Abstract
Cor pulmonale has long been described in very severe chronic obstructive pulmonary disease (COPD) and emphysema. Cross-sectional results from population-based studies show that left ventricular filling and a variety of vascular measures in the systemic circulation are abnormal in preclinical COPD and emphysema and that a predominant vascular change in COPD and emphysema is endothelial and microvascular dysfunction. These findings suggest that pulmonary vascular changes may occur early in COPD and emphysema and might contribute to pathogenesis. However, longitudinal epidemiologic studies with direct measures of the pulmonary vasculature are lacking; therefore, inferences are limited at present. New imaging-based approaches to the assessment of the pulmonary vasculature are applicable to epidemiologic studies and may help in defining the relationship of pulmonary vascular damage to progression of COPD and emphysema. These measures may also provide imaging-based surrogate markers, and novel therapeutics targeted to the pulmonary vasculature might reduce symptoms and improve function in these common diseases.
Keywords: chronic obstructive pulmonary disease, pulmonary emphysema, pulmonary hypertension, vascular disease, pulmonary vasculature
Cor Pulmonale: Pulmonary Heart Disease
Increased pulmonary vascular resistance and right heart failure have long been known to occur in very severe chronic obstructive pulmonary disease (COPD) and emphysema (1). Classic reports of cor pulmonale describe elevated pulmonary vascular resistance and right heart failure with reductions in left ventricular filling, left ventricular stroke volume, and cardiac output, but generally preserved left ventricular ejection fraction (2–4).
Clinical experience has demonstrated mild resting pulmonary hypertension in severe COPD without a significant reduction in left ventricular ejection fraction, although exercise-induced pulmonary hypertension is more common (1). In this usual schema, cor pulmonale is generally absent in mild, moderate, and even severe COPD but develops as an effect of end-stage, very severe COPD. The relationship between the FEV1 and left ventricular stroke volume can be conceptualized as resembling a hockey stick, with little effect of airflow limitation on left ventricular hemodynamics until the FEV1 is very severely reduced.
The traditional explanations for elevated pulmonary artery pressures in COPD are that tissue destruction causes loss of vasculature and that hypoxemia and acidosis cause pulmonary artery vasoconstriction (5, 6). Severe hyperinflation from air trapping is correlated with ventricular dimensions (7) and may cause pulmonary air pressure to exceed pulmonary venous pressure, but its effect on hemodynamics is debated (8). Surgical relief of hyperinflation, for example, lowers pulmonary venous but not arterial pressures (9).
Current work in basic science described elsewhere in this issue (see article by Petrache and colleagues, pp. 492–496) suggests that endothelial damage may contribute to emphysema, and recent observations of impaired left ventricular filling in preclinical emphysema and COPD provide indirect support for pulmonary vascular damage in the development of emphysema and COPD. Both revive the endothelial hypothesis of emphysema.
Pulmo Vasculare: Vascular Lung Disease
Historical Perspective
Early work noted prominent pulmonary vascular changes in emphysema and posited that vascular damage in the lungs may contribute to end-organ damage in the lungs as it does in other organs, such as the kidney. Almost 60 years ago, Abbott noted, on filling the pulmonary arteries of patients with emphysema post mortem with lipiodol, the “absence of smaller arteries in the more emphysematous areas” (10).
The prominent pathologist A. A. Liebow articulated more than 50 years ago an early construct of the endothelial hypothesis of COPD. After careful examination of the pulmonary arteries and veins of patients with emphysema, he postulated that changes in the local vascular milieu caused alveolar destruction in emphysema (11). Leading pulmonary physiologists at the time did not accept his hypothesis (12).
Difficulty in accessing the pulmonary vasculature compartment in clinical far less large-scale epidemiological studies, however, resulted in Liebow's hypothesis languishing. Contemporary imaging modalities are starting to allow the testing of his hypotheses in epidemiologic studies. To date, however, most clinical and epidemiologic studies have relied on proxy measures of pulmonary vascular structure and function. Most of these proxy measures have been defined in the systemic circulation and can be grouped into endothelial, microvascular, and macrovascular measures. This approach provides basic inferences but is limited in that abnormalities in the pulmonary circulation may or may not be reflected in the systemic circulation, and vice versa. An alternative approach is to examine blood flow into the left ventricle as a very indirect measure of pulmonary vascular resistance and pulmonary vascular damage. More recent and ongoing studies are using newer noninvasive approaches for the direct assessment of pulmonary vascular structure and function to tackle the endothelial hypothesis in epidemiologic studies more directly.
