Abstract
Eleven patients underwent controlled ovarian hyperstimulation yielding seventy-two embryos for evaluation. Mean nitric oxide metabolite (NOx) levels in the insemination media (IM) were 2.6 times higher in embryos that progressed to blastocysts by culture day (CD) 5 than those that did not (p=0.009). A comparison of the ROC curves between morphologic predictors and NOx levels revealed a trend toward a stronger association of IM NOx with blastocyst formation.
Improvements in in vitro fertilization (IVF) outcomes have largely been due to the generation of supernumary embryos allowing for multiple embryo transfer (ET). The development of extended embryo culture has resulted in increased implantation rates and the potential for reductions in multiple births through single ET (1,2). One of the major disadvantages, however, has been the worry that in approximately 40% of patients, embryos would not be available for transfer (3).
Unfortunately, culture day (CD) 3 morphologic assessment is not optimal for the prediction of blastocyst progression on CD 5 (4–5). Investigators have therefore sought quantifiable objective measures of embryonic health with a focus on metabolic activity (6–11). One such metabolic factor, nitric oxide (NO), has been implicated in a variety of biological and reproductive processes including oocyte maturation, fertilization and embryonic progression (12). Gouge et al. (13) demonstrated that NO production is essential for embryonic progression in the murine model. Chen and colleagues showed that blastocyst development in culture is inhibited by L-NAME, an NO inhibitor, in a concentration-dependent manner and SNP, an NO donor, can effectively reverse this effect (14). These studies highlight the potential importance of NO in embryogenesis.
The objective of this study was to investigate the potential relationship of NO metabolite (NOx) levels generated by human preimplantation embryos in culture with developmental capacity. Our goal was to compare the prediction of blastocyst formation on the basis of the standard subjective morphological assessment on CD 3 with an objective, quantifiable measure of metabolic function.
Eleven subfertile women between 27 and 44 years of age undergoing sonographic-guided transvaginal oocyte aspiration at Johns Hopkins Hospital between March 31st and June 26th of 2006 were included in the study. This study was approved by the Johns Hopkins Institutional Review Board. Patients were treated with either a gonadotropin releasing hormone (GnRH) agonist (-ag) or antagonist (-an) protocol. All patients received a single dose of 10,000 IU of hCG once three follicles achieved a diameter of at least 18 mm. Ultrasound-guided oocyte retrieval followed 36 hours later.
Following oocyte retrieval, metaphase II oocytes were placed in individual 80 μL droplets of IM. Intracytoplasmic sperm injection was performed on 20 of the retrieved oocytes as described by Palermo and colleagues (15). Once the zygotes were transferred to cleavage media, the IM was collected and placed in separate microcentrifuge tubes and stored at −80 ºC until processing. Embryo free media droplets were cultured under identical conditions and used as controls. Embryos were assessed morphologically on CD 3 and either prepared for ET or placed in blastocyst media. Morphological assessment of the cleavage stage embryo on CD 3 was performed through the use of a modified grading system as proposed by Veeck (16).
Media isolated from cultured embryos or control plates were thawed to room temperature. Due to the short half-life of NO, the stable metabolic products (nitrite and nitrate), referred to as NOx, were measured as described by the QuantiChrom™ Nitric Oxide Assay Kit (BioAssay Systems, Hayward CA, USA), which is an assay based on a modified Greiss reaction with a detection range of 0.1 to 50 μM. Measurements of NOx in duplicates from each sample were performed as described in the kit protocol. The NOx (μM) was calculated for each of the samples and controls. To account for non-independence of IM NOx levels in oocytes derived from the same donor, all analyses with IM NOx as the dependent variable used Generalized Estimating Equations regression methods with an exchangeable correlation structure. The average levels of IM NOx in the analysis groups were calculated as linear combinations of the regression coefficients. We examined whether IM NOx, blastomere number, or subjective grade were effective in predicting survival of the embryo to CD 5 using simple logistic regression adjusting for clustering of data by donor. Receiver Operating Characteristic (ROC) curve analysis was performed for predicting survival of the embryo to CD 5 using IM NOx, blastomere number, and subjective grade as classifying variables.
