SUMMARY
Malaria in Africa is vectored primarily by the Anopheles gambiae complex. Although the mechanisms of population persistence during the dry season are not yet known, targeting dry season mosquitoes could provide opportunities for vector control. In the Sahel, it appears likely that M-form A. gambiae survive by aestivation (entering a dormant state). To assess the role of eco-physiological changes associated with dry season survival, we measured body size, flight activity and metabolic rate of wild-caught mosquitoes throughout 1 year in a Sahelian locality, far from permanent water sources, and at a riparian location adjacent to the Niger River. We found significant seasonal variation in body size at both the Sahelian and riparian sites, although the magnitude of the variation was greater in the Sahel. For flight activity, significant seasonality was only observed in the Sahel, with increased flight activity in the wet season when compared with that just prior to and throughout the dry season. Whole-organism metabolic rate was affected by numerous biotic and abiotic factors, and a significant seasonal component was found at both locations. However, assay temperature accounted completely for seasonality at the riparian location, while significant seasonal variation remained after accounting for all measured variables in the Sahel. Interestingly, we did not find that mean metabolic rate was lowest during the dry season at either location, contrary to our expectation that mosquitoes would conserve energy and increase longevity by reducing metabolism during this time. These results indicate that mosquitoes may use mechanisms besides reduced metabolic rate to enable survival during the Sahelian dry season.
KEY WORDS: aestivation, gonotrophic cycle, malaria vector, metabolism, respiration, seasonality, temperature
INTRODUCTION
The African malaria mosquito Anopheles gambiae Giles is responsible for the majority of malaria transmission in Africa (Holstein, 1954; Lyimo and Koella, 1992; Collins et al., 2001), with yearly estimates of hundreds of millions of infections and over 780,000 deaths (WHO, 2010). Anopheles gambiae s.l. is a species complex that is widely distributed throughout sub-Saharan Africa, with high environmental variation across its range. This environmental variation can theoretically lead to adaptation of populations to their local conditions, including alteration of life histories, phenotypic plasticity, host preference and other ecological traits (Stearns, 1992; Hoffmann et al., 1995; Schlichting and Pigliucci, 1998; Chown and Gaston, 1999; Roff, 2002). Some populations of these mosquito species are located in areas with distinct wet and dry seasons, and the mechanisms of population persistence over the long, hot, dry season are still largely unknown (Holstein, 1954; Omer and Cloudsley-Thompson, 1968; Omer and Cloudsley-Thompson, 1970; Lehmann et al., 2010; Adamou et al., 2011). Knowledge of these mechanisms may generate new vector-control strategies that target mosquito populations when they are most vulnerable.
The two competing hypotheses of how local population persistence occurs are (1) local aestivation of adults during the dry season or (2) migration of adults from areas with permanent water soon after the first rains (Charlwood et al., 2000; Lehmann et al., 2010; Adamou et al., 2011). Under aestivation, adults that emerge at the end of one wet season cease reproduction, enter protective shelters, and remain in a quiescent state until the next season's rains, possibly with occasional feeding bouts to replenish resources (Yaro et al., 2012). Alternatively, under migration, mosquito populations persist year-round in areas with permanent water and ongoing reproduction, and migrate to seasonal locations once the rainy season begins each year. Recently, there has been an accumulation of evidence suggesting that, in the Sahel, aestivation is the likely strategy of the M molecular form of A. gambiae, while S-form A. gambiae and Anopheles arabiensis Patton most likely re-establish their populations by migration after the first rains (Lehmann et al., 2010; Adamou et al., 2011). However, the relative contribution of aestivation vs migration within populations is not known, and neither the underlying physiological mechanisms nor the phenotypic markers of aestivating mosquitoes have been identified.
Aestivation (summer diapause) is a state of hormonally induced dormancy that enables insects to survive harsh environmental conditions during the hot or dry season, typically via increased longevity and reduced reproduction. Unlike winter diapause, no studies have dissected the eco-physiological mechanisms underlying aestivation in mosquitoes and few have done so in other insects (Denlinger, 1986; Kostal et al., 1998; Held and Spieth, 1999; Blanckenhorn et al., 2001; Benoit, 2010). It is believed, however, that similar physiological mechanisms are involved (Masaki, 1980; Denlinger, 1986; Benoit, 2010). Accordingly, the expected physiological changes that underlie aestivation include reduced reproduction, metabolic rate, feeding response and activity level, increased desiccation and temperature resistance, and increased nutritional reserves prior to the start of aestivation, as these characterize insect diapause (Masaki, 1980; Danks, 1987; Chown and Gaston, 1999; Blanckenhorn et al., 2001; Denlinger, 2002; Gray and Bradley, 2005; Huestis and Marshall, 2006; Kessler and Guerin, 2008; Ragland et al., 2009). Many insects also exhibit morphological variation in body size and/or coloration associated with diapause (e.g. Mousseau and Roff, 1989; Musolin and Numata, 2003; Teder et al., 2010; Yamamoto et al., 2011).
Accordingly, it is expected that aestivating mosquitoes would reduce their metabolism, as lowering metabolic rate allows for decreased water loss via a lower respiration rate, conservation of limited nutritional resources and potentially increased longevity (Ishihara and Shimada, 1995; Sohal and Weindruch, 1996; Chown and Gaston, 1999; Denlinger, 2002; Gray and Bradley, 2005; Lighton, 2008). This prediction is based only on winter diapause studies (e.g. Danks, 1987; Chown and Gaston, 1999; Guppy and Withers, 1999; Denlinger, 2002; Benoit and Denlinger, 2007; Ragland et al., 2009; Ragland et al., 2010) rather than on summer diapause, as no measurements of metabolic rate during insect aestivation are known to us. In contrast to summer aestivation, metabolism during winter diapause is reduced not only by the low ambient temperatures that insects experience but also by the insect's physiology (Danks, 1987; Layne and Eyck, 1996; Guppy and Withers, 1999; Denlinger, 2002; Canzano et al., 2006; Benoit and Denlinger, 2007; Ragland et al., 2009).
Here, we evaluated seasonal variation in metabolic rate, activity level and body size of M-form A. gambiae, the only member of the complex that can be found throughout the dry season in the Sahel. Specifically, we tested the prediction that both flight activity and metabolic rate are depressed during the dry season. Further, we tested that these predictions would hold in a Sahelian population but not in a riparian one. Seasonal variation in these traits was measured from October 2009 to August 2010, facilitating comparisons among the periods just prior to the dry season (October to December 2009), throughout the dry season (January to May 2010), and in the following wet season (June to August 2010). This study extends our previous work (Huestis et al., 2011), which compared A. gambiae M-form with A. arabiensis during the late wet season (October to November) and found no significant difference in metabolic rate between the species after accounting for body size.