Systemic Endothelial Function
Direct assessment of pulmonary vascular endothelial function in vivo requires invasive catheterization and is not feasible in epidemiologic or large clinical studies. Flow-mediated dilation (FMD) of the brachial artery is a physiological test of endothelium-dependent, nitric oxide–mediated vasodilation, which has been validated against both coronary (13, 14) and pulmonary artery endothelial function (15).
We performed a study among 107 cotinine-confirmed greater than 10 pack-year former smokers nested within an ongoing prospective cohort study of smokers (16). FMD of the brachial artery was measured after 12-hour fast and 15-minute rest. Forty percent of participants had COPD. We observed significant associations of FMD with post-bronchodilator FEV1 and percent emphysema and consistent associations with diffusing capacity (DlCO).
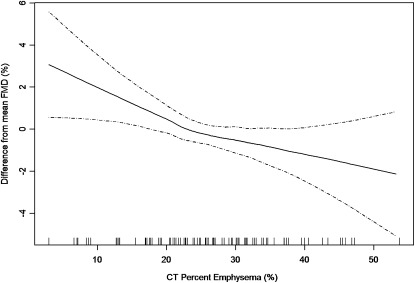
Because endothelial dysfunction was known to occur in very severe COPD (17), these observations might have been driven by the presence of a few patients with very severe COPD and not by the larger number of participants with milder or no COPD. In other words, endothelial dysfunction might have been a result of end-stage COPD and absent in milder COPD. However, to be involved in the pathogenesis of COPD and emphysema, endothelial dysfunction would be a requisite component of early, mild disease. The use of generalized additive models with smoothing function allowed the examination of this question. Generalized additive models with smoothing functions fit curves to the data, should curves be present (e.g., a hockey stick pattern) and straight lines should there be no evidence for nonlinearity. The associations of FMD with FEV1 and with percent emphysema (Figure 1) were not hockey sticks but rather were linear across the spectrum from normality to disease without statistical evidence for a hockey stick pattern (16). This finding suggests that endothelial dysfunction is a component of mild, early COPD and not just a result of end-stage cor pulmonale.
Figure 1.
Linear relationship between endothelial function measured by flow-mediated dilation of the brachial artery and percent of emphysema in former smokers. (Reprinted with permission from Reference 16.).
In addition, the relationship of FMD to the FEV1 in this study was entirely explained by percent emphysema, possibly suggesting that emphysema mediated the relationship of FMD to the FEV1. This finding is also consistent with the endothelial hypothesis, which is likely of greater relevance to emphysema than to airflow limitation.
Eickhoff and colleagues subsequently replicated these associations for lung function among 60 patients with COPD and 40 control subjects, with the additional contribution of showing independence from serum markers of inflammation (18). Likewise, Moro and colleagues found lower FMD among 44 patients with COPD without hypoxemia compared with 48 control subjects and a consistent relationship of FMD to airflow limitation (19). Both studies also found evidence for impaired nitrate-mediated dilation in COPD, which suggests subendothelial vascular dysfunction in COPD. The impairments in endothelial-dependent dilation in patients with COPD were considerably greater than those observed for endothelial-independent dilation (e.g., 31% vs. 15%, respectively, in Eickhoff and colleagues [18]) such that both authors concluded a mixed but predominantly endothelial defect in COPD.
In contrast, Maclay and colleagues found no difference in precise measures of endothelial reactivity during intrabrachial infusion of endothelium-dependent vasodilators among 18 men with COPD and 17 male control subjects (20). There were several differences between this study and the first three that might account for the diverse result. The first three measured systemic endothelial function using FMD, whereas the latter measured it using venous occlusion plethysmography. FMD reflects endothelial function in both conduit and resistance arteries, whereas venous occlusion plethysmography reflects endothelial function in resistance arteries (21). Conduit and resistance arteries in the systemic circulation differ with respect to caliber, location, and molecular characteristics (22), as they likely do in the pulmonary circulation, and only FMD has been validated against endothelial function of the pulmonary arteries, to my knowledge. Second, the strongest associations for FMD were observed for emphysema, which was not assessed in the Maclay and colleagues study (20). Third, its sample size was small, raising the possibility of a false-negative result. An additional difference was the high prevalence of inhaled corticosteroid use in that study (78% vs. 9% in ours; not reported in Eickhoff and colleagues [18] and Moro and colleagues [19]), which may be relevant because inhaled corticosteroids appear to normalize airway endothelial function (23). Whether these findings apply to systemic or pulmonary endothelial function is unknown, but they raise an intriguing explanation for the difference between the studies.
