Abstract
Ceramides are signaling sphingolipids involved in cellular homeostasis but also in pathological processes such as unwanted apoptosis, growth arrest, oxidative stress, or senescence. Several enzymatic pathways are responsible for the synthesis of ceramides, which can be activated in response to exogenous stimuli such as cytokines, radiation, or oxidative stress. Endothelial cells are particularly rich in acid sphingomyelinases, which can be rapidly activated to produce ceramides, both intracellular and at the plasma membrane. In addition, neutral sphingomyelinases, the de novo pathway and the ceramide recycling pathway, may generate excessive ceramides involved in endothelial cell responses. When up-regulated, ceramides trigger signaling pathways that culminate in endothelial cell death, which in murine lungs has been linked to the development of emphysema-like disease. Furthermore, ceramides may be released paracellularly where they are believed to exert paracrine activities. Such effects, along with ceramides released by inflammatory mediators, may contribute to lung inflammation and pulmonary edema, because ceramide-challenged pulmonary endothelial cells exhibit decreased barrier function, independent of apoptosis. Reestablishing the sphingolipid homeostasis, either by modulating ceramide synthesis or by opposing its biological effects through augmentation of the prosurvival sphingosine-1 phosphate, may alleviate acute or chronic pulmonary conditions characterized by vascular endothelial cell death or dysfunction.
Keywords: sphingolipids, apoptosis, pulmonary emphysema, acute lung injury, pulmonary circulation
Ceramides are signaling sphingolipids that consist of a sphingosine backbone and a fatty acid side chain, the length and saturation of which determines the identity of individual ceramide species. Ceramides serve either as metabolic intermediaries or building blocks of other sphingolipids, or as second messengers for functions ranging from growth arrest to apoptosis (1). The role of ceramide as a critical mediator of apoptosis has been revealed in many acute and chronic conditions, including radiation-induced injury, atherosclerosis, and Alzheimer’s dementia (2, 3). Its importance in the pathogenesis of lung diseases has been recently highlighted in reports of ceramide involvement in diverse conditions such as acute lung injury, cystic fibrosis, emphysema, lung infections, and asthma (4–6). The specificity of ceramide-triggered responses depends on the ceramide species, the enzymatic pathway responsible for its up-regulation, the subcellular niche of ceramide up-regulation, and the cell type in which this response occurs. In this report, we discuss the evidence of ceramides’ involvement in pulmonary vascular responses, in particular the mechanisms of ceramide up-regulation and the effect on endothelial cell function and lung pathology.
Ceramide Species and Enzymatic Pathways of Ceramide Synthesis in Lung Endothelial Cells
Ceramide is the generic term for multiple species, distinguished by the chain length and saturation of the N-linked fatty acid chain. With the development of sophisticated tandem mass spectrometry techniques necessary for their detection, it became evident that individual ceramide species play distinct biological roles (7, 8). Furthermore, their synthesis is regulated by several ceramide synthases (also known as longevity assurance LASS) (9), with affinity for specific fatty acids. We described the composition of ceramide species in the murine lung endothelium, which contains high levels of ceramide C16:0 (54% of total ceramides) (10), predominantly synthesized by ceramide synthases-5 and -6, followed by C24:1 (24% of total ceramides), typically a product of ceramide synthases-2 and -4 (11, 12). The significance of up-regulation of individual ceramide synthases and ceramide species in the lung remains to be elucidated. Thus far reports are implicating ceramide synthase-5 as the predominant ceramide synthase in the lung epithelium with a role in the negative regulation of surfactant synthesis (13), and C16:0 as the principal ceramide species associated with apoptosis in cultured mouse lung endothelial cells (10).