MATERIALS AND METHODS
Study sites
Our focal village was M'Piabougou, Mali (13.60°N, 7.19°W), located in the southern Sahel. The climate is characterized by a 7 month dry season from November to May with rains typically falling from June to October, as previously described (Huestis et al., 2011). M'Piabougou consists of approximately 400 houses and 1500 inhabitants, and is located over 30 km from the nearest permanent surface water source. Our secondary site was N'Gabakoro Droit (12.68°N, 7.84°W), a village located on the border of the Sahel within 100 m of the Niger River and approximately 150 km SSW of M'Piabougou. N'Gabakoro consists of approximately 800 houses and 3500 inhabitants. The climate of N'Gabakoro is similar to that of the southern Sahel, although annual precipitation is higher and the first rains fall an average of 4 weeks earlier (Yaro et al., 2012).
Data collection
Indoor-resting mosquitoes were collected each morning by gentle aspiration, housed in paper cups, and provided with water-soaked cotton pads for a minimum of 30 min prior to metabolic assays. The sex of individuals and the gonotrophic state of females were determined and single mosquitoes were then subjected to a metabolism assay as described previously (Huestis et al., 2011). Briefly, metabolic chambers consisted of 5 ml syringes attached to a 3-way stopcock. A microphone was attached at the third stopcock position. Once individuals were aspirated into the chambers, dry, CO2-free air was flowed into the chambers for a minimum of 2 min before the assay was started. After approximately 2 h, a 3 ml sample of air was injected into a FoxBox-C portable gas analyser (Sable Systems International, Las Vegas, NV, USA). To control for circadian rhythms, metabolism assays were conducted between 10:00 h and 14:00 h year-round. Mosquitoes were preserved in 80% ethanol or desiccated for further analyses (see below). Temperature was recorded at 15 min intervals using a HOBO data logger (Onset Computer Corporation, Bourne, MA, USA), and the mean of the recordings taken over the assay duration was subsequently used as a measurement of assay temperature (see Results).
The volume of CO2 produced by individual mosquitoes during the assay was calculated using ExpeData software (Sable Systems International), and analysed as previously described (Huestis et al., 2011). The resulting total CO2 production was divided by assay duration, yielding a measurement of mean volume of CO2 produced per minute (μl CO2 min–1) for each mosquito. Thus, the term ‘metabolic rate’ refers to this measurement (mean μl CO2 min–1) per mosquito throughout this paper.
Flight during metabolic measurements has a large effect on metabolic rate (Reinhold, 1999; Randall et al., 2002; Gray and Bradley, 2003; Lighton, 2008; Huestis et al., 2011), so flight was assessed by sound recordings made during each assay using microphones and portable voice recorders as previously described (Huestis et al., 2011). Briefly, bouts of flight were identified from the spectrogram of the sound recorded during each assay using Audacity 3.1 (Mazzoni, 2009). The total time each mosquito flew was determined, and the proportion of time spent flying was calculated by dividing the total flight time by the total time the mosquito was subjected to the metabolism assay. This index of flight activity is referred to as ‘flight activity’ throughout, and, because it was measured at the same time as the metabolic assays, also controls for circadian rhythms.
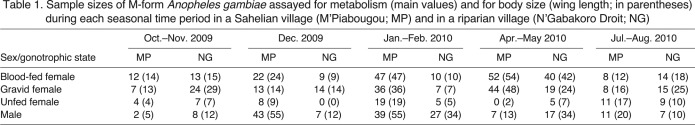
In our study area, the A. gambiae s.l. complex consists of both the M and S molecular forms of A. gambiae s.s. and A. arabiensis; their relative abundances fluctuate seasonally (Coluzzi et al., 1979; Coluzzi et al., 1985; Coluzzi et al., 2002; Touré et al., 1994; Touré et al., 1998; Lehmann et al., 2010; Adamou et al., 2011), with M-form A. gambiae being the only taxon found during the dry season (January–May). Mosquitoes were assigned to species and molecular form via standard PCR and restriction enzyme assays conducted on two legs (Fanello et al., 2002). As explained above, the present analysis includes only those identified as M-form A. gambiae. Wings were mounted and wing length was used as a measure of body size as previously described (Huestis et al., 2011). For intact mosquitoes preserved in desiccant, dry body mass was measured to 0.001 mg using a Cahn C-31 electrobalance (Cahn Instruments, Cerritos, CA, USA) after first removing all legs and wings from the mosquitoes.
Statistical analyses
To increase sample size within groups and clarify seasonal effects, the data were grouped into 2 month intervals based on weather similarity: October–November 2009, January–February 2010, April–May 2010, and July–August 2010 (see Table 1). These groupings represent the late wet season after rains stopped but larval sites were still available (October–November), the early/cool dry season when no larval sites were available (January–February), the late/hot dry season (April–May), and the mid wet season after the rain commenced (July–August), and were used throughout the analyses to follow. December represents a transitional period, when all standing water has thoroughly dried but remaining adult anophelines could still persist without an extreme extension of their lifespan (>2 months), and therefore was not grouped with any other time point.
Table 1.
Sample sizes of M-form Anopheles gambiae assayed for metabolism (main values) and for body size (wing length; in parentheses) during each seasonal time period in a Sahelian village (M'Piabougou; MP) and in a riparian village (N'Gabakoro Droit; NG)
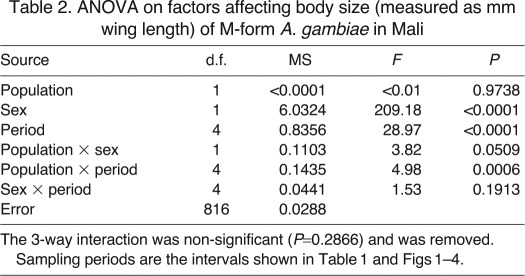
Analysis of variance (ANOVA) models were used to analyse variation in wing length, with population, sex, sampling period and their 2-way interactions as random factors; the 3-way interaction was non-significant and subsequently removed from the final analysis. Further removal of non-significant interactions did not affect the conclusions and they were therefore retained in the final model. Post hoc comparisons between means were made using REGWQ (Ryan–Einot–Gabriel–Welsch Q multiple comparison) tests in SAS 9.2 (SAS Institute, Cary, NC, USA). Least-squares means for dry body mass, flight activity and metabolic rate were calculated with analysis of covariance (ANCOVA) models using PROC GLM within SAS 9.2 (SAS Institute). This procedure accommodates comparisons of groups that differ in the mean value of other variables and/or differ in sample size. To determine the unique effect of season, we used sequential multivariate ANCOVAs, adding in the effects of seasonal temperature and other season-dependent variables such as flight activity and body size (wing length) one at a time and determining the remaining effect of season. Wing length was used as the primary indicator of body size instead of dry mass, because mass varies greatly with blood digestion or time since the last sugar meal (Lanciani, 1992; Aboagye-Antwi and Tripét, 2010; Russell et al., 2011), wing length was found to be at least as good a predictor of metabolic rate as dry body mass in our previous study (Huestis et al., 2011), and wing length measurements were available for larger sample sizes.