Hence, most of the published epidemiologic and clinical data suggest that systemic endothelial dysfunction occurs early in COPD and particularly emphysema, although the direct relevance to the pulmonary circulation, mechanisms, direction, and clinical implications of this association remain to be defined.
Systemic Microvascular Structure and Function
Multiple validated measures of small vessel and microvascular function are available for the systemic circulation. These include direct measures of retinal arteriolar and venular caliber and microalbinuria. The retinal arterioles narrow predominantly in response to hypertension and the retinal venules widen predominantly from smoking, inflammation, and diabetes; both changes independently predict cardiovascular events (24, 25). Less direct measures of systemic microvascular function include microalbuminuria, which is a measure of endothelial dysfunction, and microvascular damage in the renal circulation (26, 27), which also predicts cardiovascular events regardless of the presence of diabetes and hypertension (28, 29).
Among 3,397 participants with retinal measures in the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study, we recently found that retinal venular caliber was inversely associated with the FEV1 and FEV1/FVC ratio independent of smoking, biomarkers of inflammation, diabetes, and other risk factors for microvascular disease (30). The association was linear, as was previously found for FMD.
Polatli and others have found that microalbuminuria is increased in COPD exacerbations (31, 32), and Casanova and colleagues demonstrated greater microalbuminuria among patients with COPD compared with control subjects independent of smoking (33). They further found that microalbuminuria was particularly correlated with oxygen saturation in patients with COPD.
Together, these cross-sectional studies suggest that COPD is associated with systemic microvascular damage, as evidenced in the retinal and renal circulations and that these shared deficits in pulmonary and systemic microvascular structure and function are not simply due to shared risk factors, such as cigarette smoking. These cross-sectional associations suggest that COPD may cause systemic microvascular disease, that there is a shared susceptibility to the deleterious effects of cigarette smoking, or that endothelial dysfunction and microvascular disease in both the pulmonary and systemic circulations may contribute to COPD pathogenesis.
Systemic Macrovascular Structure and Function
In contrast to findings for systemic microvascular disease, the relationship between COPD and emphysema and systemic macrovascular measures is more varied. Measures of systemic macrovascular disease include those that approximate atherosclerosis and those that approximate large artery stiffness. Whereas atherosclerosis results predominantly from lipid deposition and related inflammation, changes in collagen and elastin matrix and composition, vascular aging, and atherosclerosis all can contribute to large artery stiffness.
Of noninvasive measures of atherosclerosis, coronary artery calcium measured on cardiac-gated computed tomography (CT) scans is, arguably, the best predictor of clinical cardiovascular events (34, 35). In the MESA Lung Study, there was no evidence of an association of either airflow limitation or percent emphysema on CT scan with either the presence or the extent of coronary artery calcium (36). These findings were similar to a smaller study of Korean men, in whom the FVC but not the FEV1/FVC ratio was associated with coronary artery calcium (41). The latter study suggests, in fact, that increased coronary artery calcium may be more of a component of restrictive rather than obstructive lung disease.
Thickening of the carotid intima media is a measure of subclinical atherosclerosis that independently predicts cardiovascular events. The common carotid intima media thickens in response to hypertension, whereas thickening of the internal carotid intima media may be a response to endothelial dysfunction and oxidative stress (37, 38). Airflow limitation was also associated in the MESA Lung Study with greater internal carotid intima media thickness among smokers but not among never smokers (36), findings that were similar to those in the Atherosclerosis Risk In Communities (ARIC) Study (39).
In contrast, percent emphysema on CT scan was associated with reduced ankle-brachial index in the MESA Lung Study regardless of and independent of smoking history (36), and emphysema fully explained the previously reported association of lung function with ankle-brachial index (39, 40). Low ankle-brachial index is a measure of atherosclerosis of the large arteries and defines peripheral vascular disease clinically. Nonetheless, endothelial dysfunction and microvascular disease are prominent features of peripheral vascular disease (41), and lower leg angioplasty in patients with peripheral vascular disease improves FMD (42). Hence, these findings may be of relevance to the pulmonary microcirculation, although validation studies are lacking. Interestingly, emphysema appeared to mediate the relationship of ankle-brachial index to lung function, reminiscent of our earlier findings for FMD (16).
