Abstract
Objective
In this study we have assessed the renal and cardiac consequences of ligature-induced periodontitis in both normotensive and nitric oxide (NO)-deficient (L-NAME-treated) hypertensive rats.
Materials and methods
Oral L-NAME (or water) treatment was started two weeks prior to induction of periodontitis. Rats were sacrificed 3, 7 or 14 days after ligature placement, and alveolar bone loss was evaluated radiographically. Thiobarbituric reactive species (TBARS; a lipid peroxidation index), protein nitrotyrosine (NT; a marker of protein nitration) and myeloperoxidase activity (MPO; a neutrophil marker) were determined in the heart and kidney.
Results
In NO-deficient hypertensive rats, periodontitis-induced alveolar bone loss was significantly diminished. In addition, periodontitis-induced cardiac NT elevation was completely prevented by L-NAME treatment. On the other hand L-NAME treatment enhanced MPO production in both heart and kidneys of rats with periodontitis. No changes due to periodontitis were observed in cardiac or renal TBARS content.
Conclusions
In addition to mediating alveolar bone loss, NO contributes to systemic effects of periodontitis in the heart and kidney.
Keywords: Periodontitis, Nitric oxide, Neutrophils, Nitrotyrosine, Lipid peroxidation, Heart, Kidney
1. Introduction
Under physiological conditions, nitric oxide (NO), an important inflammatory mediator, is the intracellular transducer of central N-methyl D-aspartate (NMDA)-receptor activation, as well as a key regulator of vascular homeostasis.1 It is well demonstrated that rodents chronically treated with NO synthase (NOS) inhibitors develop hypertension, characterized by endothelial dysfunction.2,3 Endothelial dysfunction in man can be secondary to hyperlipidemia,4 ischemia-reperfusion5 and atherosclerosis.6 More recently, periodontal disease has also been linked to endothelial dysfunction.7,8
The relationship between endogenous NO and periodontal disease involves the inducible NOS (iNOS) isoform, as demonstrated by the beneficial effects that the administration of iNOS inhibitors (such as mercaptoethylguanidine9 and aminoguanidine10) had on the disease. Furthermore, Gyurko et al. have shown that iNOS-derived NO promotes bone resorption through osteoclast differentiation during bone development, as well as after bacterial infection in Porphyromonas gingivalis-induced periodontal disease in mice.11 However, the available information on NO production by oral tissues during periodontitis is either limited or controversial. For example, it has been reported that salivary nitrite concentration in patients with periodontitis is lower than that found in healthy individuals.12 On the other hand, it has been proposed that gingival NO overproduction must occur during periodontitis, based on the high concentrations of L-arginine and L-citrulline (substrate and by-product of NO biosynthesis, respectively) found in the gingiva of periodontal patients.13 However, these data are not conclusive, considering that nitrite can also originate from the reduction of exogenous nitrate by nitrate reductase-positive colonizing bacteria,14 or that the amino acids L-arginine and L-citrulline are also part of the urea cycle pathway, of which arginase I –the rate limiting enzyme – is up-regulated in inflammation.15
The relationship between periodontal and systemic diseases has been the focus of numerous studies. For example, pre-term low birth weight has been associated with the presence of periodontitis in the mother, where periodontal overproduction of interleukin (IL)-1β, tumour necrosis factor (TNF)-α and prostaglandins is proposed to directly contribute to induction of labour.16,17 Cardiac and cerebrovascular disease pathogenesis is also related to increased IL-6, C-reactive protein and circulating neutrophils.19,20 It has been suggested that these mediators, which are also augmented during periodontitis, can increase the risk of atherosclerotic lesions in periodontal patients.20 More recently, Higashi et al. have shown that the presence of periodontitis impairs endothelium-dependent vasodilation in both healthy and hypertensive young men by decreasing NO availability.21
Considering the above mentioned cardiovascular consequences that can occur secondary to the presence of periodontitis in humans, it is important to know the extent to which this condition can affect organs directly involved in circulatory homeostasis. In this way, in this study we decided to assess the influence of periodontitis on oxidative tissue damage evaluated in heart and kidneys from normotensive and NO-deficient hypertensive rats at different stages of ligature-induced periodontitis. This animal model of hypertension is secondary to the endothelial dysfunction obtained by the administration of NOS inhibitors.2,3
2. Materials and methods
2.1. Animals
Male Wistar rats (6-week old, 160–180 g) from the local animal care facilities were kept under controlled temperature (22–25 °C) and dark/light cycle (12/12 h). They were housed in polypropylene cages in groups of 5 per cage during the whole experimental period and received standard laboratory chow and tap water ad libitum. All the experimental protocols were approved by the local Ethics Committee for Animal Experimentation.
