Abstract
Rationale
A genetic component may be involved in different stages of the progression of drug addiction. Heroin users escalate unit doses and frequency of self-administration events over time. Rats that self-administer drugs of abuse over extended sessions escalate the amount of drug infused over days.
Objectives
Using a recently developed model of extended-access self-administration allowing for subject-controlled dose-escalation of the unit dose, thus potentially escalating the unit dose and number of infusions, we compared for the first time two genetically different inbred rat strains, Fischer and Lewis.
Methods
Extended (18h/day) self-administration lasted for 14 days. Rats had access to two active levers associated with two different unit doses of heroin. If a rat showed preference for the higher unit dose, then the available doses were escalated in the following session. Four heroin unit doses were available (20, 50, 125, 250 μg/kg/infusion).
Results
Fischer rats did not escalate the unit dose of heroin self-administered; daily amount of heroin administered remained low, with a mean daily intake of 1.27±0.22 mg/kg/session. In marked contrast, Lewis rats escalated the total daily amount of heroin self-administered from 3.94±0.82 mg/kg on the day 1 to 8.95±2.2 mg/kg on day 14; almost half of the subjects preferred a higher heroin dose than Fischer rats.
Conclusion
These data are consistent with the hypothesis that Lewis rats are prone to opiate taking and escalation, and are in agreement with our previous data obtained with cocaine.
Keywords: heroin, self-administration, escalation, Fischer, Lewis
INTRODUCTION
Genetic factors may contribute to the development of drug addiction in humans (Kreek et al. 2005; Lachman 2006; Uhl 2004), and may be involved in all stages of the progression from the initial use of drugs of abuse to addiction and relapse.
Inbred rat strains have a more homogeneous genotype than outbred strains (George and Goldberg 1989; Hedrich 2006), thus, they are suitable to examine genetic factors modulating the effects of drugs. Among the inbred strains, Lewis and Fischer rats are often compared because of behavioral and neuronal molecular differences in responding to drugs of abuse. Lewis rats are more responsive to a number of drugs in several behavioral tests than Fischer rats; e.g., Lewis rats more readily acquire morphine (Ambrosio et al. 1995; Martin et al. 1999) and cocaine (Kosten et al. 1997) self-administration. Lewis rats show stronger cocaine conditioned place preference (CPP) (Kosten et al. 1994), and only Lewis, but not Fischer, rats develop nicotine CPP (Horan et al. 1997). Ethanol is a stronger positive reinforcer in Lewis than in Fischer rats (Suzuki et al. 1988).
The mesolimbic dopaminergic system is crucial for the rewarding effects of drugs, including heroin. Fischer and Lewis rats differ in a number of cellular characteristics within this system (Kosten and Ambrosio 2002). For example, level of ΔFosB is higher in the nucleus accumbens (NAc) of Lewis rats (Haile et al. 2001). Lewis rats have lower levels of D2-like dopamine receptors in the striatum and NAc core, and D3 receptor in the NAc shell and olfactory tubercle (Flores et al. 1998). In microdialysis experiments, the dopaminergic system in the NAc of Fischer rats is less responsive than that of Lewis rats to morphine, amphetamine and cocaine injections (Cadoni and Di Chiara 2007). These differences may contribute to the different rewarding impact that drugs of abuse have in these two strains.
Two of the best known preclinical models to study drug reward are the intravenous self-administration (IVSA) (Panlilio and Goldberg 2007) and the conditioned place preference (Bardo and Bevins 2000). In a continuous effort to find a model that reproduces more and more closely the behavior of human drug users, we selected the IVSA model in rodents. It is known that when IVSA sessions are longer than three hours, rats escalate the amount of drug self-administered over days (e.g., Ahmed and Koob 1998; 1999; Mantsch et al. 2001; Mantsch et al. 2003; 2004; Picetti et al. 2010; Quadros and Miczek 2009; Roth and Carroll 2004; Wee et al. 2007). Recently, in Picetti et al (2010) a new model of extended-session IVSA included the possibility for the animals to select the unit dose that they may find more rewarding or match the animal’s sensitivity to the drug positive effects, thus potentially escalating the unit dose from lower to higher doses. When IVSA sessions are prolonged to 18 hours/day for 14 days and two different doses of cocaine are available during a session, Fischer and Lewis rats behave very differently. The majority of Fischer rats preferred the two lower unit doses of cocaine available (0.2 and 0.5 mg/kg/infusion) and did not escalate the total amount of cocaine self-administered. On the contrary, most Lewis rats preferred the two higher doses of cocaine (1.25 and 2.5 mg/kg/infusion), and escalated the total amount of cocaine obtained (Picetti et al. 2010).
Because of the genetic difference between these two strains, briefly discussed above, we studied the self-administration behavior of another major drug of abuse, heroin, and applied the dose-preference and dose-escalation IVSA model. Strikingly, whereas several papers have been published on heroin IVSA in Fischer rats, none could be found on the comparison of Fischer and Lewis strains. However, a number of publications are available on the comparison of these two strains self-administering other opiates, e.g. morphine (e.g., Garcia-Lecumberri et al. 2010; Sanchez-Cardoso et al. 2009). Garcia-Lecumberri et al. (2010) reported that Lewis rats are more vulnerable to the rewarding effects of morphine and are more impulsive than Fischer rats. Here, we focused our attention on heroin IVSA to assess and compare for the first time the behavior of Fischer and Lewis rats using our new dose-escalating IVSA extended-session paradigm.