Measures of central artery stiffness, such as pulse-wave velocity, are abnormal in clinical COPD (43) and emphysema among patients with COPD (44) but have been previously reviewed in detail in this journal (45) and hence are not covered here. These measures integrate stiffness, caliber, and tortuosity. We were not able to demonstrate significant relationships between decrements in lung function or subclinical increases in percent emphysema with a more direct measure of proximal aortic stiffness, distensibility of the ascending aorta on magnetic resonance imaging (MRI) among 1,917 participants in a population-based context (46).
Findings for macrovascular disease are more varied in COPD and emphysema and range from thoroughly null for coronary artery calcium to modestly strong relationships of internal carotid intima media thickness with lung function among smokers and ankle-brachial index with emphysema. Both patterns of associations might suggest that endothelial dysfunction is an early link of the vasculature to COPD and emphysema.
Percent Emphysema, Airflow Limitation, and Impaired Left Ventricular Filling
The endothelial hypothesis of emphysema suggests that endothelial and microvascular damage increase pulmonary vascular resistance with concomitant increases in emphysema and resultant airflow obstruction. Because elevated pulmonary vascular resistance causes impaired left heart filling and reductions in stroke volume and cardiac output, we hypothesized that greater percent emphysema and lower FEV1/FVC ratio would be associated with decrements in left ventricular end-diastolic volume, stroke volume, and cardiac output.
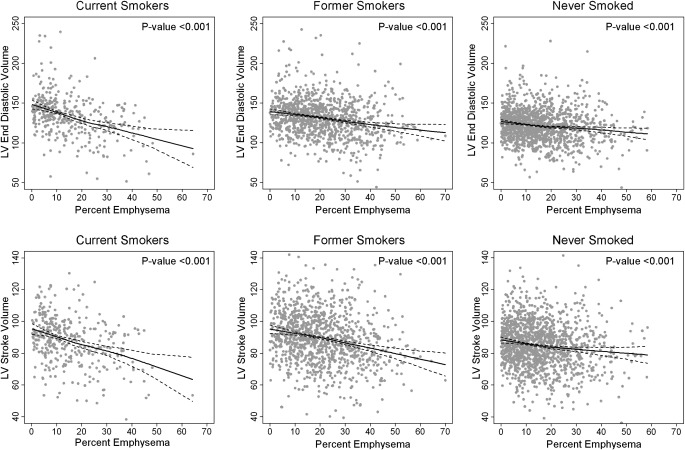
We evaluated these relationships among 2,771 participants in MESA Lung Study with left ventricular measures on MRI. We found strong, highly significant associations of the FEV1/FVC ratio and particularly percent emphysema with left ventricular end-diastolic volume, stroke volume, and cardiac output (47). These associations were linear across the spectrum of subclinical to clinical lung disease (Figure 2), which suggests that low left ventricular stroke volume and cardiac output occur not only in severe COPD but also in mild and subclinical lung disease. In contrast, there were no significant associations of lung measures with left ventricular ejection fraction.
Figure 2.
Continuous relationships of the percent emphysema to left ventricular (LV) end-diastolic volume in current smokers, past smokers, and never smokers in the Multi-Ethnic Study of Atherosclerosis. Multivariate relationships of the percent emphysema to left ventricular end-diastolic volume in current, former, and never smokers. Straight lines = smoothed regression lines adjusted for age, race/ethnicity, sex, body surface area, pack-years, urine cotinine, educational attainment, diabetes mellitus, fasting plasma glucose, body mass index, hypertension, systolic and diastolic blood pressure, C-reactive protein, fibrinogen, computed tomography scanner type, and milliampere dose. Dotted lines = 95% confidence intervals. Dots = predicted + residual values. (Reprinted with permission from Reference 47).