RESULTS
Although 1616 individuals were collected and assayed over the study duration, only 832 were M-form A. gambiae (as many late wet season individuals were A. arabiensis and many mid wet season individuals were S-form A. gambiae). Sample sizes of the final pool of M-form mosquitoes used for the rest of the analyses are shown in Table 1.
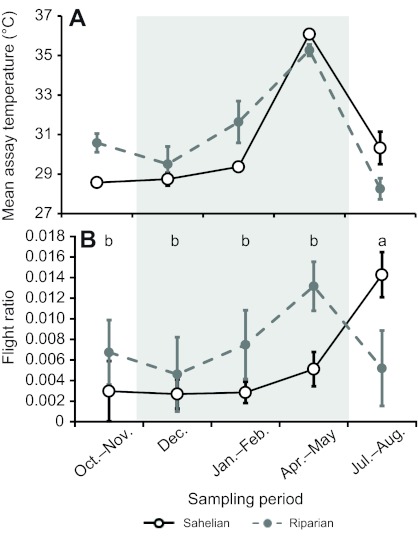
Ambient temperature is an important determinant of flight activity and metabolism (e.g. Huestis et al., 2011) and was therefore measured during the metabolism assays and incorporated into subsequent analyses. As expected for the region, a seasonal change in ambient temperature (during the assay) was detected, with highest values for both locations during the late dry season (April–May), and the lowest temperatures during the mid wet season (July–August; Fig. 1A).
Fig. 1.
Seasonal variation in temperature (A) and Anopheles gambiae flight activity (B) in our Sahelian (open circles) and riparian (filled circles) villages. Temperature is given as mean temperature during the metabolic rate assays (not daily temperature). Flight ratio was measured as percentage of time spent in flight during the metabolic rate assays, and points shown are least-squares (LS) means (accounting for variation between sexes/gonotrophic states). Error bars are ±1 s.e.m. Letters above time points in B show significance groupings (see Materials and methods) for the Sahelian location; no significant seasonal differences were found in the riparian location. The grey shaded area indicates the sampling periods during the dry season.
Seasonal variation in body size
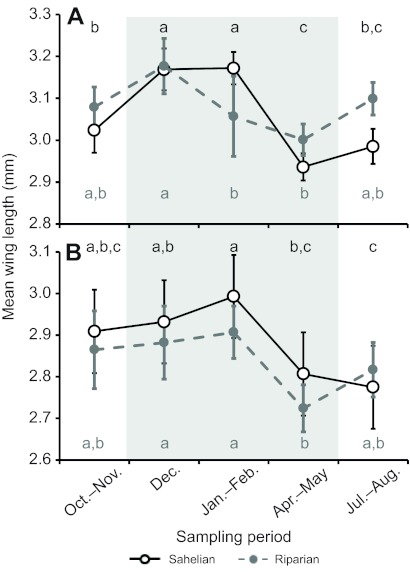
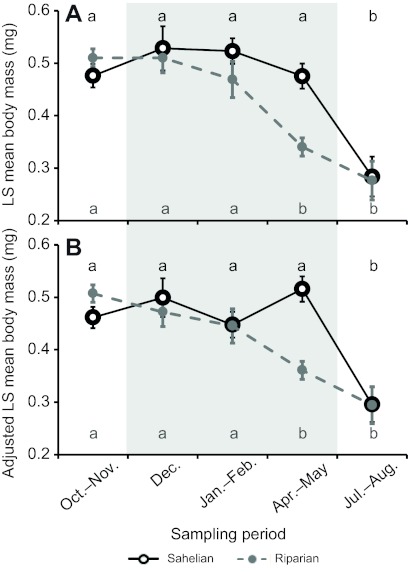
Both the Sahelian and riparian populations showed significant seasonal (sampling period) variation in body size (P<0.0001; Table 2), although the magnitude of change was greater in the Sahel for both sexes (Fig. 2). The two populations were not significantly different from each other in size (P>0.97), but there was a highly significant population × period interaction (P<0.001; Table 2), indicating that the populations respond differently to seasonal variation. In contrast, while females were larger than males overall (P<0.0001, Table 2), the sex × period interaction was not significant (P>0.19; Table 2), indicating that the sexes respond similarly to the seasonal changes. The sex × population interaction was marginally significant (P<0.051; Table 2), and may indicate that the sexes behave differently between the populations, reflecting the fact that Sahelian males are generally slightly larger than riparian males, while no clear size difference was apparent for females (Fig. 2). Lastly, the 3-way interaction was non-significant and was removed prior to the final analysis.
Table 2.
ANOVA on factors affecting body size (measured as mm wing length) of M-form A. gambiae in Mali
Fig. 2.
Seasonality of mean body size (measured as wing length) of M-form A. gambiae (A, females; B, males; note the difference in scale due to sexual dimorphism) collected from a village in the Sahel (M'Piabougou, open circles) and near the Niger River (N'Gabakoro Droit, filled circles). Error bars are ±1 s.e.m. Letters above (Sahel) and below (riparian) time points show significance groupings by REGWQ tests (see Materials and methods) within each location–sex combination. The grey shaded area indicates the sampling periods during the dry season.
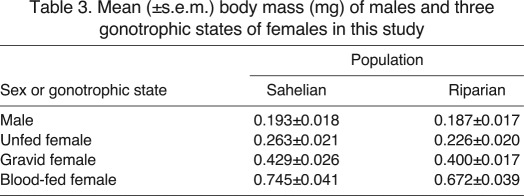
Because female mosquito body mass varies considerably with gonotrophic state, dry mass was also measured for intact specimens. The dry mass of fed females was on average 2.9 times that of unfed females, and gravid females were on average 1.7 times heavier than unfed females, providing estimates of mean dry blood-meal size and egg batch, respectively (Table 3; supplementary material Table S1). Significant seasonal variation in dry mass was found in both populations (P<0.0001). In the Sahel, mean body mass was stable and high from October to May (Fig. 3A). Interestingly, the mean dry mass remained high in April to May, when we observed a pronounced drop in wing length (Fig. 2), indicating that these mosquitoes have higher nutritional reserves relative to their body size. Indeed, once variation in wing length was accommodated, April–May individuals had the highest relative body mass (Fig. 3B). The mean dry mass was significantly lower for July–August individuals than in the rest of the year (Fig. 3), corresponding to the relatively small individuals found during this time (Fig. 2). In the riparian population, body mass was also higher in October–February than in the late dry season and wet season months (April–August; Fig. 3A). This pattern remained when wing length was included in the model (Fig. 3B).