MATERIALS AND METHODS
Animals and drug
Self-administration experimental procedures were conducted in male Fischer and Lewis rats (Charles River, Wilmington, MA). Fischer and Lewis rats weighed between 250 and 300 g (corresponding to 9–10 weeks of age), respectively, at the time of surgery. Normal chow and water were available ad libitum in both operant conditioning chambers and home cages. Rats were singly housed in a temperature-and humidity-controlled environment in the Comparative Bioscience Center of The Rockefeller University, which is an AAALAC accredited facility. The experimental protocol was approved by the Institutional Animal Care and Use Committee of The Rockefeller University.
Rats were maintained on a 12-hour reversed light/dark cycle (lights on at 0100 hours, off at 1300 hours) and the IVSA sessions were started during the dark phase of the light/dark cycle. Heroin (3,6 diacetyl-morphine-HCl) was generously provided by NIH-NIDA and was dissolved in physiological saline to a final concentration of 0.6 mg/ml.
This study began with 20 Fischer and 22 Lewis rats. Six Fischer and six Lewis rats were removed from study due to catheter failure. No rat was lost due to overdose or health-related problems.
Intravenous self-administration apparatus
Experimental chambers consisted of 12 standard self-administration boxes from Med-Associates Inc. (St. Albans, VT). Operant conditioning chambers were equipped with three retractable levers with stimulus lights (28 V DC, 100 mA, 2.5 cm diameter) located above each lever. The two active levers were mounted at the back of the chambers on opposing walls, while the inactive lever was mounted at the front. Each chamber was equipped with a multiple tone generator (Med-Associates). Water bottles mounted on the ceiling of the chambers and food-containing porcelain dishes (90 ml capacity, 77x32 mm) were available during the sessions.
Cubicles were equipped with exhaust fans that provided ventilation. Programming and data collection were performed using a computer system running MED-PC® IV software (Med-Associates).
During an IVSA session, drug doses were delivered from syringes mounted on syringe pumps (PHM-100, Med-Associates) placed outside the sound-attenuating box. Each syringe was connected via a Tygon tube to a swivel (Instech, Plymouth Meeting, PA). Another Tygon tube connected the swivel to the external portion of the cannula of the intravenous catheter.
Surgery
Surgery was conducted as described in Picetti et al. (2010). Once the catheter was implanted, it was flushed with 500 μl of heparin 30 U/ml to prevent catheter clogging and cefazolin (0.1 ml, 10 mg/ml) in saline to prevent infections. The same mixture was used to flush the catheters during the first four days after surgery.
Catheter evaluation was performed by flushing about 100 μl of a xylazine/ketamine (8/80 mg/kg) cocktail through the catheter. Rats not showing immediate signs of sedation were removed from the study. This test was performed on the day before the first self-administration session, and then once a week or when the drug self-administrating behavior deviated dramatically from that observed previously.
Heroin self-administration
Acquisition
Four to five days after surgery, rats were placed in the SA operant conditioning chambers for two hours/day, 5–6 days/week and connected to the heroin delivery pump. Pressing of either active lever resulted in the delivery of one 50-μg/kg heroin infusion (FR1, time out 40 sec). Volume and duration of infusion depended on the rat’s body weight. As a reference, for a rat weighing 300 g the infusion delivery time was 1.66 sec (~40 μl). A light cue above the pressed lever was illuminated during the infusion and the time out (TO). Responding on the inactive lever was recorded, but had no consequences.
Training was complete when self-administration behavior stabilized, i.e. when rats had three consecutive sessions with <10% variation in the total number of heroin infusions per session.
Dose-Response curve
After the acquisition training, responding to different doses of heroin was assessed using multiple-component sessions during which each rat was tested with ascending doses of heroin each session. This procedure was similar to that used for cocaine (Caine et al. 2002; Picetti et al. 2010).
Briefly, multiple-component sessions started about an hour after the onset of the dark cycle. Each session was comprised of five 25-min-long components separated by 5-min-long intervals during which levers were retracted and the chamber was illuminated. Five heroin doses (2, 20, 50, 125, 250 μg/kg/infusion) were presented in ascending order to prevent sensitization effects. Different unit doseswere administered by changing the duration of the infusion. In addition to the illumination of the light cue above the pressed lever, responding for the 20, 50, 125, 250 doses was associated with a tone cue of 1, 1.5, 2.5, 5 kHz, respectively. The duration of the tone cue equaled the duration of the infusion. Each component started with a non-contingent infusion of the appropriate dose. Rats were exposed to four multi-component sessions on consecutive days.
Extended access with subject-controlled dose-escalation
After the dose-response curve determination, rats were placed in the self-administration operant conditioning chambers for 18h starting approximately three hours after the onset of the dark cycle. The overall exposure lasted for 14 days. Each session was divided into three cycles defined by the doses of heroin associated with the active levers. Every 18h session started with a 25-min exposure (defined here as priming interval) to the active lever associated with the lower dose plus the inactive lever, followed by a 5-min TO with the levers retracted, followed by a 25-min exposure to the active lever associated with the higher dose plus the inactive lever. After a 5-min TO, the animal was exposed to the three levers for the remainder of the time. As with determination of the dose-response curve, different unit doses were administered by changing the duration of the infusion, and each 25-min long component started with a non-contingent infusion of the appropriate dose. No priming infusions were delivered at the onset of the 17-h long component with the two active levers available.