These associations were modified by smoking status (Figure 2; P interaction < 0.001). The multivariate association between percent emphysema and left ventricular end-diastolic volume was 9.2 ml (95% CI, 6.5–11.8; P = 10−11) in current smokers, 4.2 ml (95% CI, 2.8–5.4; P = 10−10) in former smokers, and 2.6 ml (95% CI, 1.4–3.7; P = 10−5) in never smokers. The magnitude of the association with left ventricular end-diastolic volume among smokers was greater, on a standard deviation basis, than all previously described risk factors, including age, and was accompanied by a reduction in left ventricular mass.
These unique results suggest that pulmonary blood flow is reduced in participants with subclinical airflow obstruction and emphysema such that left ventricular filling is impaired, with resultant reductions in stroke volume and cardiac output. Explanations for this subclinical association include early primary smoking-related pulmonary vascular damage contributing to cardiopulmonary impairment, hyperinflation (7), and concurrent accelerated aging of the lungs and heart manifest as emphysema and increased left ventricular stiffness (48). Supportive of the first explanation, recent studies confirm that left ventricular unloading due to known pulmonary vascular disease causes impaired left ventricular filling with a reversible reduction in left ventricular mass due to atrophy (49).
Pulmonary Vascular Structure and Function
Studies of excised lung tissue show significant morphological differences in the pulmonary artery endothelium of smokers with and without COPD and also in mild, moderate, and severe COPD (50, 51) in addition to attenuation of nitric oxide–mediated, endothelium-dependent relaxation (52, 53).
Such direct assessment of pulmonary vascular tissue is not feasible in large antemortem epidemiologic studies due to the invasiveness of such methods. Similarly, the definitive approach to the assessment of pulmonary vascular resistance, right heart catheterization with direct measurement of pressures, is also not feasible in large population-based studies. Catheterization is limited by invasiveness, expense, and potential insensitivity to subclinical and exercise-induced disease. Furthermore, right heart catheterization yields information on pressure but not volume or structure, omissions lamented by Cournand and Richards (54). Catheterization studies are generally small and included only severe COPD. The one study to include a sizable number of patients with mild to moderate COPD found subclinical increases in pulmonary artery pressure (55).
Recent advances in imaging technology, however, offer additional opportunities for the assessment of the pulmonary vasculature in humans. These include the use of contrast-enhanced CT imaging. This approach has demonstrated increased heterogeneity in regional perfusion parameters in smokers with emphysema compared with smokers without emphysema and persons who never smoked cigarettes (56). The size of the bolus of iodinated contrast in that study, however, required central line placement and would be inappropriate for epidemiologic studies. Nonetheless, advances in dual-source CT imaging (57) and non–contrast-enhanced approaches are overcoming this limitation.
The cross-sectional area of small pulmonary vessels on noncontrast chest CT scan correlate with FEV1, percent emphysema, DlCO, pulmonary artery pressure, and thoracic aortic calcium, a measure of systemic atherosclerosis, in patients with COPD (58–60). This latter finding provides evidence of concurrent pathology in the pulmonary and systemic circulations. In a more general sample of smokers, the total pulmonary vascular volume on noncontrast chest CT scan correlates with lung function and percent emphysema (61).
MRI has been used in large-scale epidemiologic studies (47) and gadolinium-enhanced MRI provides an opportunity to assess both cardiac and pulmonary perfusion (62–65). Hyperpolarized gas MRI provides measures of regional diffusion. Ongoing studies are likely to define directly the changes in pulmonary vasculature in varying severities of COPD and subtypes of emphysema.
Conclusions
Cor pulmonale has long been described in very severe COPD and emphysema. Cross-sectional results from population-based studies that have used a variety of vascular measures in the systemic circulation suggest that the predominant vascular change in COPD and particularly emphysema is endothelial and microvascular dysfunction. Longitudinal epidemiologic studies with direct measures of the pulmonary vasculature are lacking to date; therefore, inferences on cause and effect are limited at present. New imaging-based approaches to the assessment of the pulmonary vasculature are applicable to epidemiologic studies and may soon help in defining the relationship of pulmonary vascular damage to progression of emphysema and COPD and may provide imaging-based surrogate markers. Extant cardiovascular drugs and novel therapeutic agents targeted to the pulmonary vasculature might reduce symptoms and improve function in emphysema and COPD. Statins, for example, have long been known to improve endothelial function in humans and have recently been shown to improve pulmonary arterial endothelial function, reduce pulmonary artery pressure, and reduce emphysema in animals exposed to cigarette smoke (66).