2.2. Chemicals
All the drugs and reagents were purchased from Sigma Chem. Co. (St. Louis, MO, USA), unless otherwise stated.
2.3. Induction of periodontitis
One hundred and twenty rats were anaesthetized with ketamine (80 mg/kg, i.p.; Francotar, Virbac do Brasil Ind. e Com. Ltda, Brazil) and xylazine (16 mg/kg, i.p.; Kensol, Konig S.A., Brazil) and the lower right first molar of each rat received a 3–0 cotton ligature in a submarginal position to induce peridontitis, as previously described.22 Sham-operated animals had the ligature immediately removed after the procedure.
At the end of each experimental period, the mandibles were removed and digital X-ray images were obtained. Alveolar bone loss was estimated by the distance between the cemento-enamel junction (CEJ) and the height of alveolar bone in mesial roots surfaces of lower right first molars with the aid of the software (Computed Dental Radiography for Microsoft Windows, Shick Technologies, Inc. Long Island City, NY).
2.4. In vivo NO synthesis inhibition
Two weeks before ligature placement, half of the animals received the NO synthase inhibitor Nω-nitro L-arginine methyl ester (L-NAME) dissolved in the drinking water (200 mg/L; equivalent to approximately 45–60 mg/kg/day, as previously shown23); control animals received water alone. L-NAME treatment continued until the sacrifice of the animals at 3, 7 or 14 days following ligature placement. In this way, for each time period, 4 groups (n = 10 animals) were defined: sham (S), sham + L-NAME (S + L-NAME), ligature (L) and ligature + L-NAME (L + L-NAME). At each time point, heart and kidney samples were collected from each animal and kept at −80 °C until analyzed (see below).
2.5. Western blot analysis of nitrotyrosine-containing proteins
The presence of proteins containing 3-nitrotyrosine (NT) residues, an index of oxidative stress and protein nitration, was analyzed in the heart and kidney samples as previously described.24 Immunoreactive bands were detected by chemiluminescence (Immun-Star; Bio-Rad, USA) and their intensities were estimated by densitometric analysis (ChemImager 5500 system, Alpha Innotech Corp., USA). For all the organs, NT expression was quantified by totalling band intensities of proteins within the 27–84 kDa MW range.
2.6. Myeloperoxidase activity assay
The activity of myeloperoxidase (MPO), a hemoprotein located in azurophilic granules of neutrophils, was used as a biochemical marker for neutrophil infiltration into the studied tissues. MPO activity was measured according to the method originally described by Bradley et al.25 with some modifications26, after heating the organ homogenates at 60° during 2 h in order to inactivate endogenous catalase.27
Briefly, after homogeneizing the tissue samples in the presence of hexadecyltrimethylammonium bromide (HTAB, Sigma Chem. Co., St. Louis, EUA) in order to disrupt the granules, the tubes were centrifuged at 10,000 × g during 5 min. MPO activity was analysed in the supernantants by its capacity to catalyse the oxidation of o-dianisidine dihydrochloride (Sigma Chemical Co., St. Louis, EUA) in the presence of hydrogen peroxide (Merck, Darmstadt, Alemanha). Absorbance increase rate was monitored at 460 nm (Espectra max Plus 384, Molecular Devices Inc., Sunnyvale, EUA) and the obtained Vmax (maximum speed) parameter related to the enzyme activity using a molar extinction coefficient of 11,300 M cm−1.
2.7. Quantification of thiobarbituric acid reactive species (TBARS)
Aldehydes (mainly malondialdehyde – MDA) resulting from lipid peroxidation were quantified in the tissue homogenates after their reaction with thiobarbituric acid in acid medium, according to the method described by Bird and Draper.28 This reaction yields a pink adduct chromophore with peak absorbance at 535 nm, that is converted to MDA equivalents using a molar absorption coefficient of 1.55 × 105 M cm−1.
