Abstract
Background
Little is known regarding the association of scavenger receptor class B type I (SCARB1) single nucleotide polymorphisms (SNPs) and subclinical atherosclerosis (SCA), particularly in subjects of different racial/ethnic backgrounds. We examined this relationship in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods and Results
Forty-three SCARB1 tagging SNPs were genotyped. Baseline examinations included fasting lipids and SCA phenotypes (coronary artery calcium [CAC], and common and internal carotid artery thickness [CCIMT and ICIMT]). Examining SNP associations with different SCA phenotypes across multiple racial/ethnic groups with adjustment for multiple covariates, we found the C allele of SNP rs10846744 was associated with higher CCIMT in African American (P=0.03), Chinese (P=0.02), European American (P=0.05), and Hispanic participants (P=0.03), and was strongly associated in pooled analyses (P=0.0002). The results also showed that the association of this SNP with CCIMT was independent of lipids and other well-established cardiovascular risk factors. Stratifying by sex, there appeared to be a strong association of rs10846744 with CCIMT in females, but no genotype-sex interactions were observed.
Conclusions
Variation in SCARB1 at rs10846744 was significantly associated with CCIMT across racial/ethnic groups in MESA.
Keywords: genetics, atherosclerosis, cholesterol, lipids, prospective cohort study, genetic association
High-density lipoprotein cholesterol (HDL-C) has been established as protective against atherosclerosis and cardiovascular disease (CVD).1,2 HDL-C levels are heritable, with a recent heritability estimate of 50% in a study of twins of Northern European background.3 Polymorphisms in a number of genes involved in HDL-C regulating pathways have been shown to influence risk of subclinical atherosclerosis (SCA)4,5 and CVD.6,7
One gene, SCARB1, encodes Scavenger Receptor class B type I (SR-BI), which is a key lipoprotein receptor, binding lipoproteins with high affinity and participating in the selective transfer of neutral lipids from the lipoprotein core.8,9 In humans, studies have shown SCARB1 single nucleotide polymorphisms (SNPs) to be associated with lipid levels10–13 and lipoprotein particle size.12,14 One recent study of SCARB1 variants in the Genetics of Lipid Lowering Drugs and Diet Network trial found that the exon 1 SNP rs4238001 was associated with differences in HDL-C (P=0.01) and triglyceride levels (P=0.004) after an intervention with lipid-lowering fenofibrate.15
Several studies have suggested the effects of SCARB1 variants on lipids to be sex-specific. In one study, the rs4238001 SNP was significantly associated with higher HDL-C and lower LDL-C, but only in men.10 In postmenopausal women in the Rancho Bernardo Study, multiple SCARB1 SNPs were significantly associated with HDL-C levels among women taking estrogen therapy.16 We reported a significant association of an exon 8 synonymous coding SNP, rs5888, and a nonsynonymous coding SNP, rs5891 (V135I, valine to isoleucine substitution), with HDL-C levels in women participants in the Amish Family Diabetes Study.17 Research in mouse models has suggested sex-specific effects in the role of SCARB1 in coronary artery disease,18 and accelerated atherosclerosis has been reported in Scarb1 null female mice.19
Though the role of SR-BI in protecting against atherosclerosis has been well established in mouse models,20 its role in human susceptibility to atherosclerosis has not been extensively studied. One previous study has directly tested association of SCARB1 coding variants with measures of SCA, and observed an association of the rs4238001 SNP with internal carotid intimal medial thickness.21 Two common variants in intron 5 and exon 8 of SCARB1 were found to be associated with extreme triglyceride-to-HDL-C ratios in women with premature coronary artery disease (CAD).12 Similarly, Rodr guez-Esparragon18 identified an association of rs5888 with CAD.
Markers of SCA, including carotid intimal-medial thickness (IMT) and coronary artery calcification (CAC), have been shown to be associated with cardiovascular disease prevalence22–24 and numerous cardiovascular risk factors.22,23,25 Common carotid IMT (CCIMT), in particular, was reported in the Rotterdam Elderly Study (a study of 7,983 subjects 55 years of age or older), to be associated with risk of MI and stroke.26 The investigation of genetic associations with markers of SCA like CCIMT may help in identifying candidate genes for CVD as well as elucidating the pathological mechanisms through which these candidates may exert their role on disease risk.