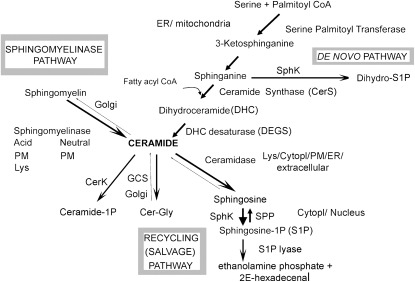
In addition to ceramide synthases, the enzymatic control of ceramide levels occurs catabolically via sphingomyelinases (SMase) or serine palmitoyltransferase (SPT) in the de novo pathway. The SMases found in the lung are the two types of acid SMases: lysosomal or soluble (also known as secreted) and the neutral SMases, which all hydrolyze membrane sphingomyelin to produce ceramide in various organelles or subcellular compartments. Of note, the endothelium contains the largest pool of acid SMase in the body (14) and may be responsible for the presence of circulating soluble SMase and ceramides, the plasma level of which was found to be elevated in systemic inflammatory states, including sepsis (15, 16). SPT, using as substrates serine and palmitoyl-CoA, regulates the first committed step in the de novo ceramide synthesis (17, 18) to form 3-ketodihydrosphingosine, an intermediate that undergoes spontaneous reduction to dihydrosphingosine. Dihydrosphingosine and specific acyl-CoA are substrates for ceramide synthases (19) to form dihydroceramides (DHC). Although differing from ceramides by just a double bond, DHC have different biological properties from ceramides and their conversion to ceramides, mediated by two DHC desaturases (20, 21), may also contribute to the control of ceramide abundance in cells. To some extent, ceramide levels are regulated through the rate of catabolism via ceramidases (22) and through the rate of recycling from sphingosine or other complex glycosphingolipids (Figure 1). Ceramide may be further phosphorylated by ceramide kinase to ceramide-1 phosphate or metabolized to sphingosine which, as a substrate for sphingosine kinases, produces sphingosine-1 phosphate (S1P). Both these metabolites have important signaling activities, suggesting that the complex network of sphingolipid-controlling enzymes may act as molecular switches. For example, these enzymes regulate the metabolism of more than one bioactive lipid with often opposite cellular functions; for example, by inducing ceramide accumulation they contribute to apoptosis, whereas by directing the synthesis of S1P they may stimulate cell growth and survival (23, 24). The ceramide/S1P balance has been established as a model of controlling cell fate in the ovary (24), immune system (25), and lung (26), including pulmonary endothelial cells. Although S1P's effects in the lung vascular function have been extensively characterized (and will not be the subject of our report), the ceramide-1 phosphate's effects in this compartment are still elusive, with reports implicating it in the regulation of innate immunity (27) via regulation of cytosolic phospholipase A2, one of critical enzymes mediating eicosanoid biosynthesis (28, 29).
Figure 1.
Schematic of ceramide-centered sphingolipid metabolism and subcellular localization of specific metabolic pathways. CerK = ceramide kinase; cytoplasm = cytopl; ER = endoplasmic reticulum; GCS = glucosylceramide synthase; ceramide-Gly = glucosylceramides; lys = lysosome; PM = plasma membrane; SPhK = sphingosine kinases; SPP = S1P phosphatase.