Table 3.
Mean (±s.e.m.) body mass (mg) of males and three gonotrophic states of females in this study
Fig. 3.
Seasonal variation in mean body mass (A) and mean body mass adjusted to mosquito wing length (B) in our Sahelian (open circles) and riparian (filled circles) villages. Values shown are least-squares (LS) means (accounting for variation between the sexes and female gonotrophic states, and unequal sample sizes). Error bars are ±1 s.e.m. Letters above (Sahel) and below (riparian) time points show significance groupings (see Materials and methods) within each location. The grey shaded area indicates the sampling periods during the dry season. Raw values across all sex/gonotrophic state groups and time points can be found in supplementary material Table S1.
Seasonal variation in flight activity
Flight activity showed significant seasonal variation (Fig. 1B), but only for the Sahelian population. Importantly, flight activity was quite low for Sahelian individuals during all time points except the mid wet season (Fig. 1B), indicating a low activity level for individuals just prior to and throughout the dry season. Interestingly, this 5-fold increase in activity level occurred when temperature was lowest, during the middle of the wet season (July–August) when these mosquitoes are predicted to capitalize on the limited reproductive opportunities. Seasonal variation in flight activity was not significant for the riparian population (Fig. 1B), and mean flight activity appears to generally parallel the mean assay temperature (Fig. 1A); thus, activity levels may reflect the temperature of the surroundings. However, the effect of temperature on flight activity was not significant in either the Sahelian (P>0.25) or riparian (P>0.8) location (not shown).
Seasonal variation in metabolic rate
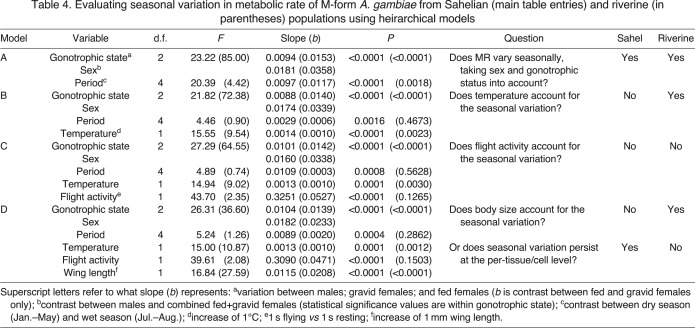
To examine the effects of season in each location and accommodate the other factors affecting metabolic rate, we used least-squares means derived from a series of ANCOVA models in which we sequentially added the effects of sampling period, temperature, flight activity and wing length (see Table 4). Raw means are provided in supplementary material Table S2. The overall effects of sex and gonotrophic state in both locations were similar to those in our previous study (Huestis et al., 2011), with the mean metabolic rate of fed females being higher than that of gravid females, and the mean metabolic rate of both fed and gravid females being higher than that of males (Table 4, model A). There was a significant effect of season (sampling period) in both locations (Table 4, model A), although the magnitude of this effect was greater in the Sahel than near the river (Fig. 4A). In the Sahel, mean metabolic rate was lowest in December (the transitional period between the wet season and the dry season) and highest in the late dry season (April–May; Fig. 4A).
Table 4.
Evaluating seasonal variation in metabolic rate of M-form A. gambiae from Sahelian (main table entries) and riverine (in parentheses) populations using heirarchical models
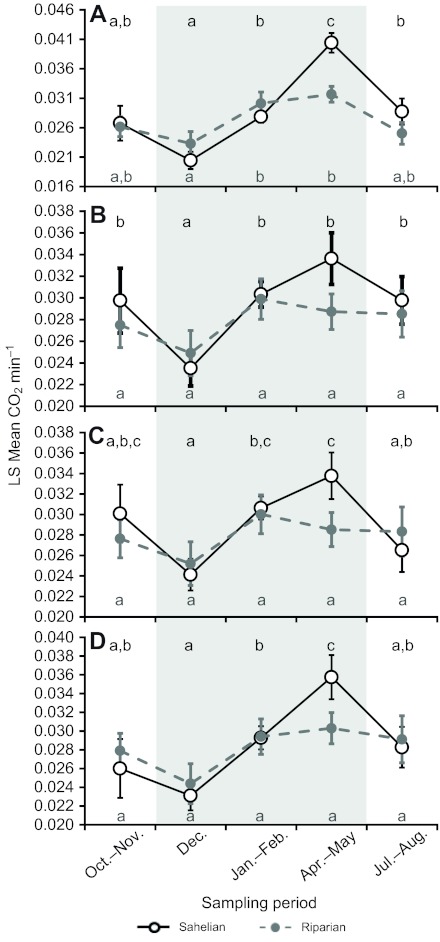
Fig. 4.
Factors affecting seasonality of metabolic rate. Factors sequentially accounted for were sex/gonotrophic state (A), temperature (B), flight ratio (C) and body size (measured as wing length; D). Values shown are least-squares (LS) means (accounting for variation in metabolic rate between sexes/gonotrophic states and unequal sample sizes). Error bars are ±1 s.e.m. Letters above (Sahel) and below (riparian) time points show significance groupings (see Materials and methods) within each location. For further statistical details, see Table 4. The grey shaded area indicates the sampling periods during the dry season. Raw values across all sex/gonotrophic state groups and time points can be found in supplementary material Table S2.
To separate seasonality from the effect of temperature on metabolic rate, mean assay temperature (Fig. 1A) was next incorporated into the models, and was highly significant in both locations (Table 4, model B). Notably, the addition of temperature into the model rendered the effect of season non-significant for the riparian population (Table 4, model B; Fig. 4B); in contrast, temperature did not change the significance of seasonality for the Sahelian population (Table 4, model B). These results suggest that ambient temperature is primarily responsible for the effect of season on metabolic rate in the riparian population but that temperature is not the only factor contributing to the seasonal differences seen in the Sahel.
To separate the effects of season and flight activity on metabolic rate, flight activity (Fig. 1B) was the next variable added into the model. In the Sahelian population, flight activity had a significant effect on metabolic rate (Table 4, model C), but was only supplemental to the other factors, as season was still highly significant (Fig. 4C). In contrast, flight activity was non-significant for the riparian population (probably reflecting the lack of significant seasonal variation in flight activity; see above), and its addition to the model had a seemingly negligible effect (Table 4, model C). Adding flight activity enables us to examine resting metabolism rather than total (resting and active) metabolism. Accordingly, unlike the riparian population, the resting metabolic rate in the Sahelian M-form showed marked seasonality over and beyond the seasonal change in temperature (Fig. 4C).