Footnotes
Supported by National Institutes of Health grants R01-HL077612, R01-HL075476, R01-HL093081, and RC1-HL100543.
Author Disclosure: R.G.B. received support for travel for presentation at Transatlantic Airways Conference from Boehringer Ingelheim and received a donation of drugs from Cenestra Health for an NIH-sponsored clinical trial.
References
- 1.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part one. Am J Respir Crit Care Med 1994;150:833–852 [DOI] [PubMed] [Google Scholar]
- 2.Frank MJ, Weisse AB, Moschoes CB, Levinson GE. Left ventricular function, metabolism and blood flow in chronic cor pulmonale. Circulation 1973;47:798. [DOI] [PubMed] [Google Scholar]
- 3.Steele P, Ellis JHJ, Van Dyke D, Sutton F, Creagh E, Davies H. Left ventricular ejection fraction in severe chronic obstructive pulmonary disease. Am J Med 1975;59:21–28 [DOI] [PubMed] [Google Scholar]
- 4.Fishman AP. Chronic cor pulmonale. Am Rev Respir Dis 1976;114:775–794 [DOI] [PubMed] [Google Scholar]
- 5.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part two. Am J Respir Crit Care Med 1994;150:1158–1168 [DOI] [PubMed] [Google Scholar]
- 6.Barbera JA, Pienado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J 2003;21:892–905 [DOI] [PubMed] [Google Scholar]
- 7.Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, Magnussen H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest 2010;138:32–38 [DOI] [PubMed] [Google Scholar]
- 8.Macklem PT. Circulatory effects of expiratory flow-limited exercise, dynamic hyperinflation and expiratory muscle pressure. Eur Respir Rev 2006;15:80–84 [Google Scholar]
- 9.Criner GJ, Scharf SM, Falk JA, Gaughan JP, Sternberg AL, Patel NB, Fessler HE, Minai OA, Fishman AP. For the national emphysema treatment trial research group. Effect of lung volume reduction surgery on resting pulmonary hemodynamics in severe emphysema. Am J Respir Crit Care Med 2007;176:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott OA, Hopkins WA. Van Fleit WE, Robinson JS. A new approach to pulmonary emphysema. Thorax 1953;8:116–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibow AA. Pulmonary emphysema with special reference to vascular change. Am Rev Respir Dis 1959;80:67–93 [DOI] [PubMed] [Google Scholar]
- 12.Fishman A. Pulmonary emphysema with special reference to vascular change. General discussion. Am Rev Respir Dis 1959;80:92–93 [DOI] [PubMed] [Google Scholar]
- 13.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 1995;26:1235–1241 [DOI] [PubMed] [Google Scholar]
- 14.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 1998;82:1535–1539, A7–8 [DOI] [PubMed] [Google Scholar]
- 15.Peinado VI, Pizarro S, Diez M, Ferrer E, Sitges M, Molins L, Catalán M, Rodríguez-Roisin R, Roca J, Barberà JA. Assessment of endothelial function in systemic and pulmonary arteries in COPD [abstract]. Presented at the international meeting of the American Thoracic Society. May 2008, Toronto [Google Scholar]
- 16.Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, Shimbo D, Stevenson L. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the emphysema and cancer action project (EMCAP) study. Am J Respir Crit Care Med 2007;176:1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, Large SR, Wells FC, Wallwork J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med 1991;324:1539–1547 [DOI] [PubMed] [Google Scholar]
- 18.Eickhoff P, Valipour A, Kiss D, Schreder M, Cekici L, Geyer K, Kohansal R, Burghuber OC. Determinants of systemic vascular function in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;178:1211–1218 [DOI] [PubMed] [Google Scholar]
- 19.Moro L, Pedone C, Scarlata S, Malafarina V, Fimognari F, Antonelli-Incalzi R. Endothelial dysfunction in chronic obstructive pulmonary disease. Angiology 2008;59:357–364 [DOI] [PubMed] [Google Scholar]
- 20.Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Newby DE, MacNee W. Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:513–520 [DOI] [PubMed] [Google Scholar]
- 21.Lauer T, Heiss C, Balzer J, Keymel S, Kelm M, Preik M, Rassaf T. Resting microvascular resistance and conduit artery tone: relevance to endothelium-dependent flow-mediated dilation. Eur J Cardiovasc Prev Rehabil 2008;15:677–682 [DOI] [PubMed] [Google Scholar]
