Abstract
A growing body of evidence indicates that oxidative stress plays a central role in the progression of chronic obstructive pulmonary disease (COPD). Chronic oxidative stress caused by cigarette smoke generates damage-associated molecular patterns (DAMPs), such as oxidatively or nitrosatively modified proteins and extracellular matrix fragments, which induce abnormal airway inflammation by activating innate and adaptive immune responses. Furthermore, oxidative stress–induced histone deacetylase 2 (HDAC2) inactivity is implicated in amplifying inflammatory responses and corticosteroid resistance in COPD. Oxidative stress also mediates disruption of innate immune defenses, which is associated with acute exacerbation of COPD. Host defense transcription factor Nuclear factor erythroid 2–related factor 2 (Nrf2) regulates a multifaceted cytoprotective response to counteract oxidative stress–induced pathological injuries. A decrease in Nrf2 signaling is associated with the progression of diseases. Recent evidence indicates that targeting Nrf2 can be a novel therapy to mitigate inflammation, improve innate antibacterial defenses, and restore corticosteroid responses in patients with COPD.
Keywords: COPD, Nrf2, bacteria, exacerbation, therapeutics
Immunologic Basis of Chronic Obstructive Pulmonary Disease
Lungs of patients with chronic obstructive pulmonary disease (COPD) are associated with persistent abnormal inflammation, characterized by accumulation of neutrophils, macrophages, and dendritic, CD8+, CD4+, NK, and B cells (1). A growing body of evidence implicates cross-talk between innate and adaptive immune response in augmenting airway inflammation and emphysema with the progression of COPD. Oxidative stress plays a critical role in driving these pathological processes. Exposure to cigarette smoke activates innate immune responses by eliciting pathogen recognition receptor signaling (such as Toll-like receptors [TLRs]) and inflammasome signaling (2). Evidence suggests that oxidative tissue injury caused by cigarette smoke may generate damage-associated molecular patterns (DAMPs) such as oxidatively or nitrosatively modified proteins, nitrosylated surfactant protein D (3), or extracellular matrix components (hyaluronic acid and fibronectin), which could elicit TLR4 signaling and activate innate immune responses (2). Activation of innate immune responses induces secretion of proinflammatory mediators (such as interleukin [IL]-1β, tumor necrosis factor [TNF]-α, IL-6, and IL-8), which mediate infiltration of neutrophils, monocytes, and macrophages into the lungs. Neutrophils and macrophages secrete reactive oxygen species (ROS), reactive nitrogen species, proteases (such as metalloproteinases [MMPs], elastase), and other proinflammatory mediators, which promote inflammation, apoptosis, and lung tissue injury. Persistent lung tissue injury, as a result of chronic exposure to cigarette smoke, cause emphysematous destruction and airway remodeling.
Results from recent studies have indicated that adaptive immune response contributes to persistent inflammatory response and emphysematous destruction in COPD. Histopathologic analysis results reveal an increase in bronchus-associated lymphoid tissue collections, an indicator of adaptive immune response, with an increase in the severity of COPD (1). In both airways and lung parenchyma, the number of CD4+ and CD8+ cells is elevated in COPD and correlates directly with decrease in lung function (1). Unequivocally, results from mouse models of chronic exposure to cigarette smoke have shown that pathogenic T cells capable of driving alveolar destruction are induced in the lungs (4). Transfer of CD3+ T cells from the lungs of mice exposed to cigarette smoke for 6 months into normal Rag2−/− mice, which are deficient in T and B cells, induced pulmonary inflammation and emphysema (4). The component that activates adaptive immune responses is still an area of intense investigation, and it is thought that DAMPs released by damaged tissue or apoptotic or necrotic cells may function as antigens and be processed by dendritic cells (DCs) and elicit adaptive immune responses. An increased accumulation of activated myeloid DCs in the lungs is associated with COPD (5). DCs may induce CD8+ and specific effector CD4+ cells (Th1 or Th17) in the lungs of patients with COPD (5). Activated CD8+ cytotoxic T cells release perforin and granzyme and may cause structural parenchymal destruction by inducing apoptosis. CD8 knockout mice showed increased resistance to cigarette smoke–induced emphysema, suggesting a crucial role of CD8+ in COPD (6). CD4+ Th1 cells secrete IFN-γ and augment innate immune responses (7). Transgenic mice have high levels of IFN-γ in their airways, which induces emphysema and supports the role of IFN-γ in COPD (8). Th17 cytokines induce secretion of chemokines and proinflammatory mediators that promote neutrophilic inflammation and thereby tissue destruction. IL-17A induces secretion of chemokine CCL20 that attracts myeloid DCs in the lungs and MMP12 from the macrophages (5). Disruption of MMP12 protects the mice from cigarette smoke–induced emphysema (9). IL-17A knockout mice showed resistance to cigarette smoke–induced emphysema, suggesting a role of Th17 in the pathogenesis of COPD (10).
