Abstract
Cigarette smoke exposure generates both acute and chronic inflammatory cell infiltrates that are operative in numerous pulmonary disorders. Cigarette smoke induces a complex signaling cascade within the lung mediated by epithelial cells, lymphocytes, macrophages, and others. The net result is a destructive and self-perpetuating inflammatory environment that is capable of creating lung diseases such as emphysema, while simultaneously fueling lung tumor growth using both matrix-dependent and -independent means.
Keywords: chronic obstructive pulmonary disease, lung cancer, inflammation
Cigarette smoking is the most common cause of preventable death in the United States and is pandemic worldwide (1). Although it is associated with many diseases, cigarette smoke exposure is most highly associated with the development of lung cancer and chronic obstructive pulmonary disease (COPD)/emphysema, the second and fourth leading causes of death in the United States, respectively. Seminal epidemiological studies from more than 2 decades ago demonstrated that COPD/emphysema and lung cancer were “linked” diseases (2, 3). That is, lung cancer incidence was shown to increase with declining FEV1, an indicator of worsening COPD. More recently, several independent studies have shown that the presence of radiographic emphysema on CT scan of the chest predicts an increase in lung cancer incidence, with airflow obstruction not being the primary risk factor (4, 5). Potential explanations for this observation are somewhat limited, given that emphysema and lung cancer are diametrically opposed diseases. For example, emphysematous lungs display alveolar capillary dropout, whereas cancers display robust angiogenesis. Emphysema is characterized by apoptosis and cell death, and cancer by uncontrolled cellular proliferation. However, it would appear that the lung epithelial cell's attempts to avoid apoptosis inadvertently generate tumor-promoting inflammation (6). It is easy to speculate, then, that the inflammatory cell infiltrates common to both diseases, and continually modified by cigarette smoke, may represent the shared mechanistic link. Herein we outline how the innate immune cell infiltrates encountered in emphysematous lungs function to accelerate the growth of lung tumors arising within this unique, cigarette smoke–generated, disease microenvironment.
Emphysema Pathogenesis
Although the definition of COPD is broad, descriptive, and allows for the inclusion of multiple disease phenotypes—airflow obstruction associated with the chronic inhalation of particulate matter (cigarette smoke)—emphysema has a strict definition. Emphysema is anatomically defined as the permanent enlargement of the peripheral airspaces distal to the terminal bronchioles. Chronic cigarette smoke exposure generates a characteristic innate and adaptive immune response (see below) culminating in the accumulation of activated macrophages and neutrophils (which release matrix-degrading proteinases) within the distal airways and peripheral airspaces of the lung. This inflammatory cell infiltrate is not the rate-limiting step in the pathogenesis of emphysema. Based on several studies performed to determine the cellular content in the bronchoalveolar lavage fluid of subjects with COPD (7), we have learned that essentially all habitual cigarette smokers display this characteristic inflammatory response. Therefore, it would appear that the development of emphysema is the result of an imbalance between the burden of inflammatory cell–derived proteinases and the adequacy of the antiproteinase shield of the host.
According to the now nearly 50-year-old proteinase–antiproteinase hypothesis, when the quantity of elastic-degrading enzymes exceeds the amount of their inhibitors, tissue destruction and emphysema result. Initially, it was assumed that there were just two variables in this equation, the neutrophil-derived serine proteinase neutrophil elastase (NE) and its physiologic inhibitor, α1-antitrypsin (A1AT). This simple concept was uniformly accepted as it readily explained the clinical observation of overwhelming lung tissue destruction encountered in A1AT-deficient subjects (8). The subsequent identification of certain members of the matrix metalloproteinase (MMP) family and other elastolytic enzymes within lung (9) has dispelled this overly straightforward view, although the basic tenants of the proteinase–antiproteinase hypothesis remain intact.