- 22.Burghuber OC, Valipour A. Knowing chronic obstructive pulmonary disease by heart: cumulating evidence of systemic vascular dysfunction. Am J Respir Crit Care Med 2009;180:487–488 [DOI] [PubMed] [Google Scholar]
- 23.Wanner A, Mendes ES. Airway endothelial dysfunction in asthma and chronic obstructive pulmonary disease: a challenge for future research. Am J Respir Crit Care Med 2010;182:1344–1351 [DOI] [PubMed] [Google Scholar]
- 24.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, Dejong PT, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med 2009;151:404–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol 2009;170:1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol 2006;17:2106–2111 [DOI] [PubMed] [Google Scholar]
- 27.Perticone F, Maio R, Tripepi G, Sciacqua A, Mallamaci F, Zoccali C. Microalbuminuria, endothelial dysfunction and inflammation in primary hypertension. J Nephrol 2007;20:S56–S62 [PubMed] [Google Scholar]
- 28.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001;286:421–426 [DOI] [PubMed] [Google Scholar]
- 29.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002;106:1777–1782 [DOI] [PubMed] [Google Scholar]
- 30.Harris B, Hoffman EA, Jacobs D, Klein R, Klein B, Wong T, Cotch MF, Jerosch-Herold M, Barr RG. Subclinical microvascular changes, lung function and CT lung density: the MESA lung study [abstract]. Am J Respir Crit Care Med 2009;179:A4521 [Google Scholar]
- 31.Komurcuoglu A, Kalenci S, Kalenci D, Komurcuoglu B, Tibet G. Microalbuminuria in chronic obstructive pulmonary disease. Monaldi Arch Chest Dis 2003;59:269–272 [PubMed] [Google Scholar]
- 32.Polatli M, Cakir A, Cildag O, Bolaman AZ, Yenisey C, Yenicerioglu Y. Microalbuminuria, von Willebrand factor and fibrinogen levels as markers of the severity in COPD exacerbation. J Thromb Thrombolysis 2008;26:97–102 [DOI] [PubMed] [Google Scholar]
- 33.Casanova C, de Torres JP, Navarro J, Aguirre-Jaime A, Toledo P, Cordoba E, Baz R, Celli BR. Microalbuminuria and hypoxemia in patients with COPD. Am J Respir Crit Care Med 2010;182:1004–1010 [DOI] [PubMed] [Google Scholar]
- 34.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345 [DOI] [PubMed] [Google Scholar]
- 35.Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, Kawut SM, Watson KE. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA lung study. Eur Respir J (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep 2009;11:21–27 [DOI] [PubMed] [Google Scholar]
- 38.Tabara Y, Kohara K, Nakura J, Miki T. Risk factor-gene interaction in carotid atherosclerosis: effect of gene polymorphisms of renin-angiotensin system. J Hum Genet 2001;46:278–284 [DOI] [PubMed] [Google Scholar]
- 39.Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis: The ARIC study. Atherosclerosis 2005;180:367–373 [DOI] [PubMed] [Google Scholar]
- 40.Engstrom G, Hedblad B, Valind S, Janzon L. Asymptomatic leg and carotid atherosclerosis in smokers is related to degree of ventilatory capacity: longitudinal and cross-sectional results from ‘men born in 1914', Sweden. Atherosclerosis 2001;155:237–243 [DOI] [PubMed] [Google Scholar]
- 41.Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP, et al. Atherosclerotic vascular disease conference: Writing GROUP III: Pathophysiology. Circulation 2004;109:2617–2625 [DOI] [PubMed] [Google Scholar]
- 42.Husmann M, Dörffler-Melly J, Kalka C, Diehm N, Baumgartner I, Silvestro A. Successful lower extremity angioplasty improves brachial artery flow-mediated dilation in patients with peripheral arterial disease. J Vasc Surg 2008;48:1211–1216 [DOI] [PubMed] [Google Scholar]
- 43.Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockcroft JR, Shale DJ. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:1259–1265 [DOI] [PubMed] [Google Scholar]
- 44.McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, Newby DE, Murchison JT, Macnee W. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:1208–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacNee W, Maclay J, McAllister DA. Cardiovascular injury and repair in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAllister D, MacNee W, Duprez DA, Hoffman EA, Vogel-Claussen J, Criqui MH, Budoff M, Jiang R, Bluemke DA, Barr RG. Pulmonary function is associated with distal aortic calcium, not proximal aortic distensibility. The MESA lung study. COPD 2011;8:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med 2010;362:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng S, Fernandes V, Bluemke D, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2009;2:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardziyenka M, Campian ME, Reesink HJ, Surie S, Bouma BJ, Groenink M, Klemens CA, Beekman L, Remme CA, Bresser P, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass: Evidence for atrophic remodeling. J Am Coll Cardiol 2011;57:921–928 [DOI] [PubMed] [Google Scholar]