The escalation procedure followed the general scheme for cocaine self-administration shown in Picetti et al. (2010). Briefly, from day 1 to day 4, one active lever delivered 20μg/kg/infusion of heroin, while the other delivered 50μg/kg/infusion (FR1, TO 40 sec). Pressing an active lever was associated with the illumination of the light cue above the same lever during the infusion and TO, and the onset of a dose-specific tone cue (see above) during the infusion. If the animal pressed the lever associated with the higher dose 70% of the time on days 3 and 4, then from day 5 the available doses became 50 and 125 μg/kg/infusion. For rats that did not fulfill this requirement, the doses were not changed until they showed 70% preference for the higher dose on two consecutive sessions.
Similarly, rats that preferred the 125-μg/kg/infusion dose on days 7 and 8 were exposed to 125 and 250μg/kg/infusion from day 9 to day 14. Again, for rats that did not meet this criterion immediately there was no change unless the criterion was met for two consecutive days.
The light/dark cycle inside the operant conditioning chambers was synchronized to the cycle in the home cages (lights on at 0100 h, off at 1300 h). After each SA session, rats were weighed and returned to their home cages for six hours.
Yoked-saline rats
Yoked-saline rats, six per strain, had surgery as described above. They were randomly paired to their heroin self-administering counterparts. They went through the same complete procedure as their heroin self-administering partners starting from the acquisition phase, but received saline (0.9%) when their paired partner pressed an active lever for heroin.
Statistical analysis
The dose-effect curves were analyzed with a two-way ANOVA (strain x dose, repeated measures) followed by planned comparison or Newman-Keuls post-hoc test. The total mean daily heroin intake was analyzed with Student’s t-test.
Statistical analysis was performed with the programs Statistica (StatSoft, Inc., Tulsa, OK) and Graphpad Prism 5 (La Jolla, CA).
RESULTS
Response to different doses of heroin, prior to extended-session exposure
Acquisition of heroin self-administration behavior (2h/session, 50μg/kg/infusion during the dark phase of the light-dark cycle) was initiated without prior training for food-reinforced responding. There was no significant difference between the two strains in days to meet acquisition criteria. During the last three days of acquisition, Fischer rats self-administered significantly less heroin, 520.2±30 μg/kg/session, than Lewis rats, 758.3±21.7 μg/kg/session (Student’s t-test p=0.003).
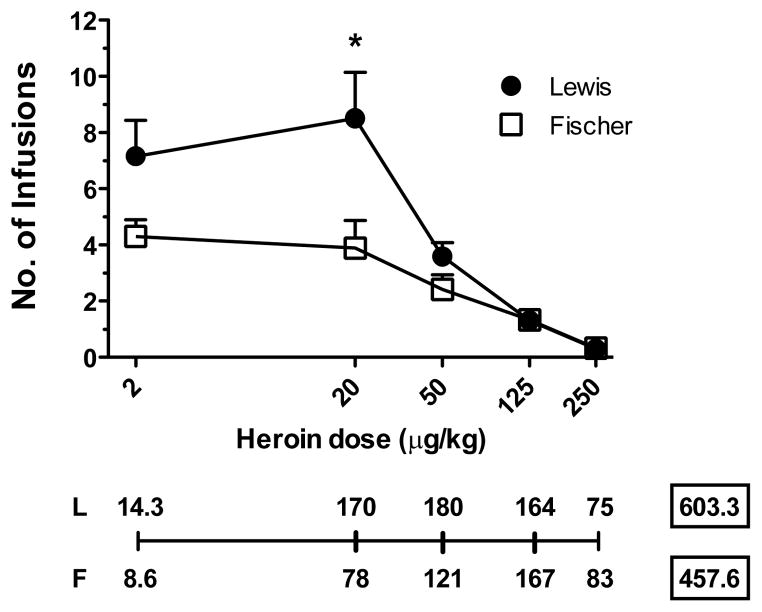
During the last three days of the acquisition, the mean number of active lever presses for Fischer rats was 10.5±0.5, whereas the mean number of inactive lever presses was 6.2±1.3 (Student’s t-test p<0.05). The mean number of active lever presses for Lewis rats was 15.8±0.5, whereas the mean number of inactive lever presses was 7.7±0.6 (Student’s t-test p<0.001). After acquisition was completed, rats were tested for their responses to a range of unit doses of heroin (2, 20, 50, 125, 250 μg/kg/infusion tested in an ascending order on the same day). The two strains showed a dose-dependent modulation of the number of infusions at different doses, (F(4,112)=27.1, p<0.0001), with a significant difference between the two strains (F(1,28)=5.6, p<0.05). There was also a significant interaction strain x dose (F(4,112)=4.0, p<0.01). Indeed, Lewis rats took significantly more infusions than Fischer rats (Fig. 1) at the dose of 20 μg/kg/infusion (planned comparison, p<0.05).