2.8. Statistical analysis
All the results are expressed as mean ± SEM (standard error of the mean). With the exception of alveolar bone loss data, all the measured variables were calculated as a percentage relative to the untreated sham group (S) for the corresponding time point. Differences between groups were analyzed by one-way ANOVA followed by the Student–Neuman–Keuls test for multiple comparisons. Values of p < 0.05 were considered significant.
3. Results
3.1. Ligature-induced bone loss
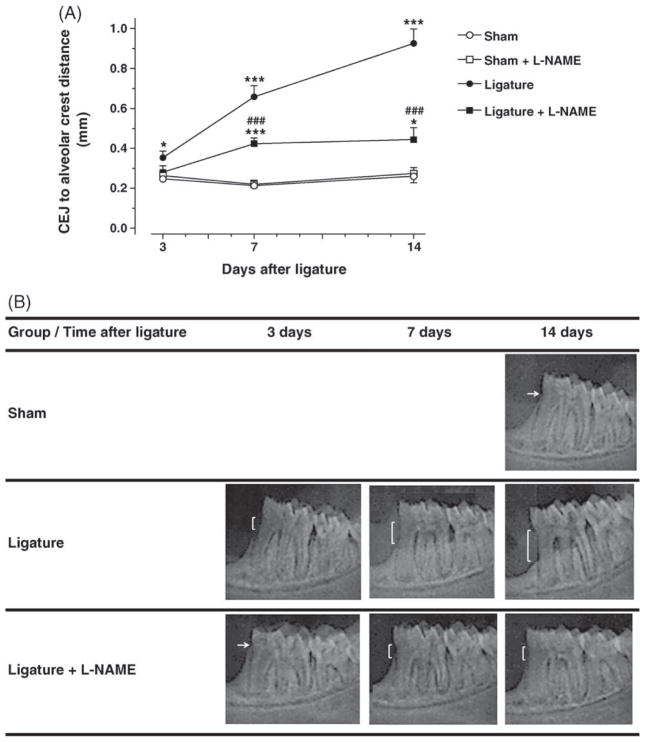
During the entire experimental period no changes in animal behaviour were observed. Body weight gain was within normal limits as confirmed by weight measurements performed 2 times a week. Significant and progressive alveolar bone loss was observed in the ligated normotensive rats in comparison with the normotensive non-ligated (sham) rats (day 3: 0.35 ± 0.03 vs. 0.25 ± 0.01 mm, p < 0.05; day 7: 0.66 ± 0.06 vs. 0.21 ± 0.01 mm, p < 0.001; day 14: 0.93 ± 0.07 vs. 0.26 ± 0.03 mm, p < 0.001; Fig. 1). L-NAME treatment significantly reduced ligature-induced bone loss at days 7 and 14 when compared to ligated normotensive animals (day 7: 0.42 ± 0.03 vs. 0.66 ± 0.06 mm, p < 0.001; day 14: 0.44 ± 0.06 vs. 0.93 ± 0.07 mm, p < 0.001). L-NAME treatment had no effect on sham animals.
Fig. 1.
Ligature-induced alveolar bone loss of the lower first molar is reduced in L-NAME-treated rats. Panel A: Measurement of the distance from the cement-enamel junction (CEJ) to the alveolar bone crest was performed from digital X-ray images of the jaws (n = 10). For each time point, ***p < 0.001 vs. the S group; #p < 0.05 and ###p < 0.001 vs. S and S + L-NAME group; ***p < 0.001 vs. L group. Panel B: Representative X-ray images obtained from each experimental group at the different time points studied following ligature placement.