Stimuli Leading to Increased Ceramides in the Pulmonary Circulation
Numerous environmental and endogenous insults lead to ceramide up-regulation in endothelial cells. Among these, inflammatory mediators, such as tumor necrosis factor (TNF)-α (10, 30); LPS (31); platelet activating factor (32); ionizing radiation (33); oxidative stress from hypoxia/reoxygenation (34), vascular endothelial growth factor (VEGF) receptor inhibition, or even excess ceramides (35–37); growth factor deprivation (26); and cigarette smoking (26, 38) are particularly relevant to lung diseases that involve pulmonary vascular or microvascular dysfunction. The mechanism of ceramide synthesis may be specific to the stimulus and the context in which it triggers cellular responses. For example, TNF-α has been shown to rapidly (within minutes) induce acid sphingomyelinase, followed by an induction of the de novo pathway of ceramide synthesis at later time points (10–24 h) (10, 31). After VEGF receptor blockade, we detected first the activation of the de novo pathway of ceramide synthesis, followed by that of soluble acid SMase, along with increased lung ceramides (26, 38). The activation of the acid SMase in endothelial cells, similar to that of neutral SMase in lung epithelial cells (39), may be redox-dependent, as shown both in vivo and in vitro (36, 38), and could also be triggered by exogenous ceramide treatment (38). Indeed, direct enrichment of lungs or cells with ceramide itself may induce the acid SMase or the de novo pathway to produce more intracellular ceramides (10, 38), or the soluble SMase to generate paracellular ceramides (26). This process may reflect a propensity for a ceramide paracrine amplification loop, whereby ceramides generated paracellularly by soluble acid SMase may signal to adjacent cells to produce more ceramides. Although ceramides are very hydrophobic, they are found in acellular biological fluids, such as plasma (16) or bronchoalveolar lavage fluid (40), likely carried by proteins or by lipid microvesicles shed from plasma membranes (41).
Significance of Ceramide-Mediated Pulmonary Vascular Responses to the Lung Pathophysiology
Up-regulation of ceramides can mediate both extrinsic and intrinsic pathways of apoptosis in various cell types, including endothelial cells, via multiple mechanisms, such as death cell receptor clustering (42) and activation of caspase-8 (43), direct activation of protein phosphatases 1 and 2a (44–46), and direct effect on mitochondrial membrane permeability (47). In addition to apoptosis, ceramides have been involved in endothelial oxidative stress (38, 48, 49), growth arrest (50, 51), cytoskeletal changes (52), and senescence (53). Through these effects, ceramides regulate major aspects of lung endothelial cell function and, not surprisingly, have been involved in the pathogenesis of several conditions associated with pulmonary vascular dysfunction.
Ceramides generated by the acid SMase pathway have been implicated in pulmonary edema induced by excessive platelet activating factor in models of acute lung injury or in response to surges in TNF-α that may occur after acute exposure to LPS in sepsis (31, 32). The mechanism by which ceramide signaling leads to lung injury involves modulation of NO signaling in endothelial cell caveoli in a fashion that appears unique to the pulmonary circulation (54). Interestingly, the proedemagenic effect of ceramides on the lung endothelium appears to be independent of apoptosis (55), the typical cellular response induced by excess ceramides. This dichotomy in the signaling effects of ceramides on the cultured lung endothelium recapitulates that of TNF-α (56), a key inflammatory cytokine that is also a potent inducer of ceramides in endothelial cells. The sustained or chronic consequences of ceramide up-regulation in the lung endothelium, induced either by direct instillation of ceramides to the lung, by a decrease in VEGF signaling, or by targeted endothelial mitochondrial damage, have been associated in vitro with increased endothelial cell apoptosis and in vivo with airspace enlargement and a phenotype consistent with lung emphysema (26, 38, 57–59). Exposure to cigarette smoke, which is the most common cause of emphysema, increases ceramides in the human lung, murine lungs, and primary lung endothelial and epithelial cells as well as alveolar macrophages in culture conditions (26, 38, 39, 60, 61). We demonstrated that increased lung ceramides are sufficient to cause alveolar endothelial and epithelial cell apoptosis, activation of macrophages, and matrix proteolysis, which altogether recapitulate the phenotype of emphysema. We also showed that up-regulation of ceramides via the de novo pathway of synthesis was necessary for apoptosis and airspace enlargement in the VEGF receptor blockade model of emphysema (26). More recently, a requirement of neutral SMase-generated ceramides has been reported in relation to cigarette smoke–induced epithelial cell apoptosis (61). The relative contribution of endothelial and epithelial cell-generated ceramides to apoptosis and airspace enlargement in response to cigarette smoke remains to be elucidated. Furthermore, the effect of accumulation of downstream metabolites of ceramide on pulmonary vascular responses and pathology has been little explored.
