Abstract
Blood vessels and lymphatic vessels in the respiratory tract play key roles in inflammation. By undergoing adaptive remodeling and growth, blood vessels undergo changes that enable the extravasation of plasma and leukocytes into inflamed tissues, and lymphatic vessels adjust to the increased fluid clearance and cell traffic involved in immune responses. Blood vessels and lymphatics in adult airways are strikingly different from those of late-stage embryos. Before birth, blood vessels in mouse airways make up a primitive plexus similar to that of the yolk sac. This plexus undergoes rapid and extensive remodeling at birth. In the early neonatal period, parts of the plexus regress. Capillaries then rapidly regrow, and with arterioles and venules form the characteristic adult vascular pattern. Lymphatic vessels of the airways also undergo rapid changes around birth, when lymphatic endothelial cells develop button-like intercellular junctions specialized for efficient fluid uptake. Among the mechanisms that underlie the onset of rapid vascular remodeling at birth, changes in tissue oxygen tension and mechanical forces associated with breathing are likely to be involved, along with growth factors that promote the growth and maturation of blood vessels and lymphatics. Whatever the mechanisms, the dynamic nature of airway blood vessels and lymphatics during perinatal development foretells the extraordinary vascular plasticity found in many diseases.
Keywords: angiogenesis, blood vessels, lymphatics, lymphangiogenesis, respiratory tract
Plasma leakage, edema, leukocyte influx, and remodeling of the airway wall are well-documented features of asthma and other inflammatory airway diseases (1–3). Growth and remodeling of blood vessels and lymphatic vessels are features of sustained inflammation, but the pathophysiological and clinical implications of this plasticity are at an early stage of understanding (4–6). Further elucidation of the causes, consequences, and reversibility of changes in airway blood vessels and lymphatics is needed to develop rational plans for exploiting them as therapeutic targets.
Angiogenesis and microvascular remodeling are features of tissue remodeling in many chronic diseases (7). Both involve endothelial cell proliferation and often occur together but represent different vascular responses and have different driving factors and consequences. Unlike angiogenesis, wherein new blood vessels grow from existing ones, microvascular remodeling examined in preclinical models involves changes in endothelial cell phenotype and structural alterations, such as vascular enlargement or transformation of capillaries into venules, without the formation of new blood vessels (8–10). The microvasculature undergoes progressive changes in structure and function as conditions worsen and disease processes evolve. Lymphatic vessels also grow and undergo remodeling in inflammation and in tumors studied in mouse models and human disease (3, 7, 11–13).
Blood vessels of the microcirculation are organized into a hierarchy of arterioles, capillaries, and venules, each having a predetermined location and distinctive structural and functional characteristics (7, 14). This hierarchical organization is superimposed on organ-specific specializations. Blood vessels of the airways and lung have many features in common with those of other organs but have some that are unique. The monolayer of thin, tightly joined endothelial cells creates the barrier that controls transendothelial flux of water, solutes, and cells. Endothelial cells of stable blood vessels in the adult seldom sprout or divide under normal conditions. Pericytes are closely associated with endothelial cells and with their tight envelopment of basement membrane form a stable, functional unit. Vascular stability depends on the close association of pericytes and endothelial cells (15, 16).
Early in embryonic development, mesodermal cells differentiate into endothelial cells. These cells form a primitive plexus of small, anastomotic, immature blood vessels that lack the hierarchy of the adult vascular system. Later in development, the primitive plexus undergoes pruning and remodeling. The product is a hierarchical network of arteries, capillaries, and veins that expands and adapts in an organ-specific manner.
Expression of the transcription factor Prox1 in the nuclei of a population of endothelial cells of the embryonic cardinal vein begins the process of lymphatic specification that culminates in the formation of the lymphatic vascular system (17–19). After the initial lymph sacs form around E9.5 in the mouse, other lymph sacs form, and the components of adult lymphatic vascular system develop and mature.
The formation of the primitive vascular plexus and lymph sacs has been the focus of multiple important studies (20–23). Other recent advances have documented key features of the development of airways and lung before and after birth (23, 24). The pulmonary and bronchial vascular networks develop through varying contributions of vasculogenesis and angiogenesis. The pulmonary circulation appears during branching morphogenesis in the pseudoglandular stage of lung development (24). The tracheobronchial vasculature appears during the canalicular stage of lung formation, when branching of the bronchial tree is almost complete, and becomes the part of the systemic circulation that provides nutrients to the trachea and conducting airways.
The blood and lymphatic vasculatures of the trachea and bronchi of adult mice have proven advantageous for studying vascular remodeling, angiogenesis, and lymphangiogenesis because of their accessibility; segmented, highly organized architecture; clear anatomical separation of capillaries from arterioles, venules, and lymphatics; and the availability of informative disease models (7, 11, 25). Capillaries have a ladder-like arrangement in the mucosa over cartilage rings, and most arterioles and venules are located between cartilage rings. Lymphatics have a segmented architecture and a distinctive distribution between cartilage rings and absence over the rings (11, 26, 27).