Fig. 1.
Dose-Response. Dose-Response curves were determined as the mean of four 5-component sessions. Each component lasted 25 min. The line below the graph shows the micrograms of heroin per kilogram self-administered for each dose in Lewis (L) and Fischer (F) rats. The numbers in squares represent the total micrograms of heroin obtained for all the doses. * p<0.05. Fischer n=14; Lewis n=16.
Subject-controlled dose-escalation and extended access to heroin self-administration
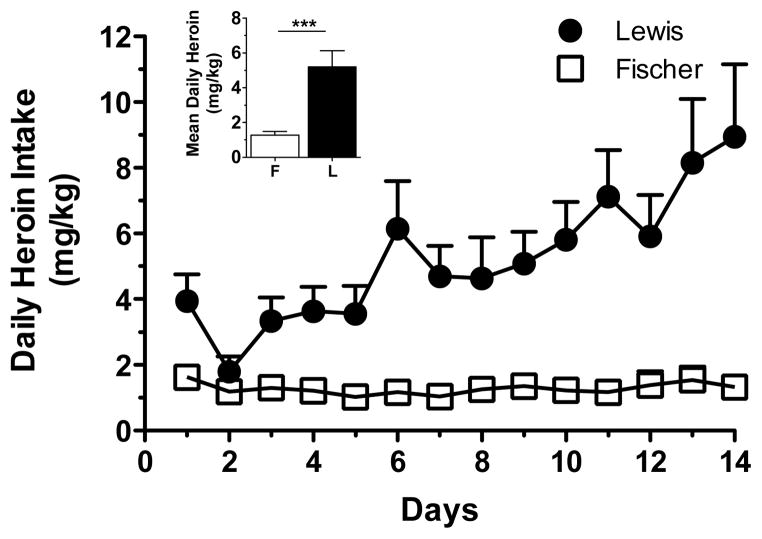
Self-administration sessions were then extended to 18 h/day, starting approximately three hours into the dark cycle, after the completion of the dose-effect study. Fischer and Lewis rats showed a striking difference in their daily heroin intake across the 14-day exposure (Fig. 2). Notably, Fischer rats did not vary their average level of heroin intake throughout the exposure. By contrast, Lewis rats began to escalate their heroin intake starting from the sixth day of exposure and had not reached a plateau by the 14th day of exposure. Lewis rats escalated their intake from 3.94±0.82 mg/kg on the first day to 8.95±2.2 mg/kg on day 14, whereas Fischer rats self-administered a low amount of heroin every day. Indeed, the average total amount of heroin taken during the 14 days of extended self-administration was significantly higher in Lewis (Fig. 2 inset), 5.2±0.9 mg/kg/session, than Fischer rats, 1.27±0.22 mg/kg/session (Student’s t-test p=0.0006).
Fig. 2.
Escalation of heroin self-administration. Daily intake of heroin during the extended IVSA sessions. The inset shows the mean daily intake.
In the context of this paper, the four unit doses available were divided into two groups: lower doses (20 and 50μg/kg/infusion), and higher doses (125 and 250μg/kg/infusion). The analysis of the unit dose preferred by each subject on day 14 revealed that 100% of Fischer rats preferred the two lower-unit doses (Table 1), while the Lewis rats were split between the 50 μg/kg/infusion dose (53.3%), and the 125 μg/kg/infusion dose (46.7%). Three Fischer rats and one Lewis rat could not be assigned to any dose because they did not meet the preference criterion (i.e., 70% of lever presses associated to one lever).
TABLE 1.
Percentages of rats preferring the different unit doses of heroin at day 14. Bold numbers are the sums of the rats of each strain preferring the two lower or the higher unit doses of heroin. Fischer n=11; Lewis n=15. Three Fischer rats and one Lewis rat could not be assigned to any dose because they did not meet the preference criteria.
| Heroin Dose (μg/kg/infusion) | 20 | 50 | Total low doses | 125 | 250 | Total high doses |
|---|---|---|---|---|---|---|
| Fischer (%) | 27 | 78 | 100 | 0 | 0 | 0 |
| Lewis (%) | 0 | 53 | 53 | 47 | 0 | 47 |
Details of the subject-controlled dose-escalation
Fig. 3 details daily activity on the active levers of the rats during the 14-day extended exposure to heroin self-administration. Each graph represents the totality of the rats tested during the 14-day IVSA, and each line represents rats exposed to a particular unit dose of heroin. Figs. 3a and 3c show that Fischer rats self-administered very little heroin throughout the extended exposure. Starting from day 5 onward, a total of 4 of the 14 Fischer rats were exposed to the 50 and 125 μg/kg/infusion doses, but the amount of heroin received from the 125 μg/kg/infusion-associated lever did not change the total amount of drug obtained. No Fischer rat escalated the unit dose to the highest unit dose available (250 μg/kg/infusion; Figs. 3a and 3c).
Fig. 3.
Daily activity on active levers. Quantity of heroin self-administered (a, b), and number of infusions (c, d) divided according to the four unit doses used, respectively. Each line represent the totality of rats exposed to a particular unit dose of heroin at any given day. Lines representing 125 and 250 μg/kg/infusion start at 5 and 12 days of IVSA, respectively, since rats started to be exposed to these doses at these time points (see Material and Methods for description of the IVSA protocol). Fischer n=14; Lewis n=16.