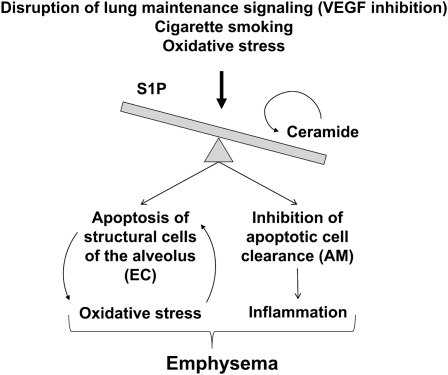
Cigarette smoke exposure may elevate ceramides either by oxidative stress, by VEGF receptor deprivation or, as recently reported, by alterations in the function of cystic fibrosis transmembrane regulator (CFTR), which in turn regulates ceramides at the plasma membrane through a process that may involve the de novo pathway (62). Our work demonstrated that in lung endothelial cells CFTR was required for proper ceramide homeostasis through a process that involved intracellular pH regulation, which in turn affected both the SMase and the de novo pathway enzymatic function (37). These findings complement the elegant work by Gulbins and colleagues, which demonstrated the importance of ceramide-induced apoptosis in epithelial cells bearing mutant CFTR in models of cystic fibrosis (63, 64). In these models, the acid SMase plays a key role in important pathogenetic processes of cystic fibrosis, controlling rates of epithelial cell apoptosis and the airways’ susceptibility to chronic lung infection (63, 64). It remains to be determined whether a similar mechanism may underlie the pathogenesis of chronic bronchitis in chronic obstructive pulmonary disease (COPD). It is possible that ceramides may be differently involved in the two major phenotypes composing COPD. In this regard, it is of interest to note that using a metabolomic approach combined with emphysema phenotyping using high-resolution computed tomography scanning of the chest, we recently reported the discovery of a sphingolipid metabolite, galabiosylceramide, which was present in all patients with emphysema but not in any of the patients with airways disease or chronic bronchitis (65). Current data indicate that the mechanisms by which ceramides contribute to emphysema development involve increased oxidative stress and structural alveolar cell apoptosis, which are linked via mutual interaction and self-amplification (38). In addition, de novo synthesized ceramides, and especially downstream production of sphingosine via acid ceramidase, profoundly inhibit the clearance of apoptotic cells by specialized alveolar macrophages (40). This is yet another self-perpetuating cycle triggered by ceramides (Figure 2) in which they both increase apoptosis of structural endothelial and epithelial cells in the lung and inhibit their clearance by macrophages, a process which may contribute to increased inflammation in the lungs of smokers and patients with COPD (66).
Figure 2.
Cartoon of proposed amplification loops triggered by disruptions of ceramide and S1P homeostasis leading to emphysema development. They include self-amplification of ceramide synthesis by ceramides produced paracellularly via soluble acid SMase; positive feedback between oxidative stress and apoptosis triggered by excess ceramides; and induction of structural lung endothelial and epithelial cells apoptosis (EC), with concomitant inhibition of their apoptotic body removal via a direct effect on alveolar macrophages (AM). This in turn may lead to spillage of intracellular contents and decreased antiinflammatory signaling by macrophages, contributing to lung inflammation. VEGF = vascular endothelial growth factor.
The involvement of endothelial cell ceramides in the pathogenesis of pulmonary vascular diseases, such as pulmonary hypertension or ischemia reperfusion injury, has not yet been elucidated, indicating a need for future investigations in these areas.