Finally, we added body size (wing length) (Huestis et al., 2011), which also varied seasonally (Fig. 2), to the previous ANCOVA models. For both the Sahelian and riparian populations, wing length had a significant effect on metabolic rate (Table 4, model D); however, the addition of wing length to the model did not change the previous results. Adding wing length enables us to examine changes in metabolic rate at the tissue level rather than the whole-individual level. Season was not significant in the riparian population (Table 4, model D; Fig. 4D). In contrast, in the Sahelian location, season was still highly significant even after the addition of temperature, flight activity and wing length into the model (Table 4, model D), and there were large differences of up to 50% in mean metabolic rate between seasons, with metabolic rate in the late dry season (April–May) being particularly high across all analyses (Fig. 4).
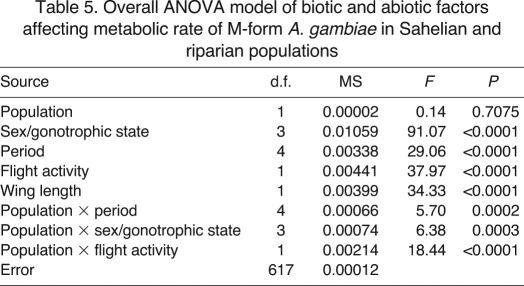
Using the above findings, we tested the effects of location and season on metabolic rate in a multivariate model that included all previous factors (sex/gonotrophic state, temperature, flight activity and wing length) and their interactions (Table 5). Location alone is not significant (P>0.7), while season (sampling period) is highly significant (P<0.0001) and the interaction of location and period is also significant (P<0.001), indicating that the two populations respond differently to the seasonal changes in their environment.
Table 5.
Overall ANOVA model of biotic and abiotic factors affecting metabolic rate of M-form A. gambiae in Sahelian and riparian populations
DISCUSSION
Little is known about the mechanisms facilitating the persistence of African anophelines throughout the dry season, yet dry season mosquitoes could represent novel targets for malaria control (Sogoba et al., 2007; Adamou et al., 2011). Here, we explored seasonal variation in metabolic rate, flight activity and body size as factors underlying the extended survival of M-form A. gambiae in the Sahelian dry season (Lehmann et al., 2010; Adamou et al., 2011). We expected that seasonal variation would be greater in the Sahelian than in the riparian population.
Generalizing from studies on winter diapause in mosquitoes (e.g. Benoit and Denlinger, 2007; Kim and Denlinger, 2009), we predicted that during the dry season, metabolic rate and flight activity would decrease while body size would increase. Our results confirmed that, for those active mosquitoes that were collected indoors throughout the year (as aestivators may be dormant inside shelters and therefore not captured), seasonal variation in all three phenotypes was greater in the Sahelian population than in the riparian population. However, only variation in flight activity exactly matched our predicted pattern. In the Sahel, body size (wing length) (Huestis et al., 2011) increased from the wet season to the early dry season, in accordance with our expectations. However, by the late dry season, wing length dropped in contrast to our expectations, while body mass remained high. For the Sahelian population at least, this shift in body size (as measured by wing length) appears to occur in the absence of any larval sites or active reproduction, and may therefore reflect different survival strategies and/or activity levels for large vs small individuals, or the arrival of small migrant mosquitoes from distant locations (see below).
Similarly, a complex pattern of seasonal variation was revealed for metabolic rate. In agreement with our prediction, mean metabolic rate of Sahelian M-form A. gambiae was suppressed in October–November, when this taxon almost vanishes from indoor collections. However, in contrast to our predictions, mean metabolic rate peaked in the late dry season, over and above its value in the middle of the wet season. These results are robust and reveal novel patterns of seasonal variation, demonstrating that unique ecophysiological mechanisms are involved in the presumed aestivation of Sahelian M-form A. gambiae. Our results emphasize the distinction between the ecophysiological processes of aestivation in A. gambiae and the classical diapause in many other insects.
Seasonal variation in wing length over a 6 month time frame has also been observed in A. arabiensis in east Africa (Russell et al., 2011), where mosquitoes were largest just after the start of the wet season, presumably due to lower competition for larval resources when densities are still low. By contrast, we observed that mean wing length of Sahelian M-form A. gambiae increased from the early (July–August) to the late (October–November) wet season and into the early dry season (December–February), and that the founding population prior to (April–May) and after (July–August) the start of the rains consists mainly of smaller individuals. Most surprisingly, mean wing length fell considerably in the middle of the dry season (April–May), in the absence of any surface water available for reproduction, as if the larger mosquitoes found earlier in the dry season were replaced by migrants or a hidden, dormant sub-population. If these mosquitoes were migrants, they would have to be capable of surviving over 10 weeks until the rains start, a feat beyond non-aestivating mosquitoes even if they are not long-distance migrants (Adamou et al., 2011). Alternatively, aestivation by Sahelian A. gambiae may involve two strategies. The larger mosquitoes that predominate from November to February represent ‘weak aestivators’, which exploit a shorter, 4 month dry season that may be broken when mango rains form new larval sites. Such mango rains typically occur every 3–5 years and are scattered throughout the Sahel, providing unique reproductive opportunities where they occur. In contrast, the smaller mosquitoes that predominate from April onwards survive the full 7 months of the dry season and employ the strategy of ‘strong aestivators’, and found the next wet season population. Heterogeneity in reproductive strategy of Sahelian M-form A. gambiae was recently noted in our study area (Yaro et al., 2012). Therefore, we hypothesize that mosquitoes may utilize alternative strategies for dry season survival and that the choice of strategy may be size dependent. Until we can sample mosquitoes from their hidden dry season shelters, the resolution of these hypotheses may be elusive.
We also found significant seasonality in body mass in the Sahel that did not identically parallel the pattern for wing length. After correcting for variation in wing length, the mean dry mass of late dry season individuals was larger than that at other times, followed by the late wet season and early dry season, with individuals found in the middle of the dry season again being smallest. These results indicate that these relatively small individuals surviving until the end of the dry season (April–May) have greater nutritional reserves (relative to their body size) than do mosquitoes at other times of the year. Considering the abundance of flowers and fruits during the dry season (Müller et al., 2010), continued availability of human hosts and the hospitable temperature range, mosquitoes may amass nutritional reserves until they can later allocate them to reproduction. It is possible that these seasonal shifts in body size are mostly due to the drying of larval sites at the end of the wet season, which in turn affects larval density and thus food availability and larval growth patterns, as it is well known that growing conditions affect adult size (Lanciani, 1992; Fischer and Fiedler, 2002; Chown and Klok, 2003; Aboagye-Antwi and Tripét, 2010; Russell et al., 2011). In the riparian population, body mass was also increased in the transition period and early dry season, but fell starting in the late dry season, indicating that aestivation predictions were not closely met.