Lewis rats showed a different pattern of behavior. Although the number of lever presses on the 20 and 50 μg/kg/infusion-associated levers doesn’t seem to be different (Fig. 3d), the amount of heroin taken from the 50 μg/kg/infusion associated lever is greater than the amount received from the lever associated with the lower dose (Fig. 3b). However, the amount of heroin received from either lever does not escalate. Starting from day 5, 12 Lewis rats were exposed to the 50–125 μg/kg/infusion associated levers, and from day 12 a total of 4 of 16 Lewis rats had access to the highest doses of heroin. Lewis rats escalated both the number of lever presses and amount of heroin self-administered from either lever associated with the two highest unit doses available (Figs. 3b and 3d).
The mean number of total daily presses on the inactive lever across the 14-day exposure to extended sessions was 16.42±0.9 by Fischer rats and 100.4±16.53 by Lewis rats. The mean number of daily presses on both active levers was 28.58±1.8 by Fischer rats and 251.8±20.43 by Lewis rats.
Hourly pattern of self-administration during extended sessions
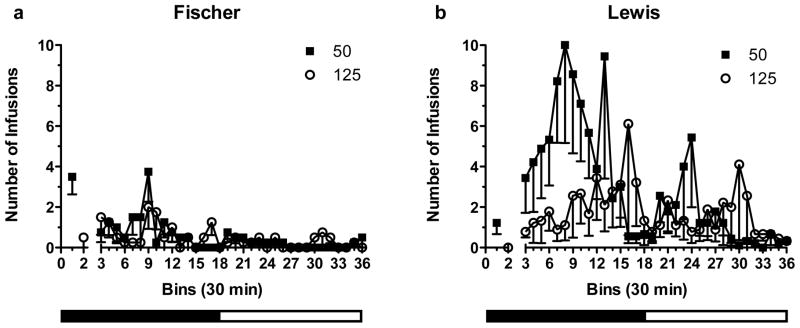
In Fig. 4a, Fischer rats show higher activity on the 50 μg/kg/infusion-associated lever on the 14th day. Most of the activity occurred during the dark phase of the light-dark cycle. The activity on both levers remained low. In Fig. 4b, Lewis rats show a much higher activity on the 50 μg/kg/infusion-associated lever during the dark phase of the session, and then decreased during the light phase. Activity on the 125 μg/kg/infusion-associated lever was lower throughout the session except towards the end of the session during the light phase.
Fig. 4.
Example of hourly self-administration pattern during day 14 of the 18 h-long sessions for the subset of rats that were exposed to the doses of 50 and 125 μg/kg/infusion. The first two bins represent the number of infusions taken during the priming intervals, whereas bins 3 to 36 show the number of infusions taken when the rats could choose between the two active levers (Fischer n=4; Lewis n=9). The bars under the graphs represent the dark (solid bar) and light (open bar) portions of the 24-hour light/dark cycle during the session.
During the priming intervals (i.e., the two 25-min intervals at the beginning of the 18-hour long sessions), Fischer rats took more infusions at both unit doses than Lewis rats. However, after the priming intervals, Fischer rats showed a decreased number of infusions from the 50-μg/kg/infusion-associated lever, whereas Lewis rats showed the opposite. Infusions from the 125-μg/kg/infusion-associated lever became irregular for Fischer rats, whereas Lewis rats increased them.
Comparison between the dose-effect curves obtained before and after the extended sessions
A subset of rats (Fischer n=4, Lewis n=8) was tested in dose-effect sessions starting the day after the last session of the extended-access self-administration. Not all the rats were used for this experiment in order to sacrifice the others 1 and 24 hours after the end of the extended-access session on day 14 for further studies.
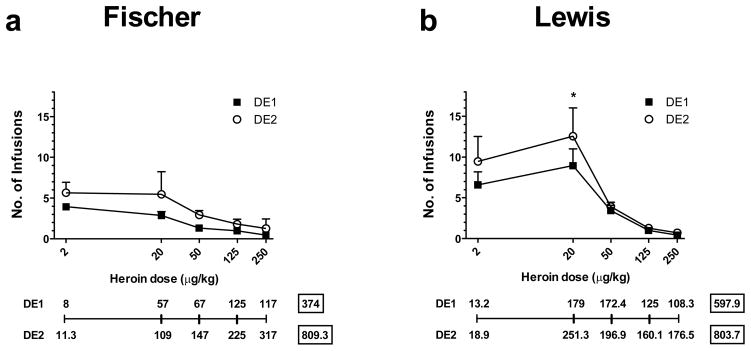
Fig. 5 shows that in both strains the dose-effect curve after 14 days of exposure to extended access IVSA has shifted upward. The shift was particularly evident at the two lowest doses of heroin used. Two-way ANOVA showed that in Lewis rats there was a significant difference between doses (F(4,28)=17.43, p<0.0001), and Newman-Keuls post-hoc test showed a significant difference between the two strains at the unit dose of 20 μg/kg/infusion (p<0.05). A similar analysis showed that there was a significant difference between doses for Fischer rats (F(4,8)=6, p<0.05).