Harnessing ceramide's proapoptotic signaling in the endothelium could be achieved by inhibiting ceramide synthesis in the context of injury or by counteracting its biological effect through concomitant up-regulation of prosurvival pathways, such as those initiated by S1P. Both acid and neutral SMase inhibitors, as well as inhibitors of the de novo pathway of ceramide synthesis, effectively inhibited ceramide-induced apoptosis in the lung in various acute or chronic injury models in vivo, as recently reviewed by Uhlig and Gulbins (5). Inhibition of ceramide synthesis in the context of normal lung homeostasis may be detrimental, as we have shown using fumonisin B1, a ceramide synthases inhibitor, which dose-dependently increased endothelial cell apoptosis, lung apoptosis, and airspace enlargement in naive mice (26). Such approach may deplete cells from ceramide normally required for proper sphingolipid metabolism, including that of generating prosurvival metabolites. The role of S1P in cell survival and proliferation has long been recognized (67), an effect that in endothelial cells could be mediated via its receptors, also known as endothelial differentiation, G-protein–coupled receptors (68). We proposed that, similar to other organs, a balance between proapoptotic ceramide and prosurvival S1P is required for the maintenance of alveolar structures in the lung (57). Treatment of mice with agonists of S1P1 receptor inhibited endothelial cell apoptosis and airspace enlargement typically induced by VEGF receptor blockade (26), suggesting the proapoptotic function of ceramide can be antagonized by engaging S1P signaling in the lung.
In addition to cell survival, S1P modulates pulmonary endothelial cell motility and barrier function, either intracellular (69) or outside-in, via specific S1P receptors such as S1P1, which typically exerts barrier protective actions (70), or S1P2, which is barrier disruptive (71). There is evidence of an intricate interplay between signaling initiated by specific S1P receptors and the presence of intracellular S1P targets. This complex S1P signaling appears to be cell-specific and may explain the wide array of cellular responses to S1P or its synthetic analogs treatment (72–74).
This brief review of the role of ceramides in the pulmonary vascular function underscores the importance and the complexity of responses orchestrated by these signaling molecules and their interconnected bioactive sphingolipids. Although several effects of ceramides and S1P have been well characterized in the lung vasculature, the role of dihydroceramides, ceramide-1 phosphate, sphingosine, and glycosphingolipids synthesized from ceramides remains unknown. The study of the sphingolipid network responses to lung injury may be facilitated by complementary metabolomic and genomic approaches and mathematical modeling in addition to judicious use of animal models of disease. Understanding the interplay and the specificity of the sphingolipid signaling network in conditions such as acute lung injury, sepsis, or emphysema is likely to yield potential biomarkers and therapeutic targets for these common and serious diseases.
Footnotes
Supported by grants R01 HL077328, R21 DA029249–01, and R01 HL090950.
Author Disclosure: I.P. received institutional grant support from the VA, AHA, the Alpha One Foundation, Quark Biotech, IUPUI Signature Center, the American Heart Association, and the BSF. D.N.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.P.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J 1998;335:465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008;9:139–150 [DOI] [PubMed] [Google Scholar]
- 3.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 2002;1585:114–125 [DOI] [PubMed] [Google Scholar]
- 4.Smith EL, Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J 2008;22:3419–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med 2008;178:1100–1114 [DOI] [PubMed] [Google Scholar]
