The importance of zinc in biology is evidenced by the growing number of disease states that directly involve aberrant levels of zinc, including neurodegeneration, inflammation, diabetes, cancer and more. Zinc is a component of an estimated 10% of all proteins1 and has a well-established role in regulation of gene expression, primarily through activation of metal-responsive transcription factor-1 (MTF1) as well as an array of trans-acting factors.2 Recently, attention has been drawn to the fact that free Zn2+ in cells has also non-genomic signaling effects that can be evoked through extracellular stimuli.3,4 The Zn2+ ion therefore fulfils the criteria for being a second messenger.5 However, delivery of its second messenger role would require three key components to be present: (1) transmission of the extracellular stimuli to the intracellular machinery, (2) mechanism for the gated release of Zn2+ into the cytosol through modification of an effector molecule and (3) the translation of Zn2+ flux into altered activity in downstream targets. Our recent finding that a zinc channel, ZIP7 (SLC39A7), can be activated by phosphorylation by protein kinase CK2 as a direct result of an extracellular stimulation provides the central component of this pathway.6
ZIP7 is the only ZIP zinc channel known to reside in the membrane of the endoplasmic reticulum.7 In response to epidermal growth factor (EGF) stimulation, CK2 activates ZIP7 through serine phosphorylation, resulting in release of free Zn2+ ions into the cytosol.6 The consequential rise in cytosolic Zn2+ concentration activates protein kinases involved in cell proliferation, which results in increased cell migration. ZIP7 is ubiquitously expressed in cells unlike many other ZIP channels which are often expressed in a tissue-specific manner. This suggests that ZIP7 may be a gatekeeper for cytosolic Zn2+ release and control Zn2+ signals in multiple cell types.8 The targets directly affected by zinc signals remain to be mapped, but protein tyrosine phosphatases (PTP) seem to be a family of proteins that are particularly affected by an increase in [Zn2+]i.4 In a recent study, it was shown that a particular PTP, PTPβ, is inhibited by Zn2+ with a Ki of 21 pM, which is well within the range of intracellular Zn2+ concentrations.9 Inhibition of PTPs is thought to be the explanation for the widespread activation of tyrosine kinases observed in cells as a result of elevated levels of Zn2+.5,8 This latter event has major implications for diseases such as cancer that rely on tyrosine kinase-driven signaling pathways for growth and delivers a new and novel area for drug development.
There are still unanswered questions concerning the full extent of the role of zinc in cell signaling. The fact that ZIP7 needs phosphorylation in order to open the gate and release Zn2+ into the cytosol raises the question of whether other ZIP channels might be gated. There are a number of predicted phosphorylation sites in other zinc transporters and channels8 that should be investigated. Few of these sites are predicted to be CK2 sites, but several other kinases are implicated, suggesting that different zinc channels may be controlled in different ways, helping to explain the diversity of zinc signaling functions and the presence of 14 ZIP proteins in humans. There is now a need to identify the direct targets of zinc signals, including how Zn2+ released in the cell can selectively target particular proteins. Protein phosphatases4,9 and the plasma membrane Ca2+-ATPase10 have been shown to be regulated by Zn2+ at physiologically relevant concentrations, but the exact nature of these interactions remains obscure. Finally, it is also unknown which phosphatase acts to dephosphorylate and presumably inactivate ZIP7, but this discovery may also provide useful avenues for clinical exploration (Fig. 1).
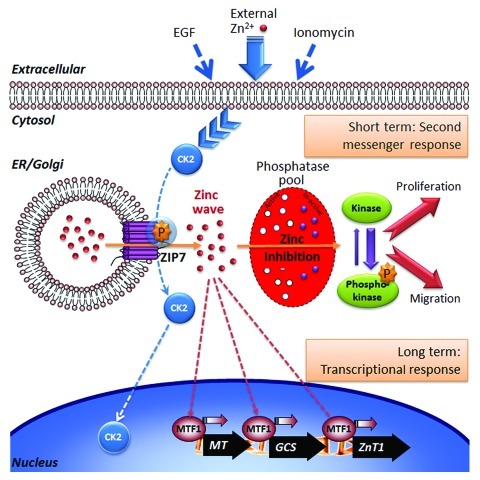
Figure 1. Schematic illustration of the second messenger signaling pathway proposed for Zn2+. Various external stimuli cause protein kinase CK2 to phosphorylate endoplasmic reticulum (ER) located zinc transporter, ZIP7 which causes the gated release of Zn2+ stored within the ER through the ZIP7 channel to create a cytosolic ‘zinc wave’. After phosphorylating its target CK2 dissociates from ZIP7 and may move into the nucleus. We postulate that elevated Zn2+ within the cytosol may selectively inhibit tyrosine phosphatases leading to prolonged tyrosine kinase activation and an observed downstream increase in pERK and pAKT resulting in cell proliferation and migration. These events, representing a short-term second messenger activity for Zn, completing within a 20 min time period, are thus temporally separated from established transcriptional impact of zinc ions which is mediated by metal transcription factor-1 (MTF-1) and is known to regulate metallothionein (MT), glutamylcysteine synthetase (GCS) and ZnT1 (SLC30A1).
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20414
References
- 1.Passerini A, et al. BMC Bioinformatics. 2007;8:39. doi: 10.1186/1471-2105-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogstrand C, et al. Biochem Soc Trans. 2008;36:1252–7. doi: 10.1042/BST0361252. [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki S, et al. J Cell Biol. 2007;177:637–45. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase H, et al. Exp Cell Res. 2003;291:289–98. doi: 10.1016/S0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 5.Beavo JA, et al. Nat Rev Mol Cell Biol. 2002;3:710–8. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 6.Taylor KM, et al. Sci Signal. 2012;5:ra11. doi: 10.1126/scisignal.2002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor KM, et al. Endocrinology. 2008;149:4912–20. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 8.Hogstrand C, et al. Trends Mol Med. 2009;15:101–11. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Wilson M, et al. J Biol Chem. 2012;287:9322–6. doi: 10.1074/jbc.C111.320796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogstrand C, et al. Toxicology. 1999;133:139–45. doi: 10.1016/S0300-483X(99)00020-7. [DOI] [PubMed] [Google Scholar]