Fig. 5.
Dose-Response before and after the 14-day-long extended IVSA exposure to heroin. a) Fischer rats. b) Lewis rats. The lines below each graph show the micrograms of heroin self-administered for each dose during the first (DE1) and second (DE2) dose-effect curves. The numbers in squares represent the total micrograms of heroin obtained for all the doses. * p<0.05.
DISCUSSION
We used a new self-administration model (Picetti et al. 2010) that allows individual rats to select the unit doses of a drug to be self-administered and to escalate these doses across days. This model simulates aspects of the behavior of humans that escalate the self-administered unit dose of heroin or cocaine over time (Kreek et al. 2009; Zernig et al. 2007) and parallels the chronic intermittent escalating doses of heroin administration in rodents (Seip et al. 2011; Zhou et al. 2008). The animals were exposed to heroin for 18 h/day for 14 days to reproduce a chronic human-like pattern of exposure. After exposure, each day rats were returned to their home cages for the rest of the day to simulate the acute withdrawal period experienced by heroin abusers. With this model, each rat regulated its dose escalation and was not experimentally forced to increase the unit dose of heroin received.
In this study we compared for the first time Fischer and Lewis rats in heroin self-administration. We selected these two strains because of their different behaviors when challenged with drugs, in order to analyze the importance of the genetic background. Moreover, we compared our behavioral results with those obtained with morphine to determine whether Lewis rats are more vulnerable than Fischer rats to heroin, as they are to morphine, regardless of the IVSA procedure adopted. This is interesting in light of the different results and conclusions reached by different laboratories on cocaine IVSA using these two strains (Freeman et al. 2009; Kosten et al. 1997; Kosten et al. 2007; Picetti et al. 2010).
We have found that in this case the behavior of these two strains is even more divergent than in the case of cocaine self-administration (Picetti et al. 2010). In the present study, Lewis rats self-administered significantly more heroin than Fischer rats by the end of the acquisition phase, whereas in the case of cocaine this difference was not significant (Picetti et al. 2010). Furthermore, the dose-effect curves differed at the two lowest doses of heroin (2 and 20 μg/kg/infusion) with Lewis rats producing more lever presses than Fischer rats. When we analyzed the amount of heroin self-administered at each dose, there was an apparent difference also at the dose of 50 μg/kg/infusion (180 μg/kg in Lewis rats vs. 121 μg/kg in Fischer rats). The dose-effect curve for Fischer rats shown herein seems very different from that obtained by Martin et al. (1998), but this apparent discrepancy could be explained by several differences in the paradigms used. In the present study, each dose was tested for 25 min under FR1 and all doses were tested on the same day, whereas in Martin et al. (1998) each dose was tested for four hours under FR10 on separate days.
When IVSA sessions were extended to 18 h/day for 14 days, the two strains behaved in a strikingly different manner. Fischer rats self-administered a low amount of heroin every day that did not vary, whereas Lewis rats escalated their intake across the 14-day exposure. This behavior parallels what we found in cocaine IVSA (Picetti et al. 2010), with one notable difference. When animals self-administered heroin, Lewis rats began self-administering the drug at a higher rate than Fischer rats from day 1. In contrast, in the case of cocaine, IVSA Lewis rats self-administered less drug than Fischer rats during the first few extended sessions and began their cocaine IVSA escalation from day 4, eventually self-administering more cocaine than Fischer rats during the last four days of the 14-day exposure (Picetti et al. 2010). With our IVSA model, Fischer rats did not escalate the quantity of heroin self-administered, whereas with a fixed dose slightly higher than the acquisition dose used in our study (60 μg/kg/infusion) without choice of unit doses and FR10 for 24 h/day, they escalated from 1.8±0.3 mg/kg/day to 5.1±1.6 mg/kg/day in seven days (Sim-Selley et al. 2000). This difference may be due to the contemporaneous presence in our paradigm of a lever delivering a low unit dose of heroin (20 μg/kg/infusion). The possibility of obtaining heroin at a low unit dose in addition to the 50-μg/kg/infusion unit dose may have induced a positive reinforcement too weak to induce the escalation in Fischer rats. It is also possible that, in our model, the 6h-daily abstinence together with the unit doses self-administered do not induce negative reinforcement (Koob and Kreek 2007) or enhance the incentive value of heroin (Hutcheson et al. 2001) in Fischer rats. It should be noted that of the four Fischer rats exposed to the 125-μg/kg/infusion unit dose, two showed a tendency toward escalating the heroin self-administered at this specific dose (data not shown). The higher amount of heroin self-administered by Lewis rats compared with Fischer rats is in agreement with the IVSA of another opioid, morphine (Garcia-Lecumberri et al. 2010; Martin et al. 1999; Sanchez-Cardoso et al. 2009). A detailed analysis of the lever-pressing activity associated with each dose and the amount of heroin taken at each dose shows that none of the Fischer rats were ever exposed to the highest unit dose of heroin, and they did not increase the lever-pressing activity and the amount of heroin self-administered across days when exposed to the other unit doses. Conversely, Lewis rats showed a higher activity, but lack of escalation with the two lowest unit doses of heroin, and increased their lever-pressing activity and the amount of drug self-administered at the two highest unit doses.