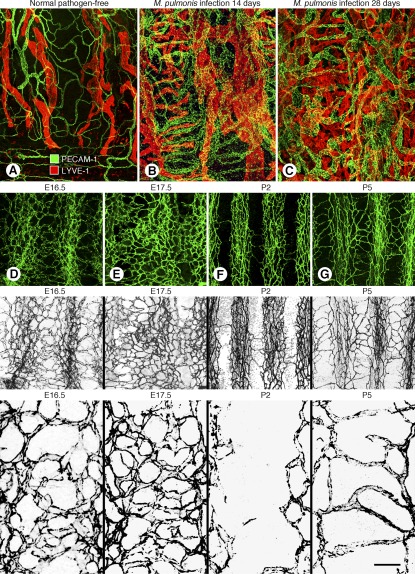
Recent studies have shown that the stereotypical architecture of the adult tracheobronchial vasculature develops around birth (28). Studies of normal mice in late gestation and early neonatal period revealed that the tracheal vasculature at E16.5 and E17.5 is a primitive embryonic plexus resembling the yolk sac vasculature and is unlike the adult airway vasculature (Figure 1) (28). The primitive plexus consists of an interconnected network of small vessels that lack the hierarchy typical of the adult. On the day of birth (P0), the primitive vascular plexus begins to undergo extensive remodeling. At P2, most of the blood vessels overlying tracheal cartilage rings have regressed, but by P5 the vasculature has regrown in a new pattern that evolves into the hierarchical, segmented, ladder-like adult vascular architecture (Figure 1) (28).
Figure 1.
(A–C) Remodeling of airway blood vessels and lymphatics in mice after Mycoplasma pulmonis infection. Confocal micrographs of mouse tracheal whole mounts stained for blood vessels (PECAM-1, green) and lymphatic vessels (LYVE-1, red). (A) Pathogen-free mouse. (B) Mouse with M. pulmonis infection of the respiratory tract for 14 days. (C) Mouse infected for 28 days. (D–G) Pre- and postnatal changes in tracheal blood vessels from E16.5 through P5. Confocal micrographs of tracheal whole mounts with blood vessels stained for PECAM-1. (D, E) At E16.5 and E17.5 the tracheal vasculature is a primitive plexus of highly anastomotic, undifferentiated blood vessels. (F) At P2 the vasculature is segmented by pruning of the primitive plexus over cartilage rings. (G) At P5 the adult vascular pattern is evident after horizontal capillaries have grown over cartilage rings and arterioles and venules form between the rings. The second row of images shows PECAM-1 staining of blood vessels in color. The third row shows the same images in grayscale to emphasize the conspicuous differences in the vasculature patterns. The bottom row of images shows higher magnification views of the vascular architecture. Scale bar at bottom right is 100 μm in top row, 80 μm in second and third rows, and 20 μm in bottom row. (A–C reprinted with permission from Reference 11; D–G reprinted with permission from Reference 28).
Among the mechanisms that underlie the rapid vascular remodeling in airways at birth, changes in tissue oxygen tension, HIF-1α expression, and mechanical forces associated with the onset of breathing are believed to be involved, along with vascular endothelial growth factor, angiopoietins, platelet-derived growth factor, and other factors that promote the growth and maturation of blood vessels (28).
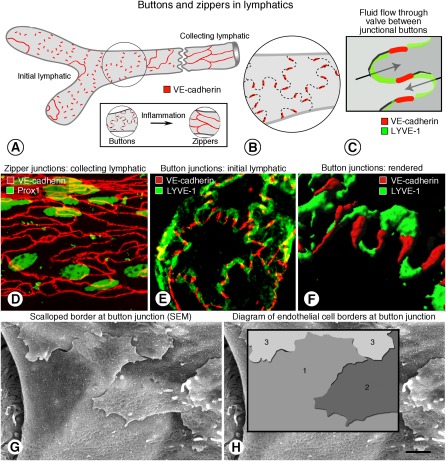
The overall architecture of airway lymphatics of mice at E16.5 is simpler but otherwise generally similar to the adult pattern, but the lymphatic endothelial cells differ from those of the adult. Initial lymphatics of mice have specialized discontinuous junctions separated by valve-like gaps at sites of fluid entry, whereas downstream collecting lymphatics have continuous junctions similar to those of blood vessels (Figure 2) (26). We have called the discontinuous, button-like junctions “buttons” and the continuous, zipper-like junctions “zippers” (26). Buttons are oriented parallel to the sides of the protruding parts of the scalloped border of lymphatic endothelial cells, where they contact the complementary part of the adjacent endothelial cell (Figure 2). Valve-like gaps located between buttons are preferential sites of fluid and cell entry into initial lymphatics (Figure 2) (26, 29). Buttons and zippers are both composed of the adherens junction protein vascular endothelial–cadherin and multiple tight junction proteins (occludin, claudin-5, ZO-1, ESAM, JAM-A) (26). Although the proteins are apparently the same in both types of junction, they have distinctly different arrangements.