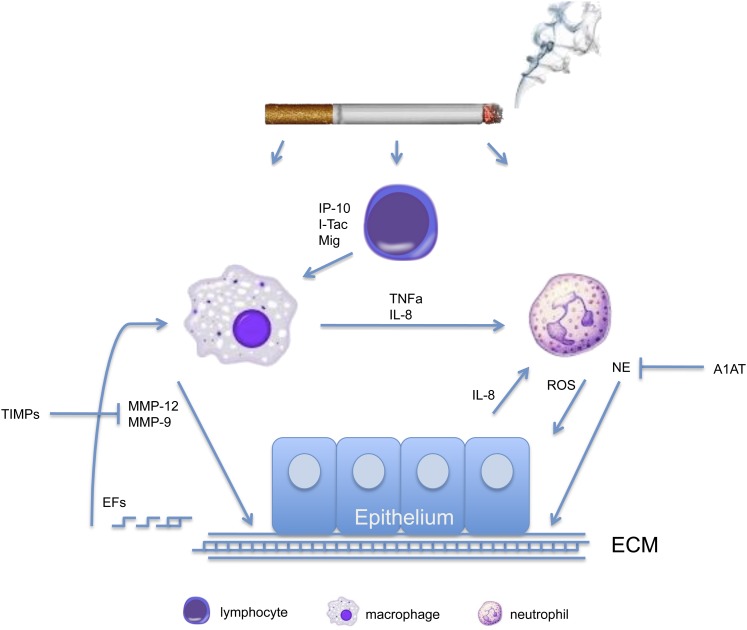
The completion of numerous studies in mice and humans has provided an increasingly clearer picture as to the generation and maintenance of the destructive inflammatory cell infiltrate characteristic of emphysema (see Figure 1). Acute cigarette smoke exposure in mice creates a mild acute lung injury characterized by typical inflammatory cytokines, such as tumor necrosis factor-α and monocyte chemotactic factor-1. However, this acute injury is short-lived. Chronic cigarette smoke exposure, as encountered in humans, reveals a significant accumulation of alveolar macrophages, CD4+ and CD8+ lymphocytes, and to a lesser extent, neutrophils. On cigarette smoke exposure, CD8+ lymphocytes release the IFN-γ–inducible chemokines MIG (CXCL-9), IP-10 (CXCL-10), and I-TAC (CXCL-11) (10). These chemokines act to induce the expression and release of matrix-degrading enzymes from resident alveolar macrophages, including the elastolytic macrophage elastase (MMP-12) (11). MMP-12, acting in concert with other inflammatory cell–derived proteinases, such as NE, destroys the rather inert and irreplaceable elastic fiber. Once the elastic recoil properties of the lung have been compromised, obstructive physiologic parameters appear, and the characteristic sequelae of emphysema ensue. Making matters worse, the degradation of elastic fibers creates novel elastic fragments that are chemotactic for monocytes (12), resulting in a positive feedback loop that promotes macrophage accumulation in the lungs of emphysematous subjects. This positive feedback loop may explain why cigarette smoke–induced inflammation frequently persists after smoking cessation. It should be noted that the inflammatory infiltrate described above might be a marker of cigarette smoke exposure and persistent bacterial infection and not necessarily the causative link between emphysema and lung cancer.
Figure 1.
Simplified schematic depicting the emphysematous disease microenvironment. As shown, emphysema is the result of an interaction between multiple cell types, cytokines/chemokines, proteinases, and proteinase inhibitors. A1AT = α1-antitrypsin; EFs = elastin fragments; IP-10 = CXCL-10; Mig = CXCL-9; I-TAC = CXCL-11; MMP = matrix metalloproteinase; NE = neutrophil elastase; ROS = reactive oxygen species; TIMPs = tissue inhibitors of metalloproteinases; TNF-α = tumor necrosis factor-α.
Lung Tumor Microenvironment
As is the case with emphysema, cigarette smoke exposure is the cause of the vast majority of lung cancers. Although cigarette smoke promotes carcinogenesis on many levels, key among these is the carcinogen-induced formation of DNA adducts, ultimately leading to the generation of activating mutations in oncogenes and the deletion of tumor-suppressor genes. Reactive oxygen species also contribute to this “genotoxic” stress generated by cigarette smoke.
The inflammatory cell composition encountered within the lung tumor microenvironment is the net result of many competing factors, especially when one accounts for the preexisting lung disease that almost uniformly accompanies a new lung cancer diagnosis. Among the competing forces are the impact of cigarette smoke exposure, the emphysematous microenvironment, the endogenous host immune response to the presence of tumor, and the manipulation of this host response by the tumor itself. In some cases, host immune cells found within the tumor microenvironment are the result of a process entirely driven by the tumor and not really a means of host defense in the least. Activating K-ras mutations, seen in approximately 25 to 50% of lung adenocarcinomas, have been shown to induce the expression of IL-8 via a nuclear factor-κB–dependent mechanism (13). Subsequently, genetically engineered mice bearing K-ras mutant lung tumors have been shown to secrete substantial quantities of CXC chemokines 1 and 2 (mouse equivalents of IL-8), which recruit neutrophils to the sites of tumorigenesis (14). Depletion of tumor-associated polymorphonuclear leukocytes in these models using an antibody approach (Gr-1) reduced the tumor burden (15), consistent with the concept that these neutrophils are tumor promoting, and not functioning for the host.
To confuse matters more, it remains unclear whether or not cigarette smoke generates a protumor inflammatory cell response. A seminal study by Witschi and colleagues examined the role of cigarette smoke exposure on lung tumor development initiated by administration of the carcinogen NNK in mice (16). Surprisingly, after coadministering NNK and cigarette smoke, the authors observed that the discontinuation of cigarette smoking led to an increase in lung tumor burden, whereas the group that continued smoking had significantly fewer tumors. The unquestioned carcinogenic effects of cigarette smoke are not the issue here, as the short-term exposure of cigarette smoke does not induce tumors in mice. Rather, it is believed that cigarette smoke generates more of a cytotoxic, or M1-type, macrophage, which is antitumor. In contrast, the majority of tumor-associated macrophages are of the tumor-promoting M2 phenotype. Additional studies will be required to tease apart the individual contributions of cigarette smoke, tumor-released cytokines/chemokines, and innate host response, on the macrophage phenotype encountered within the tumor microenvironment in vivo.