Data shown here with heroin, previously with cocaine (Picetti et al. 2010), and by others with morphine (Garcia-Lecumberri et al. 2010), seem to confirm that Fischer rats are less sensitive to the rewarding effects of drugs of abuse, or more sensitive to their rate-limiting effects. Moreover, heroin induces a greater saccharin taste aversion in Fischer rats compared to Lewis (Davis et al. 2009). Lewis rats have lower basal levels of dynorphin and Leu-enkephalin peptides in several brain nuclei involved in dopaminergic pathways (Martin et al. 1999; Nylander et al. 1995), and less binding to mu opioid receptors (MOR) than Fischer rats (Sanchez-Cardoso et al. 2007). Chronic morphine treatment affected dynorphin and Leu-enkephalin differently in these two strains, with the Lewis rats being generally not or less responsive than Fischer rats (Martin et al. 1999; Nylander et al. 1995). After morphine IVSA, MOR functionality in some brain areas, e.g., striatum and nucleus accumbens (NAc), was increased in Fischer rats, but not in Lewis rats (Sanchez-Cardoso et al. 2007). It is possible that the higher basal opioidergic tone in Fischer rats makes high opioid doses and amounts more aversive in this strain compared to Lewis rats. Dopamine receptors in the striatum and NAc (Sanchez-Cardoso et al. 2009) and dopamine levels in the NAc (Cadoni and Di Chiara 2007) are also differently affected by morphine in these two strains. After morphine IVSA, D1-and D2-like binding decreased in Lewis rats, whereas D1-like binding increased in Fischer rats (Sanchez-Cardoso et al. 2009). Two of three doses of morphine injected induced higher dopamine responsiveness in the NAc (core and shell) of Lewis rats compared to Fischer rats (Cadoni and Di Chiara 2007). Behaviorally, Lewis rats showed a stronger conditioned place preference induced by 4 mg/kg of morphine than Fischer rats (Guitart et al. 1992). The behavioral differences in response to heroin self-administration reported herein as well as the differences in response to other opiates reported by other groups may be related to the differences in the basal levels of opioid peptides and opioid receptors observed in these two strains. Further, also the different response to opioids challenges of the dopaminergic system, that interacts with the opioid one (Stinus et al. 1992), may also affect the rewarding effects of drugs and contribute to Lewis and Fischer behavioral differences.
Response to different doses of heroin was tested again with a second dose-effect curve after the last extended session on a subset of rats of both strains. The ascending limb of the Lewis rats’ curve was shifted upward. This shift may be due to sensitization to the reinforcement induced by heroin (Piazza and Deroche 2004; Zernig et al. 2004). Interestingly, the upward shift was observed also in Fischer rats, despite their lack of escalation. This shift concerned the descending limb of the dose-effect curve, and we were not able to observe the ascending limb. A finer analysis of the daily heroin intake over 14 days of these specific individuals reveals a minor, non-significant tendency toward escalation (data not shown). The proposed mechanisms behind the vertical shift of a dose-effect curve are diverse and widely debated (e.g., letter from Zernig et al. 2004 and replies in the same issue). It is certainly difficult to draw conclusions from a small group of animals such as this one. However, one may speculate that Fischer rats may require longer exposures to low doses of heroin to develop the necessary brain changes that lead to the escalation of drug intake. It may be possible that this strain of rats has strong molecular mechanisms that oppose the allostatic changes that lead to the escalation of drug intake, or that the rate-limiting effects of drugs are stronger. Further molecular analyses may elucidate this aspect.
Since it has been reported that opioids can stimulate food and water intake (Morley and Levine 1982; Reid 1985), and rats were exposed to long daily sessions of self-administration, we monitored heath factors such as body weight, food and water consumption throughout the 14 days of IVSA extended access. Body weight of heroin-exposed rats did not differ from that of yoked-saline controls. However, heroin self-administering Lewis rats consumed more food and water than their controls. No such differences were found in Fischer rats (Online Resource 1).
In conclusion, we used here a self-administration model (Picetti et al. 2010) that approximates the behavior of addicted humans who increase the unit dose of drug administered. Fischer and Lewis rats showed a dramatically different self-administration behavior, with Lewis rats escalating both unit dose and total amount of heroin administered, whereas Fischer rats did not escalate either. These results suggest that Lewis rats are more sensitive to the rewarding effects of heroin. On the contrary, Fischer rats seem to be more resistant to the subject-controlled dose-escalating self-administration of heroin starting from low unit doses. Further analysis is required to reveal the molecular changes that occurred in specific brain areas of the rats of the two strains, and, within strains, in brain areas of individual rats that preferred low vs. high unit doses of heroin.
Supplementary Material
Acknowledgments
This work was supported by grants from NIH-NIsDA P60-DA05130 (MJK) and the Carson Family Charitable Trust (MJK).
The authors thank Dr. Eduardo R. Butelman for helpful discussions during the course of the study and for reading the manuscript.
3,6 diacetyl-morphine-HCl was generously provided by the NIH-NIDA Division of Drug Supply and Analytical Services.