Figure 2.
(A–C) Buttons at sites of fluid entry in initial lymphatics in mice. (A) Schematic diagram showing distinctive, discontinuous buttons in endothelium of initial lymphatics and continuous zippers in collecting lymphatics as revealed by vascular endothelial (VE)-cadherin immunoreactivity. (B) More detailed diagram of the oak leaf–shaped endothelial cells (dashed lines) of initial lymphatics. Buttons (red) appear to be oriented perpendicular to the cell border but are in fact parallel to the sides of flaps. Most LYVE-1 immunoreactivity is at the tips of flaps. (C) Enlarged diagram of buttons showing complementary shapes of overlapping flaps of adjacent oak leaf–shaped endothelial cells. Adherens junctions at the sides of flaps are believed to direct fluid entry (arrows) through the junction-free region at the tip. (D–H) Zipper and button junctions in endothelium of lymphatics. (D) Confocal image showing VE-cadherin immunoreactivity (red) at continuous zippers in collecting lymphatic identified by Prox1 (green) in nuclei. (E) Confocal image showing VE-cadherin at buttons (red) and LYVE-1 between buttons (green) at the border of oak leaf–shaped endothelial cells of initial lymphatic. (F) Rendered version of region in E shown here at higher magnification. (G) Scanning electron microscopic image showing external surface of overlapping flaps at the junction of three endothelial cells of initial lymphatic. (H) Drawing of region in G showing the contributions of three endothelial cells. Scale bar is 10 μm in D–E, 3 μm in F, and 1.4 μm in G–H. (Reprinted with permission from Reference 26).
Lymphatics in mice at E16.5 have abundant zippers but no buttons. About 6% of the adult complement of buttons are present at E17.5, 12% at E18.5, and 35% at birth (E19.5/P0). The number increases to about 90% at P28. The number at P70 is considered 100%. Proteins associated with adherens junctions and tight junctions are present in both types of junctions throughout development, but the distribution of the proteins changes as zippers are replaced by buttons.
Studies of changes in lymphatic junctions at birth provide a better understanding of the dynamic features of lymphatics in airways of neonatal mice and offer the opportunity to identify factors that influence their growth and function during this critical period. Delineation of factors that influence lymphatic development and maturation is also important for understanding the mechanism of edema formation and resolution. Edema can occur in asthma and other inflammatory conditions of human airways and lung when the rate of plasma leakage exceeds fluid clearance through lymphatic vessels and other routes. Mucosal edema contributes to airway wall thickening and airflow obstruction in asthma (1–3).
Although much attention has been given to the contribution of blood vessel leakage to edema fluid, the cell biology underlying the clearance of the fluid through airway lymphatics has received little attention. Because lymphangiogenesis occurs in preclinical models of sustained airway inflammation (11), lymphatic growth could also occur in asthma, but the extent is unclear. Some evidence indicates that the number of lymphatics—or at least functional lymphatics—decreases in asthma (3, 13). If functional lymphatics are reduced, airway inflammation could lead to bronchial lymphedema and exaggerate airflow obstruction. Even if new lymphatics grow, the presence of edema of the airway wall would indicate that fluid leakage exceeds the clearance capacity of lymphatics and other routes.
It is unclear to what extent edema of the airways or lung results from insufficient lymphatic vessels to accommodate the plasma leakage in inflammation and to what extent lymphatics are functionally impaired in inflamed tissues. Experiments that are beginning to address these issues in preclinical models have shown that the endothelial cells of new lymphatics in inflamed airways have fewer button-like junctions necessary for efficient fluid clearance (26). If this abnormal structural feature of lymphatics is accompanied by impaired fluid uptake, it would add to the factors that can contribute to edema. It would also indicate a novel strategy for ameliorating mucosal edema by correcting lymphatic function. Verification by functional studies would support the concept that correction of defective lymphangiogenesis or lymphatic malfunction would complement the treatment of asthma and other inflammatory airway diseases.
The dynamic nature of airway blood vessels and lymphatics during perinatal development foretells the extraordinary vascular plasticity found in sustained inflammation. Because of this plasticity, blood vessels and lymphatics of the airways are sensitive indicators of environmental cues and tissue requirements that change during normal development, at birth, and in pathological conditions of the respiratory tract.