3.2. Protein nitrotyrosine content
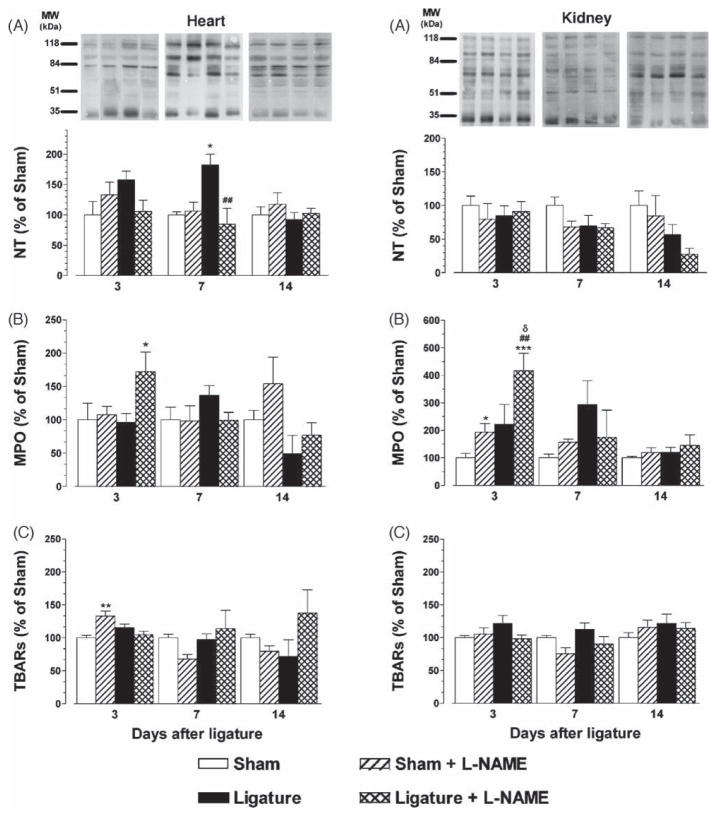
Cardiac NT content was significantly increased 7 days after ligature placement in normotensive rats (L: 183 ± 17 vs. S: 100 ± 5%; p < 0.05, Fig. 2A). As early as day 3 a slight but statistically non-significant elevation in cardiac NT was observed in ligated animals. L-NAME treatment completely abolished ligature-induced cardiac NT (L + L-NAME: 85 ± 26%, p < 0.01). Renal NT showed no significant differences among experimental groups at any time point.
Fig. 2.
Total nitrotyrosine-containing protein contents (panels A), myeloperoxidase (MPO) activity (panels B) and tiobarbituric reactive species (TBARs; panels C) expressed as % of the sham values in heart and kidney samples from control and L-NAME treated rats with ligature-induced periodontitis (n = 10). Within each time period, *p < 0.05, and ***p < 0.001 vs. the S group; ##p < 0.01 vs. the L group; δp < 0.05 vs. the S + L-NAME group.
3.3. Myeloperoxidase (MPO) activity
MPO activity was higher in the heart of ligature + L-NAME treated rats in comparison with the ligature only group at day 3 (172 ± 30 vs. 96 ± 13%; p < 0.05). Renal MPO was significantly elevated in the NO-deficient animals on day 3 (S + L-NAME: 193 ± 32% vs. S: 100 ± 17%; p < 0.01), and this increase was significantly potentiated by the presence of periodontitis (L + L-NAME: 417 ± 63% vs. S + L-NAME, p < 0.01; L + L-NAME vs. L: 222 ± 72%, p < 0.05). At days 7 and 14 no significant alterations were seen in cardiac and renal MPO.
3.4. Thiobarbituric acid reactive species (TBARS)
NO-deficient sham rats showed higher cardiac TBARs content in comparison with the normotensive sham controls on day 3 (S + L-NAME: 133 ± 8 vs. S: 100 ± 4%; p < 0.01). Ligature-induced periodontitis did not induce significant TBARS alterations. No significant differences among the groups were found in cardiac TBARS at either day 7 or 14 after ligature placement. Renal TBARS remained unchanged under all the experimental conditions.
4. Discussion
In this work we show that NO contributes to periodontal disease as periodontitis-induced bone loss is significantly reduced in L-NAME treated rats. Moreover, data presented here on periodontitis-induced changes in nitrotyrosine and myeloperoxidase levels in the heart and kidney indicate a novel role for NO in mediating systemic effects of periodontal disease. Others have reported that rats treated with selective iNOS inhibitors present not only with less alveolar bone loss secondary to periodontal disease,9 but also diminished local inflammatory markers related to oxidative stress and tissue damage, such as protein tyrosine nitration, lipid peroxidation and leukocyte infiltration.10 Moreover, it has been demonstrated that the continuous production of NO from cytokine-induced iNOS not only inhibits cGMP-mediated osteoblast growth and differentiation,29 but also favours osteoclast-mediated bone resorption11 and potentiates pro-inflammatory cytokine-induced bone loss.30,31 These observations indicate a critical role for iNOS-derived NO on periododontitis-induced bone loss and inflammation.