RP, JAC, AH, and MJK declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service, and that there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–12. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–237. [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. J Neurochem. 2007;103:487–99. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–88. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CM, Rice KC, Riley AL. Opiate-agonist induced taste aversion learning in the Fischer 344 and Lewis inbred rat strains: evidence for differential mu opioid receptor activation. Pharmacol Biochem Behav. 2009;93:397–405. doi: 10.1016/j.pbb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Res. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Kearns DN, Kohut SJ, Riley AL. Strain differences in patterns of drug-intake during prolonged access to cocaine self-administration. Behav Neurosci. 2009;123:156–64. doi: 10.1037/a0013727. [DOI] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Torres I, Marti NS, Crespo JA, Miguens M, Nicanor C, Higuera-Matas A, Ambrosio E. Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol. 2010 doi: 10.1177/0269881110367444. [DOI] [PubMed] [Google Scholar]
- George FR, Goldberg SR. Genetic approaches to the analysis of addiction processes. Trends Pharmacol Sci. 1989;10:78–83. doi: 10.1016/0165-6147(89)90083-7. [DOI] [PubMed] [Google Scholar]
- Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse. 1992;12:242–53. doi: 10.1002/syn.890120310. [DOI] [PubMed] [Google Scholar]
- Haile CN, Hiroi N, Nestler EJ, Kosten TA. Differential behavioral responses to cocaine are associated with dynamics of mesolimbic dopamine proteins in Lewis and Fischer 344 rats. Synapse. 2001;41:179–90. doi: 10.1002/syn.1073. [DOI] [PubMed] [Google Scholar]
- Hedrich HJ. Taxonomy and Stocks and Strains. In: Suckow MA, Weisbroth SH, Franklin CL, editors. The Laboratory Rat (American College of Laboratory Animal Medicine) 2. Academic Press; 2006. pp. 71–92. [Google Scholar]
- Horan B, Smith M, Gardner EL, Lepore M, Ashby CR., Jr (-)-Nicotine produces conditioned place preference in Lewis, but not Fischer 344 rats. Synapse. 1997;26:93–4. doi: 10.1002/(SICI)1098-2396(199705)26:1<93::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–7. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–44. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Haile CN, DeCaprio JL, Jatlow PI, Nestler EJ. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Res. 1997;778:418–29. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Haile CN. Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behav Neurosci. 2007;121:380–8. doi: 10.1037/0735-7044.121.2.380. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, Zhou Y, Butelman ER. Bidirectional translational research: Progress in understanding addictive diseases. Neuropharmacology. 2009;56(Suppl 1):32–43. doi: 10.1016/j.neuropharm.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM. An overview of the genetics of substance use disorders. Current Psychiatry Reports. 2006;8:133–43. doi: 10.1007/s11920-006-0013-3. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–9. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–62. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–5. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Smith JE, Dworkin SI. Training dose and session time as contextual determinants of heroin self-administration in rats. Pharmacol Biochem Behav. 1998;60:415–21. doi: 10.1016/s0091-3057(97)00599-6. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. The role of the endogenous opiates as regulators of appetite. The American Journal of Clinical Nutrition. 1982;35:757–61. doi: 10.1093/ajcn/35.4.757. [DOI] [PubMed] [Google Scholar]
- Nylander I, Vlaskovska M, Terenius L. Brain dynorphin and enkephalin systems in Fischer and Lewis rats: effects of morphine tolerance and withdrawal. Brain research. 1995;683:25–35. doi: 10.1016/0006-8993(95)00279-y. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–70. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche V. What juxtaposition, tradition and parsimony can do to vertical shifts in drug self-administration dose response functions. Psychopharmacology. 2004;171:356–359. [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology (Berl) 2010;211:313–23. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology (Berl) 2009;206:109–20. doi: 10.1007/s00213-009-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD. Endogenous opioid peptides and regulation of drinking and feeding. The American Journal of Clinical Nutrition. 1985;42:1099–132. doi: 10.1093/ajcn/42.5.1099. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long-or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, del Olmo N, Miguens M, Garcia-Lecumberri C, Ambrosio E. Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology. 2007;52:931–48. doi: 10.1016/j.neuropharm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, Miguens M, Del Olmo N, Garcia-Lecumberri C, Ambrosio E. Strain differences between Lewis and Fischer 344 rats in the modulation of dopaminergic receptors after morphine self-administration and during extinction. Neuropharmacology. 2009;57:8–17. doi: 10.1016/j.neuropharm.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Seip KM, Reed B, Ho A, Kreek MJ. Measuring the incentive value of escalating doses of heroin in heroin-dependent Fischer rats during acute spontaneous withdrawal. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci. 2000;20:4555–62. doi: 10.1523/JNEUROSCI.20-12-04555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Cador M, Le Moal M. Interaction between endogenous opioids and dopamine within the nucleus accumbens. Annals of the New York Academy of Sciences. 1992;654:254–73. doi: 10.1111/j.1749-6632.1992.tb25972.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. J Pharmacol Exp Ther. 1988;245:164–70. [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of substance abuse vulnerability: remarkable recent convergence of genome scan results. Ann N Y Acad Sci. 2004;1025:1–13. doi: 10.1196/annals.1316.001. [DOI] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–43. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A. Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate “sensitization” to “drug wanting”? Psychopharmacology. 2004;171:349–51. doi: 10.1007/s00213-003-1601-0. author reply 352–63. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008;33:226–36. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.