Insect flight activity consumes considerable energetic resources (Nayar and Van Handel, 1971; Clements, 1992; Joos et al., 1996; Gray and Bradley, 2003; Lighton, 2008; Huestis et al., 2011). Indeed, based on the caloric cost of flight, we previously calculated that by reducing flight activity, metabolic rate can be reduced 18- to 22-fold, therefore substantially reducing a mosquito's caloric requirements and the frequency of feeding (Huestis et al., 2011). In the Sahelian population, flight activity was significantly lower in the time periods just prior to and throughout the dry season, relative to that during the mid wet season (July–August), representing an approximately 5-fold decrease. These results agree closely with the expectations for aestivating mosquitoes. By contrast, we found no significant seasonal variation in flight activity for the riparian population, with the greatest magnitude of change between time periods being 2-fold; these results do not support aestivation predictions.
Unlike flight activity, the significant seasonal variation in resting metabolic rate of the Sahelian M-form did not agree with expectations for aestivating mosquitoes; namely, reduced resting metabolic rate during the dry season. In fact, it was highest during the late dry season, intermediate during the early dry season as well as during the mid wet season, and lowest in the transitional period, suggesting that aestivation is associated with a higher resting metabolic rate. This surprising result suggests that a higher resting metabolic rate may be required for extended survival without reproduction under the climate of the Sahelian dry season and that this elevated metabolic rate may represent one of the costs of aestivation. Seasonal variation in metabolic rate of the riparian M-form, in contrast, was non-significant once ambient temperature was included in the model, in agreement with the expectation of no (or reduced) aestivation where year-round breeding is possible. Consistent with this explanation, resting metabolic rate in the late dry season was higher in the Sahelian than in the riparian population, despite similarly high temperatures in the two localities.
One important caveat to this finding is that differential fuel usage can bias metabolic rate measurements when CO2 production alone is used (Sinclair et al., 2011), because the respiratory quotient varies depending on the primary energy source being burned (Lighton, 2008). Specifically, the usage of stored lipids during starvation will artificially depress the measured CO2 production relative to carbohydrate usage (Sinclair et al., 2011). However, the potential impact on our study is small because (1) the fraction of unfed females in our sample size was generally small (Table 1) and (2) CO2 production increased during the dry season (January–May) rather than decreased (as would potentially occur under starvation conditions). Therefore, we do not find it very likely that seasonal changes in fuel usage would strongly bias our findings.
Although some of our results follow aestivation predictions, the departures from these expectations are evident and necessitate reevaluation of the expectations as well as the implications of the disagreements between them and our findings. Our expectations were formed based on studies of overwintering insects and mosquitoes. However, no study has demonstrated a reduction of resting metabolic rate during aestivation of any insect species; thus, the absence of previous empirical data makes interpretation of these results more difficult. The high ambient temperatures during the Sahelian dry season may prevent or limit a reduction in metabolic rate even if it would be adaptive. Furthermore, unlike typical diapause, in which the organism ‘escapes’ an unfavourable environment (e.g. low temperatures or lack of food), it is the absence of breeding opportunity (i.e. larval sites) that drives aestivation of African anophelines. This difference can change the strategies mosquitoes implement during their aestivation. The abundance of sugar sources (e.g. Müller et al., 2010), blood and water (around houses and in wells) probably relaxes the constraints imposed by limited food and water typically available to diapausing insects. Consistent with this, we (Yaro et al., 2012) found that reproductive physiology, but not blood-feeding response, was depressed during the dry season in Sahelian M-form A. gambiae. Accordingly, we propose that during the dry season, the Sahelian M-form A. gambiae exhibit both weak and strong aestivation strategies. All aestivating mosquitoes reduce their flight activity, but smaller mosquitoes are more likely to represent ‘strong aestivators’, which survive longer while their reproduction is suppressed, their metabolism is elevated and they possess high nutritional reserves (i.e. the highest body mass relative to wing length, see above). Indeed, these results are similar to those of Djawdan et al. (Djawdan et al., 1997), who found that increased stress resistance did not lead to lower metabolic rate but rather to greater accumulation of nutritional reserves (carbohydrates and lipids) in Drosophila melanogaster. Alternatively, the smaller mosquitoes that predominated in the late dry season (April–May) with elevated metabolic rate and body mass could represent newly arrived migrants after a long-distance dispersal. They must be capable of either surviving over 10 weeks until the first rain or continuing their migration elsewhere (or dying) while new migrants continuously arrive. A source of migrants like this would have such an incredibly high mosquito density that it would be difficult to miss, yet no such candidate site is known (Adamou et al., 2011).
Importantly, all of our results pertain only to those mosquitoes that were active and located indoors; however, the majority of the population during the dry season probably shelters in hidden, outdoor sites (Lehmann et al., 2010; Adamou et al., 2011). Possibly, those mosquitoes that are active during the dry season, and therefore are able to be captured indoors, represent a mixture of any or all of the following: aestivators low on reserves that must emerge from their shelters to feed, mosquitoes breaking aestivation, non-aestivating migrants arriving from distant locations, and newly emerged mosquitoes from underground, hidden larval sites. Without finding mosquitoes during the dry season in their unknown, outdoor shelters, we cannot rule out the possibility that metabolic rate is actually reduced by those aestivating in shelters. Lastly, the documented changes in mosquito physiology during the dry season may change plasmodium–mosquito interactions and have important implications for malaria transmission (e.g. Adamou et al., 2011). Additional studies on mosquito biology during the dry season are bound to uncover new facets of the ecology of the important disease vector A. gambiae.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the villagers in both locations for their support and assistance with mosquito collections, K. Hines for use of the electrobalance, and R. Sakai, R. W. Gwadz and T. E. Wellems for logistical support. A. Diabaté, A. Molina-Cruz and two anonymous reviewers provided helpful comments on prior versions of this manuscript.
FOOTNOTES
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/215/12/2013/DC1
FUNDING
This research was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Project 107816). Deposited in PMC for release after 12 months.