Spontaneously hypertensive rats (SHR), with genetic hypertension that is not strictly related to endothelial NO inhibition, show more severe periodontitis than the normotensive controls.31 Thus the reduced periodontal bone loss we observed in L-NAME-treated rats is likely related to iNOS inhibition and not to hypertension.
Epidemiological evidences indicate that periodontal disease may aggravate pre-existing cardiovascular and/or cerebrovascular conditions.18,19,32 However, to the best of our knowledge, the role of NO in mediating systemic effects of periodontitis has not been explored. Endothelial NOS-derived NO is a key mediator in the control of vascular tonus, platelet aggregation and adhesion. In fact, long-term NOS inhibition by L-arginine analogues in rodents has proven to be a useful animal model for arterial hypertension that shares many features with human essential hypertension and other diseases dependent on endothelial dysfunction.2,3,24
MPO is a lysosomal enzyme abundant in the azurophil granules of neutrophils. It is released during respiratory burst and it catalyses the formation of hypochlorous acid from hydrogen peroxide. Both of these cytotoxic agents are implicated in bacterial killing as well as in neutrophil-mediated host tissue damage. L-NAME treatment alone resulted in increased renal MPO activity on day 3 after the simulated ligature placement procedure, which was significantly augmented in the L + L-NAME group. At the same time point, cardiac MPO activity was also significantly increased in the NO-deficient hypertensive rats with periodontis, but in this organ, either periodontitis or L-NAME treatment alone did not induce any alteration in the measured MPO activity values. For both organs, no differences among the groups were observed at later time points, suggesting that only the early phase of periodontitis, corresponding to the initial activation of neutrophils is influenced by NO.
It has been demonstrated that long-term L-NAME treatment results in severe cardiac33 and renal fibrosis in rats.3 In addition, the lack of NO potentiates neutrophil–endothelium interaction through a mechanism involving the up-regulation of integrins CD11/CD18 in neutrophils.34 Our observations are consistent with the hypothesis that L-NAME-induced endothelial activation synergizes with periodontitis-induced priming of circulating neutrophils, leading to increased neutrophil margination and potential tissue damage in the heart and kidney.
As mentioned above, it has been proposed that periodontitis can result in systemic effects mediated by locally produced inflammatory mediators (such as IL-1β, IL-6, TNF-α and interferon-γ) that reach distant organs through the circulation. In this study, hearts from normotensive animals with periodontitis showed increased protein tyrosine nitration during the first week following ligature placement. This protein modification was not observed in the L-NAME-treated animals with periodontitis, thus demonstrating the strict dependence on NO production, most likely peroxynitrite anion formation from iNOS-derived NO. In fact, it has been reported that IL-1β, a pro-inflammatory cytokine increased during periodontitis, can trigger the synthesis of iNOS in cardiac tissue.35 Interestingly, at the latest time point, all the groups are indistinguishable in terms of cardiac NT contents, which could be due to either the reversal of this protein modification by denitrases,36 and/or the involvement of other inflammatory mediators unrelated to iNOS during this advanced phase of the inflammatory process.
Several oxygen-derived reactive species are produced during periodontitis, contributing to local tissue damage.37,38 Increased cardiac TBARS content was observed in NO-deficient hypertensive rats (3 days after the ligature procedure) but not in the corresponding periodontitis group. Similarly, Sahna and co-workers have reported that hearts from rats treated with L-NAME for 2 weeks have unaltered MPO activity and augmented TBARS contents, in addition to lower quantities of reduced glutathione.39 In this work, the authors conclude that L-NAME-induced alterations are due to the oxidative stress status caused by the lack of NO, since the administration of melatonin resulted in a partial reversal of these changes by acting as a free radical scavenger. Interestingly, augmented circulating and salivary melatonin concentrations have been reported in patients with periodontitis,40 and this could account for the absence of cardiac TBARS alterations observed in the NO-deficient hypertensive animals with concomitant periodontitis. However, it is difficult to explain the absence of cardiac lipoperoxidation in L-NAME-treated animals at days 7 or 14, unless a different pattern of free radicals production (mainly hydroxyl radical) occurs at these time points.