Acute Exacerbations Drive Copd Progression
Patients with COPD frequently experience episodes of acute exacerbations, primarily caused by bacterial or viral infections, which result in significant morbidity and mortality. The frequency of exacerbations increases with the progression of the disease and is associated with rapid decrease in lung function (11). An estimated 10% of patients with frequent exacerbations account for 70% of total health care use in the United States (12). Current treatment approaches include bronchodilators alone or in combination with inhaled corticosteroids. These treatment options show mild to moderate therapeutic benefits in reducing frequency of exacerbations but do not substantially modify decrease in lung function (13). Treatment with systemic corticosteroids in hospitalized patients with COPD exacerbation produced a mild improvement in the duration of hospital stay. Compared with placebo, systemic corticosteroids reduced the hospital stay by an average of 1.2 days (14).
Bacterial infections are a frequent cause of approximately 50% of acute exacerbations. Most common bacteria associated with COPD exacerbations are nontypeable Haemophilus influenzae, Pseudomonas aeruginosa, Moraxella catarrhalis, Streptococcus pneumoniae, and Staphylococcus aureus (11). Bronchoscopic samplings have indicated bacterial colonization in patients with stable COPD; however, acquisition of new bacterial strains such as of nontypeable H influenzae and P aeruginosa are associated with more frequent exacerbation (11, 15). Despite exaggerated airway inflammation (10- to 20-fold more macrophages), bacterial colonization and frequent infection are observed in patients with COPD, suggesting a defect in innate immune defenses. The innate immune system provides the first line of defense against pathogenic insult in the lungs where it consists of an epithelial barrier, mucociliary clearance, antimicrobial peptides, and alveolar macrophages. Alveolar macrophages play a key role in phagocytic clearance of bacteria in the lungs. Oxidative stress induced by chronic exposure to cigarette smoke is linked to disruption of the lungs’ innate immune defenses (2). Alveolar macrophages from patients with COPD show impaired bacterial phagocytosis compared with that in patients who are smokers but do not have COPD (16). Impairment of bacterial phagocytosis by alveolar macrophages may result in chronic bacterial colonization. Results of studies in mouse models indicate that chronic cigarette smoke exposure impairs bacterial phagocytosis by alveolar macrophages and diminishes pulmonary bacterial clearance (17–19). The underlying mechanism for this defect is not well understood.
Around 25 to 50% of exacerbations are associated with respiratory viral infection (20, 21). It is unclear whether patients with COPD are more susceptible to respiratory viral infection, but evidence suggests that patients with frequent exacerbations are more susceptible to viral infection (21). Rhinovirus and influenza are the viruses most commonly associated with frequent COPD exacerbations (21). Rhinovirus infection in volunteers with COPD caused airflow obstruction and induced higher levels of airway inflammation and viral load compared with results in those without COPD (22). For the first time, the results of this study demonstrated the causal role of rhinovirus infection in COPD exacerbation. Impaired antiviral responses as well as higher expression of viral receptors such as intercellular adhesion molecule 1 (ICAM-1), a primary receptor for rhinovirus, as a result of chronic abnormal inflammatory response in the airways may increase the risk of viral exacerbations with the progression of COPD.