Acknowledgments
The authors thank Jonas Fuxe, Hiroya Hashizume, Amy Ni, and Erin Lashnits for their contributions to the preparation of the fluorescence, confocal, and scanning electron microscopic images.
Footnotes
Supported in part by National Institutes of Health grants HL24136, HL59157, and HL96511 from the National Heart, Lung, and Blood Institute (D.M.) and a postdoctoral fellowship from the Lymphatic Research Foundation (L.-C.Y.).
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960;13:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson JW, Hii S. The importance of the airway microvasculature in asthma. Curr Opin Allergy Clin Immunol 2006;6:51–55 [DOI] [PubMed] [Google Scholar]
- 3.Ebina M. Remodeling of airway walls in fatal asthmatics decreases lymphatic distribution: beyond thickening of airway smooth muscle layers. Allergol Int 2008;57:1–10 [DOI] [PubMed] [Google Scholar]
- 4.Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy 2010;65:946–958 [DOI] [PubMed] [Google Scholar]
- 5.Ribatti D, Puxeddu I, Crivellato E, Nico B, Vacca A, Levi-Schaffer F. Angiogenesis in asthma. Clin Exp Allergy 2009;39:1815–1821 [DOI] [PubMed] [Google Scholar]
- 6.Paredi P, Barnes PJ. The airway vasculature: recent advances and clinical implications. Thorax 2009;64:444–450 [DOI] [PubMed] [Google Scholar]
- 7.McDonald DM. Angiogenesis and vascular remodeling in inflammation and cancer: biology and architecture of the vasculature. : Figg WD, Folkman J, Angiogenesis: an integrative approach from science to medicine. New York: Springer; 2008. pp. 15–31 [Google Scholar]
- 8.Thurston G, Maas K, Labarbara A, McLean JW, McDonald DM. Microvascular remodelling in chronic airway inflammation in mice. Clin Exp Pharmacol Physiol 2000;27:836–841 [DOI] [PubMed] [Google Scholar]
- 9.Fuxe J, Lashnits E, O'Brien S, Baluk P, Tabruyn SP, Kuhnert F, Kuo C, Thurston G, McDonald DM. Angiopoietin/tie2 signaling transforms capillaries into venules primed for leukocyte trafficking in airway inflammation. Am J Pathol 2010;176:2009–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabruyn SP, Colton K, Morisada T, Fuxe J, Wiegand SJ, Thurston G, Coyle AJ, Connor J, McDonald DM. Angiopoietin-2-driven vascular remodeling in airway inflammation. Am J Pathol 2010;177:3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 2005;115:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007;6:273–286 [DOI] [PubMed] [Google Scholar]
- 13.El-Chemaly S, Levine SJ, Moss J. Lymphatics in lung disease. Ann N Y Acad Sci 2008;1131:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baluk P, Falcón BL, Hashizume H, Sennino B, McDonald DM. Cellular actions of angiogenesis inhibitors on blood vessels. : Marmé D, Fusenig N, Tumor angiogenesis: basic mechanisms and cancer therapy. New York: Springer Publishing Company; 2007. pp. 557–576 [Google Scholar]
- 15.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 2006;312:623–629 [DOI] [PubMed] [Google Scholar]
- 16.Fuxe J, Tabruyn SP, Colton K, Zaid H, Adams A, Baluk P, Lashnits E, Morisada T, Le T, O'Brien S, et al. Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation. Am J Pathol 2011;178:2897–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver G. Lymphatic vasculature development. Nat Rev Immunol 2004;4:35–45 [DOI] [PubMed] [Google Scholar]
- 18.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires prox1 activity. Genes Dev 2008;22:3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver G, Srinivasan RS. Lymphatic vasculature development: current concepts. Ann N Y Acad Sci 2008;1131:75–81 [DOI] [PubMed] [Google Scholar]
- 20.Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science 1999;284:1635–1639 [DOI] [PubMed] [Google Scholar]
- 21.Chinoy MR. Lung growth and development. Front Biosci 2003;8:d392–d415 [DOI] [PubMed] [Google Scholar]
- 22.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006;133:1611–1624 [DOI] [PubMed] [Google Scholar]
- 23.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature 2008;453:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparrow M, Weichselbaum M. The development of the bronchial circulation. Arch Physiol Biochem 2003;111:353–355 [DOI] [PubMed] [Google Scholar]
- 25.McDonald DM. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol 1994;266:L61–L83 [DOI] [PubMed] [Google Scholar]
- 26.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007;204:2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest 2009;119:2954–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni A, Lashnits E, Yao LC, Baluk P, McDonald DM. Rapid remodeling of airway vascular architecture at birth. Dev Dyn 2010;239:2354–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med 2009;206:2925–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]