Neutrophils and Neutrophil Elastase as the mechanistic link
Based on the fact that neutrophils and NE are present in the disease microenvironments encountered in emphysema and lung cancer, our group undertook studies to determine the role of NE within the lung tumor microenvironment. Using the lox-stop-lox (LSL)-K-ras mouse model of lung adenocarcinoma, we demonstrated that NE deficiency protected the mice against tumor burden and mortality (17). Additional studies revealed that NE promotes lung tumor growth via directly promoting tumor cell proliferation. NE accomplishes this by gaining access to tumor endosomes, degrading target substrates, and activating the PI3K signaling within tumor cells, ultimately leading to increased proliferation. We were able to demonstrate the therapeutic implications of this work by using a small molecular weight NE inhibitor. Administration of ONO-5046 to LSL-K-ras tumor-bearing mice reduced the tumor burden by threefold.
Recent clinical and epidemiological reports also implicate the NE:A1AT axis in lung cancer development. Taniguchi and colleagues have reported the presence of two separate single-nucleotide polymorphisms within the NE promoter that increase lung cancer risk (18). Reporter assays demonstrated that both SNPs increased NE expression/activity, consistent with the above studies. With respect to A1AT deficiency, there has not been a report of increased lung cancer risk. This is not surprising when one considers that the life expectancy of A1AT-deficient subjects is only approximately 50 years of age. Also, given that these patients develop early-onset emphysema, they typically consume substantially fewer cigarettes over their lifetimes and subsequently have reduced exposure to carcinogens. In contrast, subjects heterozygous for a mutant A1AT allele (usually S or Z) display a twofold increase in lung cancer risk (19). The authors speculate that transient imbalances in proteinase/antiproteinase content will arise in these subjects on exposure to acute stressors, such as infections.
It appears, then, that NE is a unique entity, in that it promotes both the tissue destruction observed in emphysema and the progression of lung cancers, making it an attractive therapeutic target for lung cancer that arises within emphysematous lungs.
Conclusions
Cigarette smoking is the major risk for the development of both COPD/emphysema and lung cancer. The disease microenvironment encountered in emphysema appears to play a tumor-promoting role, most likely by augmenting the growth of lung tumors arising within the disease locale. The exact nature of the tumor microenvironment is likely to be the result of a competition between cigarette smoke, innate and adaptive immune responses, and local-regional disease microenvironments, all manipulated by the release of tumor-derived cytokines and chemokines. Additional study should identify components of the tumor microenvironment, such as NE, that promote both emphysema formation and lung cancer growth.
Supplementary Material
Footnotes
Supported by National Institutes of Health Grant R01HL108979.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Peto R, Chen ZM, Boreham J. Tobacco–the growing epidemic. Nat Med 1999;5:15–17. [DOI] [PubMed] [Google Scholar]
- 2.Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med 1986;105:503–507. [DOI] [PubMed] [Google Scholar]
- 3.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med 1987;106:512–518. [DOI] [PubMed] [Google Scholar]
- 4.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932–1938. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med 2008;178:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Qu P, Wu L, Li B, Du H, Yan C. Api6/AIM/Spα/CD5L overexpression in alveolar type II epithelial cells induces spontaneous lung adenocarcinoma. Cancer Res 2011;71:5488–5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:822–826. [DOI] [PubMed] [Google Scholar]
- 8.Larsson C. Natural history and life expectancy in severe alpha1-antitrypsin deficiency, Pi Z. Acta Med Scand 1978;204:345–351. [DOI] [PubMed] [Google Scholar]
- 9.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 10.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 2007;178:8090–8096. [DOI] [PubMed] [Google Scholar]
- 11.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med 2004;1:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest 2006;116:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 2004;6:447–458. [DOI] [PubMed] [Google Scholar]
- 14.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006;25:2105–2112. [DOI] [PubMed] [Google Scholar]
- 15.Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol 2003;163:2221–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE. The carcinogenicity of environmental tobacco smoke. Carcinogenesis 1997;18:575–586. [DOI] [PubMed] [Google Scholar]
- 17.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 2010;16:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taniguchi K, Yang P, Jett J, Bass E, Meyer R, Wang Y, Deschamps C, Liu W. Polymorphisms in the promoter region of the neutrophil elastase gene are associated with lung cancer development. Clin Cancer Res 2002;8:1115–1120. [PubMed] [Google Scholar]
- 19.Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet WR, Wampfler JA, Thibodeau SN, Katzmann JA, Allen MS, Midthun DE, et al. Alpha1-antitrypsin deficiency carriers, tobacco smoke, chronic obstructive pulmonary disease, and lung cancer risk. Arch Intern Med 2008;168:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.