Conversely, in the kidney, no significant alterations in TBARS content were observed in response to either periodontitis, L-NAME treatment, or their combination. This may be due to high catalase content present in renal tissues, leading to hydrogen peroxide decomposition and prevention of the formation of hydroxyl radical through the Fenton and/or Haber–Weiss reactions and hence, lipid peroxidation.27
In our animal model, unilateral ligature-induced periodontitis leads to a moderate localized form of periodontal disease. Periodontal disease in man often presents with more than one periodontal pocket, bleeding or a chronic abscess. In this way, our experimental conditions may underestimate the local and systemic tissue damage present in human periodontitis.
In conclusion, our data support a key role for NO in the development of local alveolar bone loss in periodontal disease. In addition, our observations strongly suggest that NO mediates systemic effects of periodontitis on heart and kidney, which may be due to the transient bacteremia (or the circulating toxins) and/or the immunological activation caused by the abundant number of oral microorganisms that occur during periodontal disease. In addition, the significance of these systemic alterations is strongly dependent on both the organ and the stage of the periodontal inflammatory process, and may parallel some of the biochemical changes observed in certain diseases (such as cardiovascular disorders or bacterial pneumonia).
Acknowledgments
Funding: The authors thank the financial support from the State of São Paulo Research Foundation (FAPESP; grant 2002/00300-0 and fellowships for BSH, RMP and SAT), National Council for Scientific and Technological Development (CNPq; grant 475549/2004-0 and fellowships for SKC and MNM) and USPHS grants DE16933 (RG) and DE15566 (TVD) from the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Ethical approval: All the experimental protocols were approved by the Animal Research Ethics committee of the University of São Paulo, Brazil (Protocol number CEEA 058/03).
References
- 1.Kendall HK, Marshall RI, Bartold PM. Nitric oxide and tissue destruction. Oral Dis. 2001;7(1):2–10. [PubMed] [Google Scholar]
- 2.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90(1):278–81. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992;20(3):298–303. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Shimokawa H, Vanhoutte PM. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ Res. 1989;64(5):900–14. doi: 10.1161/01.res.64.5.900. [DOI] [PubMed] [Google Scholar]
- 5.Ku DD. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982;218(4572):576–8. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- 6.Vanhoutte PM, Shimokawa H. Endothelium-derived relaxing factor and coronary vasospasm. Circulation. 1989;80(1):1–9. doi: 10.1161/01.cir.80.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–20. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 8.Higashi y, Goto C, Hidaka T, Soga J, Nakamura S, Fujii Y, et al. Oral infection-inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis. 2009;206(2):604–10. doi: 10.1016/j.atherosclerosis.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Lohinai Z, Benedek P, Feher E, Gyorfi A, Rosivall L, Fazekas A, et al. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br J Pharmacol. 1998;123(3):353–60. doi: 10.1038/sj.bjp.0701604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Paola R, Marzocco S, Mazzon E, Dattola F, Rotondo F, Britti D, et al. Effect of aminoguanidine in ligature-induced periodontitis in rats. J Dent Res. 2004;83(4):343–8. doi: 10.1177/154405910408300414. [DOI] [PubMed] [Google Scholar]
- 11.Gyurko R, Shoji H, Battaglino RA, Boustany G, Gibson FC, 3rd, Genco CA, et al. Inducible nitric oxide synthase mediates bone development and P. gingivalis-induced alveolar bone loss. Bone. 2005;36(3):472–9. doi: 10.1016/j.bone.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Auer A, Aleksic J, Ivic-Kardum M, Auer J, Culo F. Nitric oxide synthesis is decreased in periodontites. J Clin Periodontol. 2001;28(6):565–8. doi: 10.1034/j.1600-051x.2001.028006565.x. [DOI] [PubMed] [Google Scholar]
