Song YM, Park KC, Choi SH, Kim ND, Yoon JH, Mungai PT, Schumacker PT, et al. Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J Biol Chem 2010;285:11584–11595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim KH, Park JY, Jung HJ, Kwon HJ. Identification and biological activities of a new antiangiogenic small molecule that suppresses mitochondrial reactive oxygen species. Biochem Biophys Res Commun 2011;404:541–545 [DOI] [PubMed] [Google Scholar]
- 57.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 2008;10:1687–1698 [DOI] [PubMed] [Google Scholar]
- 58.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 2007;101:258–267 [DOI] [PubMed] [Google Scholar]
- 59.Pache JC, Carnesecchi S, Deffert C, Donati Y, Herrmann FR, Barazzone-Argiroffo C, Krause KH. NOX-4 is expressed in thickened pulmonary arteries in idiopathic pulmonary fibrosis. Nat Med 2011;17:31–32 [DOI] [PubMed] [Google Scholar]
- 60.Weissmann N, Zeller S, Schafer RU, Turowski C, Ay M, Quanz K, Ghofrani HA, Schermuly RT, Fink L, Seeger W, et al. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 2006;34:505–513 [DOI] [PubMed] [Google Scholar]
- 61.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res 2006;98:1314–1322 [DOI] [PubMed] [Google Scholar]
- 63.Emerling BM, Platanias LC, Black E, Nebreda AR, Davis RJ, Chandel NS. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol Cell Biol 2005;25:4853–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]