In this study, we examined SNPs genotyped in SCARB1 and markers of SCA in the four racial/ethnic groups of the MESA study to determine: (a) whether or not genetic variation in SCARB1 is associated with SCA; (b) whether the role of SCARB1 variants in SCA may be mediated via their effects on lipid levels; and (c) whether the associations of SCARB1 variants with SCA are modified by gender.
Methods
Study Design
The recruitment and design of the MESA study has been previously described.27 The MESA study is a longitudinal prospective cohort study designed to investigate the prevalence and progression of subclinical cardiovascular disease. Beginning in July 2000, MESA enrolled 3601 women and 3213 men aged 45 to 84 years without previous evidence of CVD from four racial/ethnic groups. The racial/ethnic composition is 38% European American (EUA), 28% African American (AFA), 22% Hispanic (HIS), and 12% Asian (of Chinese descent) (CHN), with participants from 6 U.S. communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; upper Manhattan, New York; and St. Paul, Minnesota). Institutional Review Board approval was obtained at all sites. The current analysis examines a sample (n = 2,757) from the overall baseline MESA cohort who were randomly selected from the 6,814 MESA participants for genotyping. All participants in this subgroup provided written consent to the use of their DNA and collection of phenotype data.
Genotyping and Laboratory Analyses
See online Supplement, which includes complete description of polymorphisms (Supplemental Table 1).
Statistical Analysis
To test the association of SCARB1 SNPs with SCA markers (CCIMT, ICIMT, and CAC), genotypic tests of association were performed assuming an additive genetic model. Pairwise SNP linkage disequilibrium (LD) was estimated with r2 and D’ within each racial/ethnic group (AFA, CHN, EUA, and HIS) using Haploview v4.028 (Supplemental Figures 1 and 2), with haplotype blocks defined by the Gabriel et al. method.29 Genotypic associations of the quantitative traits CCIMT and ICIMT and the binary trait CAC were examined in 2,757 participants (712 AFA, 630 CHN, 711 EUA, 704 HIS) using linear and logistic regression models adjusting for age, sex, and MESA recruitment site (model 1) stratified by racial/ethnic group. To determine if the effects of any variants demonstrating associations with SCA phenotypes may be moderated by the role of SCARB1 variation in lipid levels, initial genotypic associations were followed up with additional adjustment for multiple covariates (HDL-C, LDL-C, TG) and other cardiovascular risk factors ( body mass index [BMI], glucose levels, and hypertension [HTN]) (model 2). Genotypic analyses were performed using PLINK software.30 To account for multiple hypothesis testing and allow statistically significant association at α=0.05, empirical p-values from simulation were estimated in PLINK using the ‘--mperm’ option and are reported here for each independent test of association using 20,000 simulation replicates. All p-values reported for tests of association are empirical p-values unless otherwise stated, and are denoted by Pe. These empirical p-values were estimated across the gene utilizing a max(T) approach to better account for the examination of multiple phenotypes in multiple racial/ethnic groups. For any SNPs demonstrating associations with the same phenotype across three or more groups at or below the empirically-adjusted α=0.05, we performed joint modeling adjusting for racial/ethnic group and including a term for genotype-race interaction.
Results
MESA Baseline Characteristics
Baseline characteristics of subjects in the four racial/ethnic groups of the MESA cohort genotyped for SCARB1 SNPs are shown in Table 1. For the entire group, the mean age with standard deviation was 61.6 ± 9.9 years, there were more females than males (54%), and the mean BMI with standard deviation was 28.0 ± 5.5 kg/m2. Mean levels of most lipids and SCA markers differed significantly between the four ethnic groups (P<0.0001 to P<0.001). Of the SCA phenotypes, AFA had the highest mean CCIMT and ICIMT measurements, while the CHN group had the lowest levels. However, CAC frequency was highest in EUA and the lowest in AFA.
Table 1.