- 6.Masini E, Giannini L, Nistri S, Cinci L, Mastroianni R, Xu W, Comhair SA, Li D, Cuzzocrea S, Matuschak GM, et al. Ceramide: a key signaling molecule in a guinea pig model of allergic asthmatic response and airway inflammation. J Pharmacol Exp Ther 2008;324:548–557 [DOI] [PubMed] [Google Scholar]
- 7.Kroesen BJ, Jacobs S, Pettus BJ, Sietsma H, Kok JW, Hannun YA, de Leij LF. BcR-induced apoptosis involves differential regulation of C16 and C24-ceramide formation and sphingolipid-dependent activation of the proteasome. J Biol Chem 2003;278:14723–14731 [DOI] [PubMed] [Google Scholar]
- 8.Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol 2004;55:257–267 [DOI] [PubMed] [Google Scholar]
- 9.Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J 2005;390:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medler TR, Petrusca DN, Lee PJ, Hubbard WC, Berdyshev EV, Skirball J, Kamocki K, Schuchman E, Tuder RM, Petrache I. Apoptotic sphingolipid signaling by ceramides in lung endothelial cells. Am J Respir Cell Mol Biol 2008;38:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkataraman K, Riebeling C, Bodennec J, Riezman H, Allegood JC, Sullards MC, Merrill AH, Jr, Futerman AH. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem 2002;277:35642–35649 [DOI] [PubMed] [Google Scholar]
- 12.Riebeling C, Allegood JC, Wang E, Merrill AH, Jr, Futerman AH. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J Biol Chem 2003;278:43452–43459 [DOI] [PubMed] [Google Scholar]
- 13.Xu Z, Zhou J, McCoy DM, Mallampalli RK. Lass5 is the predominant ceramide synthase isoform involved in de novo sphingolipid synthesis in lung epithelia. J Lipid Res 2005;46:1229–1238 [DOI] [PubMed] [Google Scholar]
- 14.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, Tabas I. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem 1998;273:4081–4088 [DOI] [PubMed] [Google Scholar]
- 15.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, et al. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci USA 2000;97:8681–8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res 2003;44:754–761 [DOI] [PubMed] [Google Scholar]
- 17.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta 2003;1632:16–30 [DOI] [PubMed] [Google Scholar]
- 18.Yasuda S, Nishijima M, Hanada K. Localization, topology, and function of the LCB1 subunit of serine palmitoyltransferase in mammalian cells. J Biol Chem 2003;278:4176–4183 [DOI] [PubMed] [Google Scholar]
- 19.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become Cers (ceramide synthases)? Insights into the regulation of ceramide synthesis. J Biol Chem 2006;281:25001–25005 [DOI] [PubMed] [Google Scholar]
- 20.Causeret C, Geeraert L, Van der Hoeven G, Mannaerts GP, Van Veldhoven PP. Further characterization of rat dihydroceramide desaturase: tissue distribution, subcellular localization, and substrate specificity. Lipids 2000;35:1117–1125 [DOI] [PubMed] [Google Scholar]
- 21.Stiban J, Fistere D, Colombini M. Dihydroceramide hinders ceramide channel formation: implications on apoptosis. Apoptosis 2006;11:773–780 [DOI] [PubMed] [Google Scholar]
- 22.Rosen H, Liao J. Sphingosine 1-phosphate pathway therapeutics: a lipid ligand-receptor paradigm. Curr Opin Chem Biol 2003;7:461–468 [DOI] [PubMed] [Google Scholar]
- 23.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta 2002;1585:193–201 [DOI] [PubMed] [Google Scholar]
- 24.Tilly JL, Kolesnick RN. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochim Biophys Acta 2002;1585:135–138 [DOI] [PubMed] [Google Scholar]
- 25.Baumruker T, Prieschl EE. Sphingolipids and the regulation of the immune response. Semin Immunol 2002;14:57–63 [DOI] [PubMed] [Google Scholar]
- 26.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graf C, Rovina P, Tauzin L, Schanzer A, Bornancin F. Enhanced ceramide-induced apoptosis in ceramide kinase overexpressing cells. Biochem Biophys Res Commun 2007;354:309–314 [DOI] [PubMed] [Google Scholar]
- 28.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J Biol Chem 2005;280:17601–17607 [DOI] [PubMed] [Google Scholar]
- 29.Wijesinghe DS, Lamour NF, Gomez-Munoz A, Chalfant CE. Ceramide kinase and ceramide-1-phosphate. Methods Enzymol 2007;434:265–292 [DOI] [PubMed] [Google Scholar]