Pulmonary inflammation is augmented during bacterial or viral exacerbations and is characterized by heightened oxidative stress and innate and adaptive immune responses. Bronchoalveolar fluid analysis results showed elevated levels of oxidative stress (23), cytokines (IL-8), neutrophils, and CD8+ and CD4+ cells in the airways of patients experiencing exacerbation compared with those in patients with stable COPD (23, 24). Because of the increase in the levels of these pathological drivers of COPD, frequent exacerbations are associated with accelerated decrease in lung function, so prevention or reduction of exacerbation frequency is a major goal in COPD therapy.
NRF2 Host Defense Pathway, Oxidative Stress, and COPD
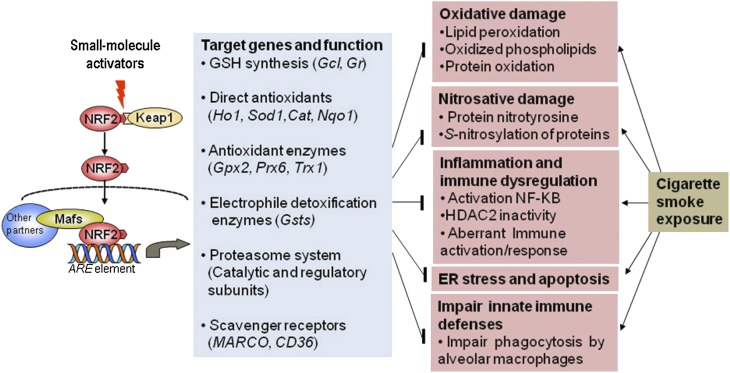
Nrf2 plays a central role in attenuating oxidative stress induced by a wide variety of environmental stressors including cigarette smoke by inducing expression of a broad spectrum of cytoprotective defense mechanisms (25). Under normal conditions, Nrf2 is held in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), ubiquitinated by Cul3-Keap1 E3 ligase complex, and degraded by means of the proteasome pathway (25). In response to oxidative stress, reactive cysteines in Keap1 are modified, presumably resulting in a conformation change in Keap1 structure, leading to a decrease in E3 ligase activity, stabilization and translocation of Nrf2 to the nucleus, and robust induction of cytoprotective genes. Exposure to cigarette smoke increases the expression of Nrf2-regulated cytoprotective genes, which include GSH biosynthesizing enzymes, antioxidant enzymes, NADPH-regenerating enzymes, and xenobiotic detoxification enzymes; induces proteasome system in the lungs (Figure 1); and attenuates oxidative damage (26). Conversely, disruption of Nrf2 impairs induction of this cytoprotective defense program and exaggerates oxidative stress, nitrosative stress, inflammation, endoplasmic reticulum (ER) stress, apoptosis, and emphysema in mice after exposure to cigarette smoke (26, 27). Results from recent studies indicate that Nrf2 may protect against dysregulation of inflammatory-immune responses induced by pathogenic insult. Bacterial (28) or viral (influenza [29] or RSV [30]) infection induced greater pulmonary inflammation and lung tissue injury in Nrf2-disrupted mice than in wild-type mice. Lipopolysaccharide (LPS), a TLR4 ligand, induced a hyperinflammatory response in Nrf2-deficient macrophages or neutrophils when compared with the response in wild-type cells (31). The Nrf2 signaling pathway suppressed ROS-mediated TLR4 activation and attenuated NF-κB and interferon response factor 3 proinflammation signaling in macrophages after LPS stimulation (32). Conversely, constitutive activation of Nrf2 by means of tissue-specific disruption of Keap1 diminished oxidative stress, apoptosis, and inflammation in the lungs of mice after cigarette smoke exposure (33). TLR4 signaling and NF-κB activity was suppressed in Keap1-disrupted macrophages (32).
Figure 1.