REFERENCES
- Aboagye-Antwi F., Tripét F. (2010). Effects of larval growth condition and water availability on desiccation resistance and its physiological basis in adult Anopheles gambiae sensu stricto. Malar. J. 9, 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamou A., Dao A., Timbiné S., Kassogué Y., Yaro A. S., Diallo M., Traoré S. F., Huestis D. L., Lehmann T. (2011). The contribution of aestivating mosquitoes to the persistence of Anopheles gambiae in the Sahel. Malar. J. 10, 151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J. B. (2010). Water management by dormant insects: comparisons between dehydration resistance during summer aestivation and winter diapause. In Aestivation: Molecular and Physiological Aspects, Progress in Molecular and Subcellular Biology Series (ed. Navas C. A., Carvalho J. E.), pp. 209-230 Berlin: Springer; [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Denlinger D. L. (2007). Suppression of water loss during adult diapause in the northern house mosquito Culex pipiens. J. Exp. Biol. 210, 217-226 [DOI] [PubMed] [Google Scholar]
- Blanckenhorn W. U., Henseler C., Burkhard D. U., Briegel H. (2001). Summer decline in populations of the yellow dung fly: diapause or quiescence? Physiol. Entomol. 26, 260-265 [Google Scholar]
- Canzano A. A., Krockenberger A. A., Jones R. E., Seymour J. E. (2006). Rates of metabolism in diapausing and reproductively active tropical butterflies, Euploea core and Euploea sylvester (Lepidoptera: Nymphalidae). Physiol. Entomol. 31, 184-189 [Google Scholar]
- Charlwood J. D., Vij R., Billingsley P. F. (2000). Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of East Africa. Am. J. Trop. Med. Hyg. 62, 726-732 [DOI] [PubMed] [Google Scholar]
- Chown S. L., Gaston K. J. (1999). Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol. Rev. 74, 87-120 [Google Scholar]
- Chown S. L., Klok C. J. (2003). Altitudinal body size clines: latitudinal effects associated with changing seasonality. Ecography 26, 445-455 [Google Scholar]
- Clements A. N. (1992). The Biology of Mosquitoes, Vol. 1, Development, Nutrition, and Reproduction. London: Chapman and Hall; [Google Scholar]
- Collins F. H., Kamau L., Ranson H. A., Vulule J. M. (2001). Molecular entomology and prospects for malaria control. Bull. World Health Organ. 78, 1412-1423 [PMC free article] [PubMed] [Google Scholar]
- Coluzzi M., Sabatini A., Petrarca V., Di Deco M. A. (1979). Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 73, 483-497 [DOI] [PubMed] [Google Scholar]
- Coluzzi M., Petrarca V., Di Deco M. A. (1985). Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Bollettino di Zoologia 52, 45-63 [Google Scholar]
- Coluzzi M., Sabatini A., della Torre A., Di Deco M. A., Petrarca V. (2002). A polytene chromosome analysis of the Anopheles gambiae species complex. Science 298, 1415-1418 [DOI] [PubMed] [Google Scholar]
- Danks H. V. (1987). Insect Dormancy: an Ecological Perspective. Ottawa: Biological Survey of Canada; [Google Scholar]
- Denlinger D. L. (1986). Dormancy in tropical insects. Annu. Rev. Entomol. 31, 239-264 [DOI] [PubMed] [Google Scholar]
- Denlinger D. L. (2002). Regulation of diapause. Annu. Rev. Entomol. 47, 93-122 [DOI] [PubMed] [Google Scholar]
- Djawdan M., Rose M. R., Bradley T. J. (1997). Does selection for stress resistance lower metabolic rate? Ecology 78, 828-837 [Google Scholar]
- Fanello C., Santolamazza F., della Torre A. (2002). Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med. Vet. Entomol. 16, 461-464 [DOI] [PubMed] [Google Scholar]
- Fischer K., Fiedler K. (2002). Reaction norms for age and size at maturity in response to temperature: a test of the compound interest hypothesis. Evol. Ecol. 16, 333-349 [Google Scholar]
- Gray E. M., Bradley T. J. (2003). Metabolic rate in Culex tarsalis (Diptera: Culicidae): age, size, activity, and feeding effects. J. Med. Entomol. 40, 903-911 [DOI] [PubMed] [Google Scholar]
- Gray E. M., Bradley T. J. (2005). Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. Am. J. Trop. Med. Hyg. 73, 553-559 [PubMed] [Google Scholar]
- Guppy M., Withers P. (1999). Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol. Rev. Camb. Philos. Soc. 74, 1-40 [DOI] [PubMed] [Google Scholar]
- Held C., Spieth H. R. (1999). First evidence of pupal summer diapause in Pieris brassicae L.: the evolution of local adaptedness. J. Insect Physiol. 45, 587-598 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. A., Sgro C. M., Lawler S. H. (1995). Ecological population genetics: the interface between genes and the environment. Annu. Rev. Genet. 29, 349-370 [DOI] [PubMed] [Google Scholar]
- Holstein M. H. (1954). Biology of Anopheles gambiae: Research in French West Africa. Geneva: World Health Organization; [Google Scholar]
- Huestis D. L., Marshall J. L. (2006). Interaction between maternal effects and temperature affects diapause occurrence in the cricket Allonemobius socius. Oecologia 146, 513-520 [DOI] [PubMed] [Google Scholar]
- Huestis D. L., Yaro A. S., Traoré A. I., Adamou A., Kassogué Y., Diallo M., Timbiné S., Dao A., Lehmann T. (2011). Variation in metabolic rate of Anopheles gambiae and A. arabiensis in a Sahelian village. J. Exp. Biol. 214, 2345-2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M., Shimada M. (1995). Trade-off in allocation of metabolic reserves: effects of diapause on egg production and adult longevity in a multivoltine bruchid, Kytorhinus sharpianus. Funct. Ecol. 9, 618-624 [Google Scholar]
- Joos B., Lighton J. R. B., Harrison J. F., Suarez R. K., Roberts S. P. (1996). Effects of ambient oxygen tension on flight performance, metabolism, and water loss of the honeybee. Physiol. Zool. 70, 167-174 [DOI] [PubMed] [Google Scholar]
- Kessler S., Guerin P. M. (2008). Responses of Anopheles gambiae, Anopheles stephensi, Aedes aegypti, and Culex pipiens mosquitoes (Diptera: Culicidae) to cool and humid refugium conditions. J. Vector Ecol. 33, 145-149 [DOI] [PubMed] [Google Scholar]
- Kim M., Denlinger D. L. (2009). Decrease in expression of beta-tubulin and microtubule abundance in flight muscles during diapause in adults of Culex pipiens. Insect Mol. Biol. 18, 295-302 [DOI] [PubMed] [Google Scholar]