- 30.Slowik MR, De Luca LG, Min W, Pober JS. Ceramide is not a signal for tumor necrosis factor-induced gene expression but does cause programmed cell death in human vascular endothelial cells. Circ Res 1996;79:736–747 [DOI] [PubMed] [Google Scholar]
- 31.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards CK, III, Schuchman EH, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med 1997;186:1831–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, et al. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 2004;10:155–160 [DOI] [PubMed] [Google Scholar]
- 33.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 2000;60:321–327 [PubMed] [Google Scholar]
- 34.Therade-Matharan S, Laemmel E, Carpentier S, Obata Y, Levade T, Duranteau J, Vicaut E. Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol 2005;289:R1756–R1762 [DOI] [PubMed] [Google Scholar]
- 35.Pautz A, Franzen R, Dorsch S, Boddinghaus B, Briner VA, Pfeilschifter J, Huwiler A. Cross-talk between nitric oxide and superoxide determines ceramide formation and apoptosis in glomerular cells. Kidney Int 2002;61:790–796 [DOI] [PubMed] [Google Scholar]
- 36.Zhang AY, Yi F, Jin S, Xia M, Chen QZ, Gulbins E, Li PL. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid Redox Signal 2007;9:817–828 [DOI] [PubMed] [Google Scholar]
- 37.Noe J, Petrusca D, Rush N, Deng P, Vandemark M, Berdyshev E, Gu Y, Smith P, Schweitzer K, Pilewsky J, et al. CFTR regulation of intracellular pH and ceramides is required for lung endothelial cell apoptosis. Am J Respir Cell Mol Biol 2009;41:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, et al. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 2008;295:L44–L53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy M, Khan E, Careaga M, Goldkorn T. Neutral sphingomyelinase 2 is activated by cigarette smoke to augment ceramide-induced apoptosis in lung cell death. Am J Physiol Lung Cell Mol Physiol 2009;297:L125–L133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrusca DN, Gu Y, Adamowicz JJ, Rush NI, Hubbard WC, Smith PA, Berdyshev EV, Birukov KG, Lee CH, Tuder RM, et al. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J Biol Chem 2010;285:40322–40332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber LC, Jungel A, Distler JH, Moritz F, Gay RE, Michel BA, Pisetsky DS, Gay S, Distler O. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis 2007;12:363–374 [DOI] [PubMed] [Google Scholar]
- 42.Perrotta C, De Palma C, Falcone S, Sciorati C, Clementi E. Nitric oxide, ceramide and sphingomyelinase-coupled receptors: a tale of enzymes and messengers coordinating cell death, survival and differentiation. Life Sci 2005;77:1732–1739 [DOI] [PubMed] [Google Scholar]
- 43.Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem 2007;282:7416–7423 [DOI] [PubMed] [Google Scholar]
- 44.Santoro MF, Annand RR, Robertson MM, Peng YW, Brady MJ, Mankovich JA, Hackett MC, Ghayur T, Walter G, Wong WW, et al. Regulation of protein phosphatase 2A activity by caspase-3 during apoptosis. J Biol Chem 1998;273:13119–13128 [DOI] [PubMed] [Google Scholar]
- 45.Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M, Henry T, Yang E. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for bad-mediated apoptosis. Mol Cell Biol 2003;23:6350–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SW, Kim HJ, Chun YJ, Kim MY. Ceramide produces apoptosis through induction of p27(kip1) by protein phosphatase 2A-dependent Akt dephosphorylation in PC-3 prostate cancer cells. J Toxicol Environ Health A 2010;73:1465–1476 [DOI] [PubMed] [Google Scholar]
- 47.Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr 2005;37:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Forstermann U. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 2002;106:2250–2256 [DOI] [PubMed] [Google Scholar]
- 49.Didion SP, Faraci FM. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arterioscler Thromb Vasc Biol 2005;25:90–95 [DOI] [PubMed] [Google Scholar]
- 50.Spyridopoulos I, Mayer P, Shook KS, Axel DI, Viebahn R, Karsch KR. Loss of cyclin A and G1-cell cycle arrest are a prerequisite of ceramide-induced toxicity in human arterial endothelial cells. Cardiovasc Res 2001;50:97–107 [DOI] [PubMed] [Google Scholar]