Demographic characteristics of SCARB1 genotyped participants in the study sample (Mean ± SD or Number (Percent)) and nominal P-value from ANOVA.
| African American | Chinese | European American | Hispanic | P-value | |
|---|---|---|---|---|---|
| Number of subjects | 711 | 630 | 712 | 704 | |
| Females (%) | 391 (55.0%) | 335 (53.2%) | 380 (53.4%) | 381 (54.1%) | 0.90 |
| Age (yr) | 61.5 ± 9.9 | 62.4 ± 10.4 | 61.5 ± 10.4 | 61.1 ± 10.1 | 0.20 |
| BMI (kg/m2) | 30.1 ± 5.8 | 23.9 ± 3.3 | 27.8 ± 5.3 | 29.5 ± 5.1 | <0.0001 |
| Waist Circ. (cm) | 101.0 ± 14.5 | 86.9 ± 10.0 | 97.8 ± 15.1 | 100.4 ± 12.8 | <0.0001 |
| Glucose (mmol/L) | 5.9 ± 1.8 | 5.9 ± 1.5 | 5.4 ± 1.1 | 6.1 ± 2.2 | <0.0001 |
| Hypertension | 397 (55.8%) | 231 (36.7%) | 277 (38.9%) | 295 (41.9%) | <0.001 |
| Lipid Levels | |||||
| HDL-C (mmol/L) | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.4 ± 0.4 | 1.2 ± 0.3 | <0.0001 |
| LDL-C (mmol/L) | 3.0 ± 0.9 | 3.0 ± 0.7 | 3.0 ± 0.8 | 3.1 ± 0.8 | 0.04 |
| Total Cholesterol (mmol/L) | 4.9 ± 1.0 | 5.0 ± 0.8 | 5.1 ± 0.9 | 5.2 ± 1.0 | <0.0001 |
| Triglycerides (mmol/L) | 1.2 ± 0.7 | 1.6 ± 1.0 | 1.5 ± 0.9 | 1.8 ± 1.3 | <0.0001 |
| SCA | |||||
| CCIMT (mm) | 0.91 ± 0.19 | 0.82 ± 0.17 | 0.87 ± 0.21 | 0.86 ± 0.18 | <0.0001 |
| ICIMT (mm) | 1.10 ± 0.61 | 0.88 ± 0.48 | 1.09 ± 0.59 | 1.01 ± 0.56 | <0.0001 |
| CAC present (%) | 300 (42.2%) | 311 (49.4%) | 393 (55.2%) | 316 (44.9%) | <0.001 |
To convert glucose to mg/dl divide by 0.0555; to convert cholesterol to mg/dl divide by 0.0259; to convert TG to mg/dl divide by 0.0113
Associations of SCARB1 SNPs with markers of SCA
We first examined the associations of SNPs (Figure 1) with SCA phenotypes after adjusting for age, sex, and MESA center. While a number of significant SNP associations were observed in each racial/ethnic group for all SCA phenotypes examined, no significant SNP associations were observed across all groups for either ICIMT or CAC. Similar results were observed when the associations were adjusted for lipid levels and other major CVD risk factors (model 2) (Supplemental Figure 3).
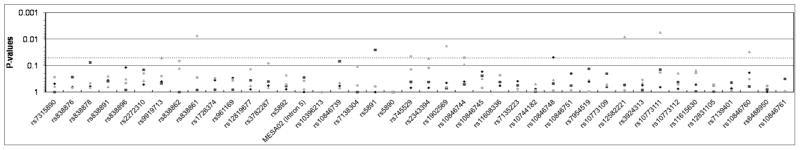
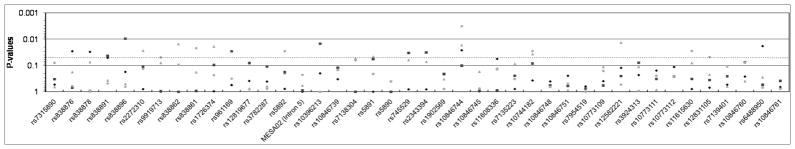
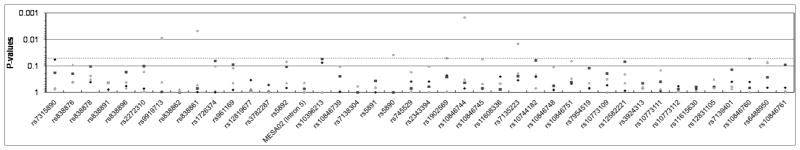
Figure 1.
SCARB1 SNP associations with SCA phenotypes (A: CAC; B: CCIMT; C: ICIMT) in MESA participants under an additive genetic model (model 1). Empirically-estimated p-values for AFA are shown with gray triangles, CHN with gray diamonds, EUA with black squares, and HIS with black circles. The light grey line demarcates P=0.05.