Targeting host defense by up-regulating Nrf2 may inhibit pathological drivers of COPD. Activation of Nrf2 by small molecules increases genes that encode direct antioxidants (heme oxygenase 1 [Ho-1], superoxide dismutase1 [SOD1], catalase [cat], NADPH quinone reductase 1 [Nqo1]), GSH biosynthesizing enzymes (glutamate-cysteine ligase), electrophile detoxification enzymes (glutathione S-transferase, [Gsts]), catalytic and regulatory members of the proteasome system, and scavenger receptors (MARCO and CD36). This multifaceted cytoprotective response suppresses oxidative stress, endoplasmic reticulum (ER) stress, inflammation, immune dysregulation, and disruption of innate immune defenses induced by chronic cigarette smoke exposure.
The Nrf2-directed antioxidant response decreases in peripheral lung tissue and alveolar macrophages in patients with COPD compared with that in patients without COPD. A decrease in Nrf2 activity with progression of COPD was linked to a decrease in Nrf2 protein and is related to changes in the activity of its positive regulator, DJ-1 and negative regulators, Keap1 and BACH1 (34–36). Nrf2 activity showed an inverse correlation with markers of oxidative damage in COPD (35). Diminished Nrf2-regulated antioxidant defenses in alveolar macrophages and airway epithelium in patients with COPD may contribute to the amplification of inflammatory response during acute exacerbations.
Improving lung antioxidant defenses might be a promising approach to protect against oxidative stress–driven pathophysiology in patients with COPD. Results from clinical trials in which the investigators used pharmacological antioxidants such as N-acetyl cysteine and vitamin E showed a modest or no effect on FEV1 decrease (37, 38). This reduced performance of pharmacological antioxidants may be attributed to poor bioavailability or insufficient protection at the lung tissue level. On the contrary, activation of host defenses by Nrf2 may be a promising approach to induce a broad spectrum of cytoprotective defenses in the lungs.
Targeting NRF2: Experimental Therapeutics for Preventing Acute Exacerbations
Interventions aiming to curb the infectious cause of COPD involve two main approaches: the use of antibiotics to prevent and treat infectious exacerbations of COPD, and the use of corticosteroids to control the overall inflammatory response that is a hallmark of COPD pathogenesis. The long-term use of the antibiotic azithromycin has some benefit in preventing acute exacerbations of COPD; however, it was associated with an increase in the incidence of colonization with antibiotic-resistant bacteria (39). Corticosteroids are the most commonly prescribed medications for COPD. Steroids can be successful in reducing inflammation, but steroid resistance is a common phenomenon in patients with COPD (40). The prolonged use of corticosteroids has also been associated with an increased risk of pneumonia. Results of a meta-analysis indicated a 34% increased risk of developing bacterial pneumonia in patients with COPD treated with inhaled corticosteroid therapy (41). Commonly used therapeutic interventions have resulted in either impaired immune function or increased susceptibility to antibiotic-resistant bacteria. It may be beneficial to develop effective therapeutic approaches to prevent COPD exacerbations through enhancement of pulmonary innate immune defenses in patients with COPD and through immunomodulation to prevent hyperinflammatory responses.
Oxidative stress plays a central role in disrupting innate immune defenses and amplifying inflammation in COPD (2). Recently published data from our laboratory provide a strong rationale for targeting Nrf2 as a novel therapeutic approach to prevent bacterial exacerbations and hyperinflammation in COPD. To test the hypothesis that enhanced oxidative stress associated with Nrf2 deficiency impairs the clearance of respiratory infections, we identified a previously unknown role of Nrf2 in regulating expression of the scavenger receptor macrophage receptor with collagenous structure (MARCO). MARCO aids in the binding and uptake of bacteria, oxidized low-density lipoproteins, and environmental particles (42, 43). MARCO is highly expressed in tissue (liver or lung) macrophages in response to bacterial infection, and genetic ablation of MARCO enhances bacterial colonization, inflammation, and diminished survival in a model of pneumococcal pneumonia (43). Activation of Nrf2 by small-molecule sulforaphane enhances the bacterial phagocytic ability of alveolar macrophages in both patients with COPD and mice exposed to cigarette smoke (28). An increase in the surface expression of MARCO correlated with improved bacterial clearance in mice exposed to cigarette smoke. Promoter analysis results demonstrated that Nrf2 regulates transcriptional expression of MARCO in macrophages.