- 51.Smith EL, Schuchman EH. Acid sphingomyelinase overexpression enhances the antineoplastic effects of irradiation in vitro and in vivo. Mol Ther 2008;16:1565–1571 [DOI] [PubMed] [Google Scholar]
- 52.Gupta N, Nodzenski E, Khodarev NN, Yu J, Khorasani L, Beckett MA, Kufe DW, Weichselbaum RR. Angiostatin effects on endothelial cells mediated by ceramide and RhoA. EMBO Rep 2001;2:536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venable ME, Yin X. Ceramide induces endothelial cell senescence. Cell Biochem Funct 2009;27:547–551 [DOI] [PubMed] [Google Scholar]
- 54.Kuebler WM, Yang Y, Samapati R, Uhlig S. Vascular barrier regulation by PAF, ceramide, caveolae, and NO - an intricate signaling network with discrepant effects in the pulmonary and systemic vasculature. Cell Physiol Biochem 2010;26:29–40 [DOI] [PubMed] [Google Scholar]
- 55.Lindner K, Uhlig U, Uhlig S. Ceramide alters endothelial cell permeability by a nonapoptotic mechanism. Br J Pharmacol 2005;145:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2001;280:L1168–L1178 [DOI] [PubMed] [Google Scholar]
- 57.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, Schweitzer KS, Skobeleva A, Rajashekhar G, Hubbard WC, et al. Stimulation of sphingosine 1 phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med 2010;181:344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le A, Zielinski R, He C, Crow MT, Biswal S, Tuder RM, Becker PM. Pulmonary epithelial neuropilin-1 deletion enhances development of cigarette smoke-induced emphysema. Am J Respir Crit Care Med 2009;180:396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cantin AM. Cellular response to cigarette smoke and oxidants: adapting to survive. Proc Am Thorac Soc 2010;7:368–375 [DOI] [PubMed] [Google Scholar]
- 60.Bodas M, Min T, Vij N. Critical role of CFTR dependent lipid-rafts in cigarette smoke induced lung epithelial injury. Am J Physiol Lung Cell Mol Physiol 2011;7:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filosto S, Castillo S, Danielson A, Franzi L, Khan E, Kenyon N, Last J, Pinkerton K, Tuder R, Goldkorn T. Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. Am J Respir Cell Mol Biol 2011;44:350–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodas M, Min T, Mazur S, Vij N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. J Immunol 2011;186:602–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gulbins E, Grassme H. Ceramide and cell death receptor clustering. Biochim Biophys Acta 2002;1585:139–145 [DOI] [PubMed] [Google Scholar]
- 64.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 2008;14:382–391 [DOI] [PubMed] [Google Scholar]
- 65.Reisdorph N, Kim YI, Armstrong M, Mahaffey S, Powell R, Reisdorph R, Bowler R. Metabolic and biomarker profiles associated with high resolution CT phenotypes of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2011;8:204–204a [Google Scholar]
- 66.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 2006;129:1673–1682 [DOI] [PubMed] [Google Scholar]
- 67.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol 1999;147:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res 2004;94:724–734 [DOI] [PubMed] [Google Scholar]
- 69.Berdyshev EV, Gorshkova I, Usatyuk P, Kalari S, Zhao Y, Pyne NJ, Pyne S, Sabbadini RA, Garcia JG, Natarajan V. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS ONE 2011;6:e16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J 2005;19:1646–1656 [DOI] [PubMed] [Google Scholar]
- 71.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1p2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 2007;27:1312–1318 [DOI] [PubMed] [Google Scholar]
- 72.Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE 2007;2007:re8 [DOI] [PubMed] [Google Scholar]
- 73.Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JG. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal 2007;19:1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA 2005;102:9270–9275 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]