Several variants demonstrated associations with CCIMT across multiple racial/ethnic groups, specifically intron 1 SNPs rs10846744 and rs10744182. For SNP rs10846744, the C allele was significantly associated with higher CCIMT (Table 2) in the AFA (Pe =0.02), CHN(Pe =0.03), and HIS (Pe =0.003) groups, with a non-significant trend in the same direction in the EUA group as well (Pe =0.10).
Table 2.
Genotypic means by CCIMT (in mm) and linear regression coefficients (Betas) and empirical P-values for the association of SCARB1 SNP rs10846744 and CCIMT by racial/ethnic group under multiple models of adjustment. Model 1 includes adjustment for age, sex, and MESA center, while model 2 includes adjustment for these covariates, and also lipid levels (HDL-C, LDL-C, TC, and TG) and other major CVD risk factors (BMI, serum glucose, and HTN).
| CCIMT by Genotype (mm ± SD)
|
Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Racial/Ethnic Group | Minor Allele (Frequency) | C/C | G/C | G/G | Beta | P- value | Beta | P-value |
| African American | G (0.40) | 0.92 ± 0.20 | 0.92 ± 0.19 | 0.87 ± 0.17 | −0.022 | 0.02 | −0.021 | 0.03 |
| Chinese | G (0.42) | 0.84 ± 0.19 | 0.82 ± 0.16 | 0.81 ± 0.16 | −0.019 | 0.03 | −0.020 | 0.02 |
| European American | C (0.35) | 0.90 ± 0.17 | 0.88 ± 0.21 | 0.86 ± 0.21 | 0.019 | 0.10 | 0.023 | 0.05 |
| Hispanic | C (0.18) | 0.89 ± 0.19 | 0.86 ± 0.19 | 0.83 ± 0.17 | 0.025 | 0.003 | 0.019 | 0.03 |
To more fully confirm this relationship, the genotypic association of rs10846744 with CCIMT was examined by pooling data across all racial/ethnic groups. The C allele was significantly associated with higher CCIMT across all groups at Pe =0.0002 (nominal P=2.5×10−5) after adjustment for ethnicity. Genotype-race interactions were modeled to test for heterogeneity between genotypic associations between racial/ethnic groups, however no significant interaction was observed (Pe =0.98).
To determine if the association of rs10846744 with CCIMT might be mediated through lipids or through other cardiovascular risk factors, we examined the association of this SNP with CCIMT under model 2 adjustments, and observed the change in regression coefficient values within each racial group (Table 2). The values of regression coefficients changed modestly across most racial/ethnic groups, and significant association with CCIMT remained for the C allele of rs10846744 SNP across all of the four racial/ethnic groups (AFA Pe =0.03; CHN Pe=0.02; EUA Pe =0.05; HIS Pe =0.03).
We found limited LD between rs10846744 and other SCARB1 SNPs in the AFA, CHN, or HIS groups (Supplement Figures 1 and 2), therefore we did not examine any haplotypic or other genotypic associations in these groups. However, in the EUA group, we found that two SNPs in an LD block with rs10846744 showed evidence of similar association with CCIMT, with rs2343394 significantly associated (Pe =0.02) and rs1902560 associated with borderline significance (Pe =0.08) with CCIMT under model 2 adjustments (data not shown).
Sex-stratified association of SNPs with SCA markers
We examined if sex-specific effects were present in the association of SCARB1 SNPs with SCA phenotypes by testing genotype-sex interactions, modeled additively and adjusted for age, and MESA site (model 1), as shown in Supplemental Table 2. No statistically significant genotype-sex interactions were observed across multiple racial/ethnic groups. Examining differences in patterns of association stratifying by sex, associations of individual SNPs with CAC (Supplemental Table 3), CCIMT (Supplemental Table 4), and ICIMT (Supplemental Table 5) suggested stronger associations of SCARB1 variation with SCA among women compared to men. Few significant associations within each sex were observed across multiple racial/ethnic groups, with a notable exception. The rs10846744 SNP, which demonstrated significant associations in the overall analysis with CCIMT, also demonstrated significant associations with CCIMT among females in the same groups [AFA (Pe =0.02), CHN (Pe =0.009), and HIS (Pe =0.009)]; however, no significant genotype-sex interactions were observed (AFA Pe =0.55; CHN Pe =0.18; EUA Pe =0.90; HIS Pe =0.82).