Eleven patients with a mean age of 34.3 years (range: 27–44 years) underwent oocyte aspiration. The level of NOx was assessed in the IM of 72 individually cultured embryos with 27 and 45 cultured for 3 or 5 days, respectively. The five day culture resulted in 27 blastocysts, 26 of which had media analyzed post insemination/injection.
No significant differences were noted between IM NOx of embryos fertilized via ICSI (1.00 ± 1.22 μM) or IVF (0.73 ± 0.72 μM) (p= 0.849). There was no significant difference in mean IM NOx when considering CD 3 blastomere number or morphologic grade. The IM NOx produced from embryos that were eventually transferred or cryopreserved was 0.630 μM, compared to embryos that were ultimately discarded, −0.204 μM (p=0.053). The mean NOx level in the IM was 2.6 times higher in embryos that progressed to blastocysts by CD 5 than those that did not (1.33 ± 0.51 vs. 0.52 ± 0.84 μM, p=0.009).
While we did not find differences between IM NOx levels, morphologic grade and blastomere number at CD 3, others have shown that embryonic morphology can be of assistance in predicting blastocyst progression on CD 5 (17). Therefore, given that we found that embryos with higher IM NOx levels progressed compared to embryos with lower IM NOx levels, we used logistic regression and ROC to compare IM NOx levels, morphologic grade and blastomere number as predictors for blastocyst progression. Analysis revealed that only the IM NOx level and CD 3 blastomere number were significantly associated with progression to the blastocyst stage by CD 5 (p<0.02).
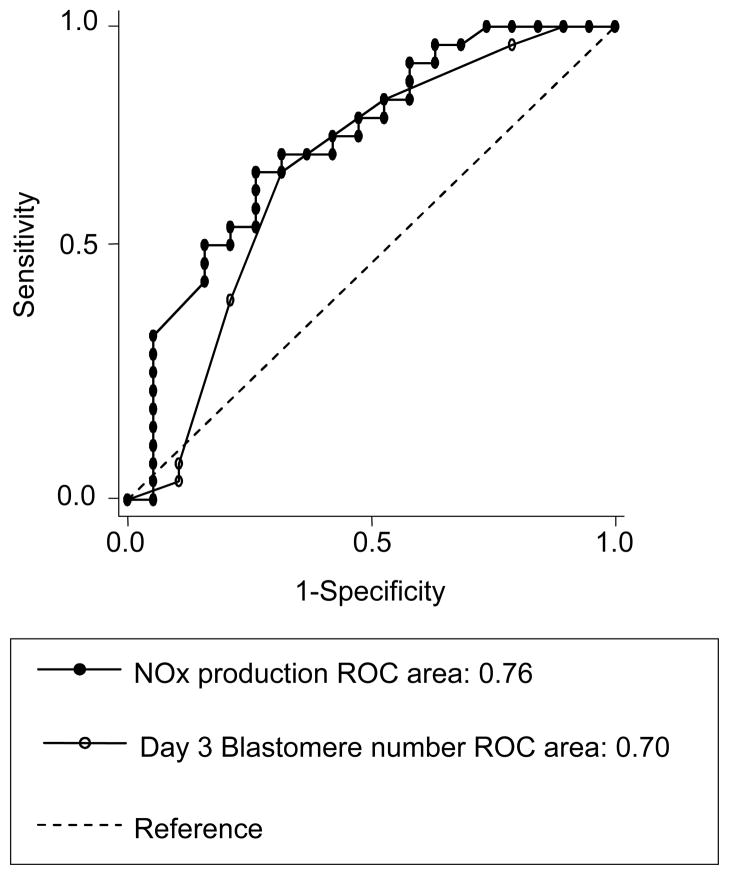
Due to the fact that CD 3 morphologic grade was found to have little predictive value, this parameter was omitted from the model utilized to generate the final ROC. Figure 1 displays the ROC generated from IM NOx and CD 3 blastomere number, highlighting the predictive nature of the two parameters. IM NOx alone revealed a sensitivity of 73.1% with associated specificity of 68.4%, utilizing a cutoff of IM NOx greater than or equal to 0.136 μM. A CD 3 blastomere number of at least six yielded a sensitivity of 69.2% with associated specificity of 68.4%. Statistical analysis revealed that the decision for extended culture to day 5 was not dependent on CD 3 morphology. Although there was no significant difference between the predictive potential of IM NOx and blastomere number on CD 3, a comparison of the curves revealed a trend toward a stronger association of IM NOx with blastocyst formation by CD 5.