LPS stimulation induces greater expression of IL-8 in macrophages from patients with COPD than from patients without COPD (44). The synthetic glucocorticoid dexamethasone failed to suppress LPS-induced IL-8 expression in COPD macrophages (44). A reduction in HDAC2 activity in COPD is linked to amplification of inflammation and corticosteroid resistance (44). HDAC2 inactivity results in epigenetic changes that augment LPS-induced expression of inflammatory cytokines (IL-8 and IL-6) in COPD macrophages and in mice exposed to cigarette smoke (28). HDAC2 inactivity impairs glucocorticoid receptor–mediated transrepression of NF-κB activity and causes corticosteroid resistance (28). Oxidative and nitrosative stress–induced post-translational modifications such as phosphorylation of serine/threonine residues (45) and S-nitrosylation or nitration of tyrosine residues in HDAC2 protein induce HDAC2 inactivity (46, 47). S-nitrosylation of HDAC2 is a predominant mechanism involved in HDAC2 inactivity in COPD macrophages (47). Impaired induction of Nrf2-regulated antioxidants might contribute to HDAC2 inactivity (46). An increase in GSH by activation of Nrf2 with small-molecule sulforaphane restored HDAC2 activity, largely by means of denitrosylation, and improved dexamethasone-induced repression of inflammatory cytokines (46).
Oxidative stress is thought to modify antiviral responses and virus-mediated inflammatory responses and tissue injury. Nrf2-null mice show increased sensitivity to RSV (30)- or influenza (29)-mediated airway inflammation and morbidity, which is further worsened by cigarette smoke exposure. Recent evidence suggests that RSV infection could dampen Nrf2 signaling (48). Conversely, enhancing Nrf2 signaling by means of sulforaphane reduced virus replication and inflammation in lungs of mice infected with RSV (30). Sulforaphane treatment also increased antiviral responses and reduced influenza virus replication in nasal epithelial cells derived from healthy human volunteers (49). Therefore, therapies that increase Nrf2 signaling could be beneficial in reducing viral exacerbation in patients with COPD.
Conclusions
Our current understanding of COPD indicates that a complex interaction of oxidative stress and immunologic abnormalities are associated with the progression of COPD. Enhancing host defense by targeting the Nrf2 pathway holds a great deal of promise in providing a multifaceted approach to improving immune response and protection against oxidative stress in patients with COPD (Figure 1). Sulforaphane, a phytochemical present in broccoli and cruciferous vegetables, increased Nrf2-regulated antioxidants in COPD alveolar macrophages and attenuated oxidative stress, nitrosative stress, and inflammation. Future clinical trials will determine its effectiveness in preventing exacerbations and mortality in patients with COPD.
Supplementary Material
Footnotes
Supported by National Institutes of Health grant HL081205 (S.B); National Heart, Lung, and Blood Institute Specialized Centers of Clinically Oriented Research grant P50HL084945 (S.B); National Institute of Environmental Health Sciences Disease Investigation Through Specialized Clinically-Oriented Ventures in Environmental Research grant P50ES015903 (S.B); the Flight Attendant Medical Research Institute (S.B and R.K.T); and National Institute of Environmental Health Sciences grants P50ES015903 and ES03819.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 2.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011;378:1015–1026. [DOI] [PubMed] [Google Scholar]
- 3.Guo CJ, Atochina-Vasserman EN, Abramova E, Foley JP, Zaman A, Crouch E, Beers MF, Savani RC, Gow AJ. S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol 2008;6:e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motz GT, Eppert BL, Wesselkamper SC, Flury JL, Borchers MT. Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2−/− mice. Am J Respir Crit Care Med 2010;181:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med 2009;1:4ra10. [DOI] [PubMed] [Google Scholar]
- 6.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 2007;178:8090–8096. [DOI] [PubMed] [Google Scholar]
- 7.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med 2004;1:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, Shapiro SD, Elias JA. Interferon gamma induction of pulmonary emphysema in the adult murine lung. J Exp Med 2000;192:1587–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS ONE 2011;6:e20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–471. [DOI] [PubMed] [Google Scholar]
- 12.Pandey MK, Kumar S, Thimmulappa RK, Parmar VS, Biswal S, Watterson AC. Design, synthesis and evaluation of novel PEGylated curcumin analogs as potent Nrf2 activators in human bronchial epithelial cells. Eur J Pharm Sci 2011;43:16–24. [DOI] [PubMed] [Google Scholar]