Discussion
We had three objectives for this study: (1) to determine whether or not variants of SCARB1 are associated with SCA in MESA, (2) whether the role of SCARB1 variants in SCA may be mediated via their effects on lipid levels, and (3) whether the association of SCARB1 variants with SCA was modified by gender. We have now shown that certain SCARB1 variants are associated with SCA, specifically the C allele of rs10846744 with CCIMT. For the rs10846744 variant, its association with CCIMT did not appear to be mediated through traditional lipid measurements. We did observe that this SNP was more strongly associated with CCIMT in females than males across all four MESA racial/ethnic groups.
That we observed significant associations of a number of SCARB1 SNPs with different SCA measurements in the four racial/ethnic groups is consistent with results from previous work.21 Following up a genomewide linkage analysis with fine-mapping and association testing in SCARB1, Fox et al.21 found that those homozygous for the rare allelic variants of the nonsynonymous SNP rs4238001 had lower carotid IMT. In our study, we observed a number of SNPs associated with CCIMT, ICIMT, or CAC in different racial/ethnic groups, but only the rs10846744 was significantly associated with CCIMT across multiple groups. The lack of multiple genetic associations with SCA consistent across the four groups may be due to differences in the LD structure between the groups; however, it is not known if and to what extent variation at multiple sites in SCARB1 may affect SCARB1 function. Contrastingly, variation at rs10846744 may represent the strongest direct or indirect association signal (especially in the pooled analysis) for a causal allele associated with CCIMT.
We did not find that traditional lipid measurements affected the association of rs10846744 with CCIMT. It is known that SR-BI can affect nitric oxide synthesis in the vessel wall27; thus, the effects of SR-BI on atherosclerosis might not all be directly mediated by lipid effects. Alternatively, the influence of SCARB1 variants on SCA might not be adequately explained by traditional lipid measurements, but instead via lipid subfractions or other measures of lipoprotein compositional changes.
We17 and others16 had previously reported significant differences in the association of SCARB1 variants with lipids stratified either by gender or genotype-sex interaction. Here we observe sex-specific association of rs10846744 with CCIMT, which is more strongly associated in women across multiple MESA racial/ethnic groups; however, with this exception, limited additional evidence in the MESA cohort was observed for sex differences in the associations of SCARB1 variants with SCA. It is possible that the lack of sex differences observed is due to diminished power in the analyses after stratifying by sex, although it is also possible that true sex-specific effects in SCARB1 (especially for rs10846744) may not be easily explained by the data. The availability of results from the larger CARE study within the next year should provide sufficient power to answer the question of whether associations of SCARB1 variants with SCA are modified by gender. Given evidence from previous work, such as a study of SR-BI knockout mice that showed that female SR-BI knockout mice are more prone to accelerated atherosclerosis and infertility than males,31 further examination of sex differences in the effect of SCARB1 variants on SCA is merited.
The limitations of our study merit discussion. We were unable to examine SCARB1 associations with CVD events since not enough events have accrued yet in the subgroup of MESA participants with genotyping results. However, it should be noted that other studies have found that small differences in carotid IMT to be associated with significant differences in risk for MI and stroke,32,33 suggesting that the association of rs10846744 with CCIMT might also have effects on cardiovascular disease risk. It is also unclear whether certain SNPs with strong associations to SCA phenotypes in only one racial/ethnic group but not in others are the result of Type I error, or if they represent true associations. In order to reduce the likelihood of observing false associations, we performed simulations randomly shuffling phenotypes in order to derive p-values corrected for multiple hypothesis testing, accounting for number of SNPs and phenotypes examined. This approach can limit the likelihood of observing false associations due to low minor allele frequencies and, as it is not as overly conservative as the Bonferroni correction (which would be α*=0.0001 in this study), it is less likely to exclude true associations. Another inherent limitation in confirming whether associations in one racial/ethnic group but not another are true are the vast differences in the LD background between the MESA racial/ethnic groups. Despite these limitations, at least one SNP, rs10846744, demonstrated consistency and uniform directionality in its association with CCIMT across multiple ethnic groups, in both primary and sex-stratified analyses suggesting a low likelihood of type 1 error with this result. Furthermore, to verify this observed association, after confirming the absence of race/ethnicity-genotype interactions, we also pooled genotypes and tested association in the four groups to increase statistical power.