- Kostal V., Sula J., Simek P. (1998). Physiology of drought tolerance and cold hardiness of the Mediterranean tiger moth Cymbalophora pudica during summer diapause. J. Insect Physiol. 44, 165-173 [DOI] [PubMed] [Google Scholar]
- Lanciani C. A. (1992). Photoperiod and the relationship between wing length and body weight in Anopheles quadrimaculatus. J. Am. Mosquito Control Assoc. 8, 297-300 [PubMed] [Google Scholar]
- Layne J. R., Jr, Eyck C. T. (1996). The effects of temperature and season on the O2S consumption of third-instar larvae of the goldenrod gall fly (Eurosta solidaginis). Physiol. Entomol. 21, 71-75 [Google Scholar]
- Lehmann T., Dao A., Yaro A. S., Adamou A., Kassogué Y., Diallo M., Sékou T., Coscaron-Arias C. (2010). Aestivation of the African malaria mosquito, Anopheles gambiae in the sahel. Am. J. Trop. Med. Hyg. 83, 601-606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighton J. R. B. (2008). Measuring Metabolic Rates: a Manual for Scientists. Oxford: Oxford University Press; [Google Scholar]
- Lyimo E. O., Koella J. C. (1992). Relationship between body size of adult Anopheles gambiae s.l. and infection with the malaria parasite Plasmodium falciparum. Parasitology 104, 233-237 [DOI] [PubMed] [Google Scholar]
- Masaki S. (1980). Summer diapause. Ann. Rev. Entomol. 25, 1-25 [Google Scholar]
- Mazzoni D. (2009). Audacity 1. 3. 9, a free digital audio editor. http://audacity.sourceforge.net
- Mousseau T. A., Roff D. A. (1989). Adaptation to seasonality in a cricket: patterns of phenotypic and genotypic variation in body size and diapause expression along a cline in season length. Evolution 43, 1483-1496 [DOI] [PubMed] [Google Scholar]
- Müller G. C., Beier J. C., Traore S. F., Toure M. B., Traore M. M., Bah S., Doumbia S., Schlein Y. (2010). Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar. J. 9, 262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolin D. L., Numata H. (2003). Photoperiodic and temperature control of diapause induction and colour change in the southern green stink bug Nezara viridula. Physiol. Entomol. 28, 65-74 [Google Scholar]
- Nayer J. K., Van Handel E. (1971). The fuel for sustained mosquito flight. J. Insect Physiol. 17, 471-481 [Google Scholar]
- Omer S. M., Cloudsley-Thompson J. L. (1968). Dry season biology of Anopheles gambiae Giles in the Sudan. Nature 217, 879-880 [Google Scholar]
- Omer S. M., Cloudsley-Thompson J. L. (1970). Survival of female Anopheles gambiae Giles through a 9-month dry season in Sudan. Bull. World Health Organ. 42, 319-330 [PMC free article] [PubMed] [Google Scholar]
- Ragland G. J., Fuller J., Feder J. L., Hahn D. A. (2009). Biphasic metabolic rate trajectory of pupal diapause termination and post-diapause development in a tephritid fly. J. Insect Physiol. 55, 344-350 [DOI] [PubMed] [Google Scholar]
- Ragland G. J., Denlinger D. L., Hahn D. A. (2010). Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 107, 14909-14914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D. J., Burggren W., French K. (2002). Eckert Animal Physiology: Mechanisms and Adaptations, 5th edn. New York: W. H. Freeman and Company; [Google Scholar]
- Reinhold K. (1999). Energetically costly behavior and the evolution of resting metabolic rate in insects. Funct. Ecol. 13, 217-224 [Google Scholar]
- Roff D. A. (2002). Life History Evolution. Sunderland, MA: Sinauer Associates, Inc; [Google Scholar]
- Russell T. L., Lwetoijera D. W., Knols B. G. J., Takken W., Killeen G. F., Ferguson H. M. (2011). Linking individual phenotype to density-dependent population growth: the influence of body size on the population dynamics of malaria vectors. Proc. R. Soc. Lond. B 278, 3142-3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting C. D., Pigliucci M. (1998). Phenotypic Evolution: a Reaction Norm Perspective. Sunderland, MA: Sinauer Associates, Inc; [Google Scholar]
- Sinclair B. J., Bretman A., Tregenza T., Tomkins J. L., Hosken D. J. (2011). Metabolic rate does not decrease with starvation in Gryllus bimaculatus when changing fuel use is taken into account. Physiol. Entomol. 36, 84-89 [Google Scholar]
- Sogoba N., Doumbia S., Vounatsou P., Baber I., Keita M., Maiga M., Traoré S. F., Touré A., Dolo G., Smith T., Ribeiro J. M. C. (2007). Monitoring of larval habitats and mosquito densities in the Sudan savanna of Mali: implications for malaria vector control. Am. J. Trop. Med. Hyg. 77, 82-88 [PubMed] [Google Scholar]
- Sohal R. S., Weindruch R. (1996). Oxidative stress, caloric restriction, and aging. Science 273, 59-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S. C. (1992). The Evolution of Life Histories. Oxford: Oxford University Press; [Google Scholar]
- Teder T., Esperk T., Remmel T., Sang A., Tammaru T. (2010). Counterintuitive size patterns in bivoltine moths: late-season larvae grow larger despite lower food quality. Oecologia 162, 117-125 [DOI] [PubMed] [Google Scholar]
- Touré Y. T., Petrarca V., Traoré S. F., Coulibaly A., Maiga H. M., Sankaré O., Sow M., Di Deco M. A., Coluzzi M. (1994). Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s.str. in Mali, West Africa. Genetica 94, 213-223 [DOI] [PubMed] [Google Scholar]
- Touré Y. T., Petrarca V., Traoré S. F., Coulibaly A., Maiga H. M., Sankaré O., Sow M., Di Deco M. A., Coluzzi M. (1998). The distribution and inversion polymorphism of chromosomally recognized taxa of the Anopheles gambiae complex in Mali, West Africa. Parassitologia 40, 477-511 [PubMed] [Google Scholar]
- World Health Organization (WHO) (2010). World Malaria Report 2010. Geneva: World Health Organization; [Google Scholar]
- Yamamoto K., Tsujimura Y., Kometani M., Kitazawa C., Islam A. T. M. F., Yamanaka A. (2011). Diapause pupal color diphenism induced by temperature and humidity conditions in Byasa alcinous (Lepidoptera: Papilionidae). J. Insect Physiol. 57, 930-934 [DOI] [PubMed] [Google Scholar]
- Yaro A. S., Traoré A., Huestis D. L., Adamou A., Timbiné S., Kassogué Y., Diallo M., Dao A., Traoré S. F., Lehmann T. (2012). Seasonal variation in reproductive physiology of Anopheles gambiae in the Sahel. J. Insect Physiol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.