Figure 1.
ROC analysis: IM NOx, CD 3 blastomere number and progression to the blastocyst stage by CD 5.
To the best of our knowledge, this is the first study to examine the relationship between NOx levels in the IM media of individually cultured human preimplantation embryos and blastocyst progression. It appears that when considering CD 3 morphology and NOx, NOx was the strongest predictor of blastocyst formation and is an assessment made approximately 48 hours earlier. The level of NOx did not differ according to fertilization technique and may therefore be a reflection of the metabolic activity of the oocyte or zygote.
The study has several limitations that deserve mention. The sample population limits our ability to see significant differences with regard to final embryo disposition or pregnancy. Furthermore, although the NOx levels did not differ with regard to fertilization technique, one cannot assume that gamete manipulation does not potentially result in increases in NOx. Furthermore, the study utilizes blastocyst progression as a measure of embryonic health, whereas live birth would be optimal.
Battaglia et al. were the first to demonstrate secretion of NOx in human preimplantation embryos. They showed that embryonic mean NOx concentrations were significantly higher in embryos transferred that resulted in pregnancy when compared to nonpregnant patients, p=0.02 (18). This study differs from ours in that all embryos in the former were transferred at the 2–4 cell stage, hence limiting the ability to comment on sequential morphology or blastocyst progression over time.
The presence of NO synthase (NOS) in each reproductive compartment has been demonstrated in the literature (19–21). Chen and colleagues proposed a biphasic relationship of NO, with apoptosis induced through a cGMP independent pathway at high NO levels (14).
Although the reasoning behind a beneficial role of higher levels of NOx in the media of human preimplantation embryos remains unclear, this study has demonstrated that the production of NOx does correlate with blastocyst progression, even more so than the standard morphologic criteria. This objective, quantitative measurement obtained early in the developmental process can potentially impact embryo selection at an early stage.
Acknowledgments
We would like to acknowledge the dedication to scientific discovery exemplified by the Johns Hopkins IVF laboratory personnel, specifically through the efforts of Kathleen Broman and Brett Glazar. This work was supported through a grant from the National Institutes of Health to Dr. Annabelle Rodriguez (HL075646).
Financial Support: NIH RO1 HL075646
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–82. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 2.Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–46. doi: 10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- 3.Scholtes MC, Zeilmaker GH. Blastocyst transfer in day-5 embryo transfer depends primarily on the number of oocytes retrieved and not on age. Fertil Steril. 1998;69:78–83. doi: 10.1016/s0015-0282(97)00450-0. [DOI] [PubMed] [Google Scholar]
- 4.Milki AA, Hinckley MD, Gebhardt J, Dasig D, Westphal LM, Behr B. Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril. 2002;77:1191–5. doi: 10.1016/s0015-0282(02)03104-7. [DOI] [PubMed] [Google Scholar]
- 5.Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1998;13:2869–73. doi: 10.1093/humrep/13.10.2869. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DK, Lane M, Stevens J, Schoolcraft WB. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil Steril. 2001;76:1175–80. doi: 10.1016/s0015-0282(01)02888-6. [DOI] [PubMed] [Google Scholar]