- 13.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361:449–456. [DOI] [PubMed] [Google Scholar]
- 14.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, Anderson P, Morgan NA; Department of Veterans Affairs Cooperative Study Group. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1999;340:1941–1947. [DOI] [PubMed] [Google Scholar]
- 15.Chin CL, Manzel LJ, Lehman EE, Humlicek AL, Shi L, Starner TD, Denning GM, Murphy TF, Sethi S, Look DC. Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers. Am J Respir Crit Care Med 2005;172:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenson CS, Garlipp MA, Grove LJ, Maloney J, Sethi S. Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease. J Infect Dis 2006;194:1375–1384. [DOI] [PubMed] [Google Scholar]
- 17.Phipps JC, Aronoff DM, Curtis JL, Goel D, O'Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect Immun 2010;78:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marti LP, Regueiro V, Morey P, Hood DW, Saus C, Sauleda J, Agusti AG, Bengoechea JA, Garmendia J. Nontypeable Haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect Immun 2009;77:4232–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stampfli MR. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2004;170:1164–1171. [DOI] [PubMed] [Google Scholar]
- 20.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2004;1:115–120. [DOI] [PubMed] [Google Scholar]
- 22.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 2011;183:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005;60:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355–2365. [DOI] [PubMed] [Google Scholar]
- 25.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116. [DOI] [PubMed] [Google Scholar]
- 26.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 2004;114:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, et al. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med 2009;180:1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med 2011;3:78ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yageta Y, Ishii Y, Morishima Y, Masuko H, Ano S, Yamadori T, Itoh K, Takeuchi K, Yamamoto M, Hizawa N. Role of Nrf2 in host defense against influenza virus in cigarette smoke-exposed mice. J Virol 2011;85:4679–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med 2009;179:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 2006;351:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, Reddy SP, Remick D, Biswal S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med 2011;184:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blake DJ, Singh A, Kombairaju P, Malhotra D, Mariani TJ, Tuder RM, Gabrielson E, Biswal S. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. Am J Respir Cell Mol Biol 2010;42:524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goven D, Boutten A, Lecon-Malas V, Marchal , Somme J, Amara N, Crestani B, Fournier M, Leseche G, et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 2008;63:916–924. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 2008;178:592–604. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2008;39:673–682. [DOI] [PubMed] [Google Scholar]
- 37.Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, van Schayck CP, Olivieri D, Del Donno M, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005;365:1552–1560. [DOI] [PubMed] [Google Scholar]
- 38.Rautalahti M, Virtamo J, Haukka J, Heinonen OP, Sundvall J, Albanes D, Huttunen JK. The effect of alpha-tocopherol and beta-carotene supplementation on COPD symptoms. Am J Respir Crit Care Med 1997;156:1447–1452. [DOI] [PubMed] [Google Scholar]
- 39.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet 2009;373:1905–1917. [DOI] [PubMed] [Google Scholar]
- 41.Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanno S, Furuyama A, Hirano S. A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol Sci 2007;97:398–406. [DOI] [PubMed] [Google Scholar]
- 43.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 2004;200:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 2006;203:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol 2009;40:464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malhotra D, Thimmulappa RK, Mercado N, Ito K, Kombairaju P, Kumar S, Ma J, Feller-Kopman D, Wise R, et al. Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients. J Clin Invest 2011;121:4289–4302. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Osoata GO, Yamamura S, Ito M, Vuppusetty C, Adcock IM, Barnes PJ, Ito K. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem Biophys Res Commun 2009;384:366–371. [DOI] [PubMed] [Google Scholar]
- 48.Hosakote YM, Jantzi PD, Esham DL, Spratt H, Kurosky A, Casola A, Garofalo RP. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 2011;183:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic Biol Med 2011;51:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.