- 50.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. Cd8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:822–826 [DOI] [PubMed] [Google Scholar]
- 51.Santos S, Peinado VI, Ramirez J, Morales-Blanhir J, Bastos R, Roca J, Rodriguez-Roisin R, Barbera JA. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1250–1256 [DOI] [PubMed] [Google Scholar]
- 52.Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol 1998;274:L908–L913 [DOI] [PubMed] [Google Scholar]
- 53.Dinh-Xuan AT, Pepke-Zaba J, Butt AY, Cremona G, Higenbottam TW. Impairment of pulmonary-artery endothelium-dependent relaxation in chronic obstructive lung disease is not due to dysfunction of endothelial cell membrane receptors nor to L-arginine deficiency. Br J Pharmacol 1993;109:587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards DW., Jr Nobel lecture. 1956. The contributions of right heart catheterization to physiology and medicine, with some observations on the physiopathology of pulmonary heart disease [accessed January 11, 2011]. Available from: http://nobelprize.org/nobel_prizes/medicine/laureates/1956/richards-lecture.html
- 55.Kessler R, Faller M, Weitzenblum E. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:219–224 [DOI] [PubMed] [Google Scholar]
- 56.Alford SK, van Beek EJ, McLennan G, Hoffman EA. Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proc Natl Acad Sci USA 2010;107:7485–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoey ETD, Mirsadraee S, Pepke-Zaba J, Jenkins DP, Gopalan D, Screaton NJ. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. AJR Am J Roentgenol 2011;196:524–532 [DOI] [PubMed] [Google Scholar]
- 58.Matsuoka S, Washko GR, Dransfield MT, Yamashiro T, San Jose Estepar R, Diaz A, Silverman EK, Patz S, Hatabu H. Quantitative CT measurement of cross-sectional area of small pulmonary vessel in COPD: correlations with emphysema and airflow limitation. Acad Radiol 2010;17:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuoka S, Washko GR, Yamashiro T, Estepar RSJ, Diaz A, Silverman EK, Hoffman E, Fessler HE, Criner GJ, Marchetti N, et al. ; National Emphysema Treatment Trial Research Group Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med 2010;181:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuoka S, Yamashiro T, Diaz A, San José Estépar R, Ross JC, Silverman EK, Kobayashi Y, Dransfield MT, Bartholmai BJ, Hatabu H, et al. The relationship between small pulmonary vascular alteration and aortic atherosclerosis in chronic obstructive pulmonary disease: quantitative CT analysis. Acad Radiol 2011;18:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grout R, Alford SK, Barr RG, Hoffman EA. Total pulmonary vascular volume: a new COPD phenotype correlated with quantitative CT and PFT measures of emphysema [abstract]. Am J Respir Crit Care Med 2010;181:A1529 [Google Scholar]
- 62.Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circulation 1990;82:1–7 [DOI] [PubMed] [Google Scholar]
- 63.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome x detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948–1953 [DOI] [PubMed] [Google Scholar]
- 64.Pedersen MR, Fisher MT, van Beek EJ, Pedersen MR, Fisher MT, van Beek EJR. MR imaging of the pulmonary vasculature–an update. Eur Radiol 2006;16:1374–1386 [DOI] [PubMed] [Google Scholar]
- 65.Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, Lechtzin N, Girgis RE, Mathai SC, Goldstein TA, et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology 2011;258:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright JL, Zhou S, Preobrazhenska O, Marshall C, Sin DD, Laher I, Golbidi S, Churg AM. Statin reverses smoke-induced pulmonary hypertension and prevents emphysema but not airway remodeling. Am J Respir Crit Care Med 2011;183:50–58 [DOI] [PubMed] [Google Scholar]