- 7.Houghton FD, Leese HJ. Metabolism and developmental competence of the preimplantation embryo. Europ J Obstet Gynecol Reprod Bio. 2004;115S:S92–6. doi: 10.1016/j.ejogrb.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Devreker F, Hardy K, Van den Bergh M, Winston J, Biramane J, Englert Y. Noninvasive assessment of glucose and pyruvate uptake by human embryos after intracytoplasmic sperm injection and during the formation of pronuclei. Fertil Steril. 2000;73:947–54. doi: 10.1016/s0015-0282(00)00472-6. [DOI] [PubMed] [Google Scholar]
- 9.Devreker F, Englert Y. In vitro development and metabolism of the human embryo up to the blastocyst stage. Europ J Obstet Gynecol Reprod Bio. 2000;92:51–6. doi: 10.1016/s0301-2115(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 10.Brison DR, Houghton FD, Falconer D, Roberts SA, Hawkhead J, Humpherson PG, Lieberman BA, Leese HJ. Identification of viable embryos in IVF by non-invasive measurement of amino acid turnover. Hum Reprod. 2004;19:2319–24. doi: 10.1093/humrep/deh409. [DOI] [PubMed] [Google Scholar]
- 11.Houghton FD, Hawkhead JA, Humpherson PG, Hogg JE, Balen AH, Rutherford AJ, Leese HJ. Non-invasive amino acid turnover predicts human embryo developmental capacity. Hum Reprod. 2002;17:999–1005. doi: 10.1093/humrep/17.4.999. [DOI] [PubMed] [Google Scholar]
- 12.Thaler CD, Epel D. Nitric oxide in oocyte maturation, ovulation, fertilization, cleavage and implantation: A little dab’ll do ya. Curr Pharm Des. 2003;9:399–409. doi: 10.2174/1381612033391748. [DOI] [PubMed] [Google Scholar]
- 13.Gouge RC, Marshburn P, Gordon BE, Nunley W, Huet-Hudson YM. Nitric oxide as a regulator of embryonic development. Biol Reprod. 1998;58:875–9. doi: 10.1095/biolreprod58.4.875. [DOI] [PubMed] [Google Scholar]
- 14.Chen HW, Jiang WS, Tzeng CR. Nitric oxide as a regulator in preimplantation embryo development and apoptosis. Fertil Steril. 2001;75:1163–71. doi: 10.1016/s0015-0282(01)01780-0. [DOI] [PubMed] [Google Scholar]
- 15.Palermo GD, Cohen J, Alikani M, Adler A, Rosenwaks Z. Intracytoplasmic sperm injection: a novel treatment for all forms of male factor infertility. Fertil Steril. 1995;63:1231–40. doi: 10.1016/s0015-0282(16)57603-1. [DOI] [PubMed] [Google Scholar]
- 16.Veeck LL. An atlas of human gametes and conceptuses: An illustrated reference for assisted reproductive technology. Parthenon Publishing; New York: 1999. [Google Scholar]
- 17.Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The graduated embryo score (GES) predicts blastocyst formation and pregnancy rate from cleavage-stage embryos. Hum Reprod. 2001;16:1970–5. doi: 10.1093/humrep/16.9.1970. [DOI] [PubMed] [Google Scholar]
- 18.Battaglia C, Ciotti P, Notarangelo L, Fratto R, Facchinetti F, de Aloysio D. Embryonic production of nitric oxide and its role in implantation: a pilot study. J Assist Reprod Genet. 2003;20:449–54. doi: 10.1023/B:JARG.0000006706.21588.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telfer J, Lyall F, Norman JE, Cameron IT. Identification of nitric oxide synthase in human uterus. Hum Reprod. 1995;10:19–23. doi: 10.1093/humrep/10.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Rosselli M, Dubey RK, Rosselli MA, Macas E, Fink D, Lauper U, Keller PJ, Imthurn B. Identification of nitric oxide synthase in human and bovine oviduct. Mol Hum Reprod. 1996;2:607–12. doi: 10.1093/molehr/2.8.607. [DOI] [PubMed] [Google Scholar]
- 21.Rosselli M, Imthurm B, Macas E, Keller PJ, Dubey RK. Circulating nitrite/nitrate levels increase with follicular development: indirect evidence for estradiol mediated NO release. Biochem Biophys Res Commun. 1994;202:1543–52. doi: 10.1006/bbrc.1994.2107. [DOI] [PubMed] [Google Scholar]