It is also unclear if differences in the associations between racial/ethnic groups represent true differences between the groups, perhaps as a result of undetected gene-gene or gene-environment interactions, which could lead to variability in the significant associations of SCARB1 SNPs with lipid and/or SCA phenotypes. A potentially relevant gene-gene interaction previously observed is that of PON1 and SCARB1 on CVD in men.18 However, a more probable source of inconsistency is that the genotyped SNPs were not the true causal variants, but are in strong LD with the variants at the causal locus. With the large differences observed in allele frequencies in the LD backgrounds between the racial/ethnic groups, it is possible that a SNP could be a strong marker for the effect at a causal SNP on one LD background, but not another. It is also possible that these associations are false-positives, which suggests that further examination of these associations in other studies with data on subclinical atherosclerosis is needed.
In summary, this study suggests that variation in SCARB1 is likely to have a role in SCA. However, the exact mechanism of this relationship and to what degree it may differ by sex remain unclear, and more work is needed to confirm this relationship and to determine if it increases risk for incident CVD.
Supplementary Material
Cardiovascular diseases constitute a major public health problem. Based on the findings in mice that deficiency of the scavenger receptor class B type I (SR-BI) receptor was associated with altered lipoprotein profile and accelerated atherosclerosis, we examined the association of variation in the SR-BI gene (SCARB1) with lipid phenotypes and measures of subclinical atherosclerosis (SCA) in participants of the Multi-Ethnic Subclinical Atherosclerosis (MESA) study. The results of our study demonstrate that variation within the SCARB1 gene is associated with lipid phenotypes and SCA. One variant, rs10846744, was significantly associated with common carotid intimal thickening across the different ethnic/racial groups, with a particularly strong association in women.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding Sources: This work was supported by a NIH grant, HL075646, to A. Rodriguez. A.C. Naj was supported by NHLBI training grant T32 HL07024. This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 and RO1 HL071205 (MESA Family) from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Disclosures None
References
- 1.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. Jama. 1986;256:2835–8. [PubMed] [Google Scholar]
- 2.Sharrett AR, Ballantyne CM, Coady SA, Heiss G, Sorlie PD, Catellier D, Patsch W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–13. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 3.Goode EL, Cherny SS, Christian JC, Jarvik GP, de Andrade M. Heritability of longitudinal measures of body mass index and lipid and lipoprotein levels in aging twins. Twin Res Hum Genet. 2007;10:703–11. doi: 10.1375/twin.10.5.703. [DOI] [PubMed] [Google Scholar]
- 4.Tsai MY, Johnson C, Kao WH, Sharrett AR, Arends VL, Kronmal R, Jenny NS, Jacobs DR, Jr, Arnett D, O'Leary D, Post W. Cholesteryl ester transfer protein genetic polymorphisms, HDL cholesterol, and subclinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benton JL, Ding J, Tsai MY, Shea S, Rotter JI, Burke GL, Post W. Associations between two common polymorphisms in the ABCA1 gene and subclinical atherosclerosis: Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;193:352–60. doi: 10.1016/j.atherosclerosis.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Borggreve SE, Hillege HL, Wolffenbuttel BH, de Jong PE, Zuurman MW, van der Steege G, van Tol A, Dullaart RP. An increased coronary risk is paradoxically associated with common cholesteryl ester transfer protein gene variations that relate to higher high-density lipoprotein cholesterol: a population-based study. J Clin Endocrinol Metab. 2006;91:3382–8. doi: 10.1210/jc.2005-2322. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. Jama. 2008;299:1265–76. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–20. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 9.Rigotti A, Trigatti B, Babitt J, Penman M, Xu S, Krieger M. Scavenger receptor BI--a cell surface receptor for high density lipoprotein. Curr Opin Lipidol. 1997;8:181–8. doi: 10.1097/00041433-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Acton S, Osgood D, Donoghue M, Corella D, Pocovi M, Cenarro A, Mozas P, Keilty J, Squazzo S, Woolf EA, Ordovas JM. Association of polymorphisms at the SR-BI gene locus with plasma lipid levels and body mass index in a white population. Arterioscler Thromb Vasc Biol. 1999;19:1734–43. doi: 10.1161/01.atv.19.7.1734. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy JJ, Lewitzky S, Reeves C, Permutt A, Glaser B, Groop LC, Lehner T, Meyer JM. Polymorphisms of the HDL receptor gene associated with HDL cholesterol levels in diabetic kindred from three populations. Hum Hered. 2003;55:163–70. doi: 10.1159/000073986. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy JJ, Lehner T, Reeves C, Moliterno DJ, Newby LK, Rogers WJ, Topol EJ. Association of genetic variants in the HDL receptor, SR-B1, with abnormal lipids in women with coronary artery disease. J Med Genet. 2003;40:453–8. doi: 10.1136/jmg.40.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu LA, Ko YL, Wu S, Teng MS, Peng TY, Chen CF, Lee YS. Association between a novel 11-base pair deletion mutation in the promoter region of the scavenger receptor class B type I gene and plasma HDL cholesterol levels in Taiwanese Chinese. Arterioscler Thromb Vasc Biol. 2003;23:1869–74. doi: 10.1161/01.ATV.0000082525.84814.A9. [DOI] [PubMed] [Google Scholar]
- 14.Osgood D, Corella D, Demissie S, Cupples LA, Wilson PW, Meigs JB, Schaefer EJ, Coltell O, Ordovas JM. Genetic variation at the scavenger receptor class B type I gene locus determines plasma lipoprotein concentrations and particle size and interacts with type 2 diabetes: the framingham study. J Clin Endocrinol Metab. 2003;88:2869–79. doi: 10.1210/jc.2002-021664. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Ordovas JM, Gao G, Province M, Straka RJ, Tsai MY, Lai CQ, Zhang K, Borecki I, Hixson JE, Allison DB, Arnett DK. The SCARB1 gene is associated with lipid response to dietary and pharmacological interventions. J Hum Genet. 2008;53:709–17. doi: 10.1007/s10038-008-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richard E, von Muhlen D, Barrett-Connor E, Alcaraz J, Davis R, McCarthy JJ. Modification of the effects of estrogen therapy on HDL cholesterol levels by polymorphisms of the HDL-C receptor, SR-BI: the Rancho Bernardo Study. Atherosclerosis. 2005;180:255–62. doi: 10.1016/j.atherosclerosis.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Roberts CG, Shen H, Mitchell BD, Damcott CM, Shuldiner AR, Rodriguez A. Variants in scavenger receptor class B type I gene are associated with HDL cholesterol levels in younger women. Hum Hered. 2007;64:107–13. doi: 10.1159/000101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Esparragon F, Rodriguez-Perez JC, Hernandez-Trujillo Y, Macias-Reyes A, Medina A, Caballero A, Ferrario CM. Allelic variants of the human scavenger receptor class B type 1 and paraoxonase 1 on coronary heart disease: genotype-phenotype correlations. Arterioscler Thromb Vasc Biol. 2005;25:854–60. doi: 10.1161/01.ATV.0000157581.88838.03. [DOI] [PubMed] [Google Scholar]
- 19.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–5. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1732–8. doi: 10.1161/01.ATV.0000091363.28501.84. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Cupples LA, Chazaro I, Polak JF, Wolf PA, D'Agostino RB, Ordovas JM, O'Donnell CJ. Genomewide linkage analysis for internal carotid artery intimal medial thickness: evidence for linkage to chromosome 12. Am J Hum Genet. 2004;74:253–61. doi: 10.1086/381559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, Bommer W, Price TR, Gardin JM, Savage PJ. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23:1752–60. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 23.Mannami T, Konishi M, Baba S, Nishi N, Terao A. Prevalence of asymptomatic carotid atherosclerotic lesions detected by high-resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study. Stroke. 1997;28:518–25. doi: 10.1161/01.str.28.3.518. [DOI] [PubMed] [Google Scholar]
- 24.Bots ML, Breslau PJ, Briet E, de Bruyn AM, van Vliet HH, van den Ouweland FA, de Jong PT, Hofman A, Grobbee DE. Cardiovascular determinants of carotid artery disease. The Rotterdam Elderly Study. Hypertension. 1992;19:717–20. doi: 10.1161/01.hyp.19.6.717. [DOI] [PubMed] [Google Scholar]
- 25.Poli A, Tremoli E, Colombo A, Sirtori M, Pignoli P, Paoletti R. Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients. A new model for the quantitation and follow-up of preclinical atherosclerosis in living human subjects. Atherosclerosis. 1988;70:253–61. doi: 10.1016/0021-9150(88)90176-1. [DOI] [PubMed] [Google Scholar]
- 26.Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7:403–22. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 27.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 28.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 29.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 30.Purcell S, Neales B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A. 1999;96:9322–7. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 33.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.