- 13.Matejjka M, Partyka L, Ulm C, Solar P, Singer H. Nitric oxide is increased in periodontal disease. J Periodontal Res. 1998;33(8):517–8. doi: 10.1111/j.1600-0765.1998.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 14.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1(6):546–51. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 15.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167(11):6533–44. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 16.Collins JG, Windley HW, III, Arnold RR, Offenbacher S. Effects of a Porphyromonas gingivalis infection on inflamatory mediator response in pregnancy outcome in hamsters. Infect Immun. 1994;62(10):4356–61. doi: 10.1128/iai.62.10.4356-4361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1995;67(10):1103–13. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 18.Berk B, Weintraub W, Alexander Elevation of C-reactive protein in “active” coronary disease. Am J Cardiol. 1990;65(3):168–72. doi: 10.1016/0002-9149(90)90079-g. [DOI] [PubMed] [Google Scholar]
- 19.Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, et al. Elevated levels of interleukin-6 in unstable angina. Circulation. 1996;94(5):874–7. doi: 10.1161/01.cir.94.5.874. [DOI] [PubMed] [Google Scholar]
- 20.Loos BG, Craandijk J, Hoek FJ, Dillen PMEW, Velden U. Elevation of systemic markers related to cardiovascular diseases in peripheral blood of periodontitis patients. J Periodontol. 2000;71(10):1528–34. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 21.Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51(2):446–53. doi: 10.1161/HYPERTENSIONAHA.107.101535. [DOI] [PubMed] [Google Scholar]
- 22.Sallay K, Sanavi F, Ring I, Pham P, Behling UH, Nowotny A. Alveolar bone destruction in the immunosuppressed rat. J Periodontal Res. 1982;17(3):263–74. doi: 10.1111/j.1600-0765.1982.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 23.Muscará MN, Vergnolle N, Lovren F, Triggle CR, Elliott SN, Asfaha S, et al. Selective cyclo-oxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br J Pharmacol. 2000;129:1423–30. doi: 10.1038/sj.bjp.0703232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teixeira SA, Castro GM, Papes F, Martins ML, Rogério F, Langone F, et al. Expression and activity of nitric oxide synthase isoforms in rat brain during the development of experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 2002;99(1):17–25. doi: 10.1016/s0169-328x(01)00341-2. [DOI] [PubMed] [Google Scholar]
- 25.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 26.Gomes DA, Pires JR, Zuza EP, Muscara MN, Herrera BS, Spolidorio LC, et al. Myeloperoxidase as inflammatory marker of periodontal disease: experimental study in rats. Immunol Invest. 2009;38(2):117–22. doi: 10.1080/08820130802457503. [DOI] [PubMed] [Google Scholar]
- 27.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23(3):179–86. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 28.Bird RP, Draper AH. Comparative studies on different methods of malondyaldehyde determination. Meth Enzymol. 1984;90:105–10. doi: 10.1016/s0076-6879(84)05038-2. [DOI] [PubMed] [Google Scholar]
- 29.Mancini L, MoradiI-Bidhendi N, Becherini L, Martineti V, Mancintyre I. The biphasic effects of nitric oxide in primary rat osteoblasts are cGMP dependent. Biochem Biophys Res Commun. 2000;274(2):477–81. doi: 10.1006/bbrc.2000.3164. [DOI] [PubMed] [Google Scholar]
- 30.Ralson SH, Todd D, Helfrish MH, Benjamin N, Grabowski PS. Human osteoblastic-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology. 1994;135(1):330–6. doi: 10.1210/endo.135.1.7516867. [DOI] [PubMed] [Google Scholar]
- 31.Leite CL, Redings CA, Vasques EC, Meyrelles SS. Experimental-induced periodontitis is exacerbated in spontaneously hypertensive rats. Clin Exp Hypertens. 2005;27(6):523–31. doi: 10.1081/CEH-200067688. [DOI] [PubMed] [Google Scholar]
- 32.Angeli F, Verdecchia P, Pellegrino C, Pellegrino RG, Pellegrino G, Prosciutti L, et al. Association between periodontal disease and left ventricle mass in essential hypertension. Hypertension. 2003;41(3):488–92. doi: 10.1161/01.HYP.0000056525.17476.D7. [DOI] [PubMed] [Google Scholar]
- 33.Moreno H, Jr, Piovesan Nathan L, Costa SKP, Metze K, Antunes E, Zatz R, et al. Enalapril does not prevent the myocardial ischemia caused by the chronic inhibition of nitric oxide synthesis. Eur J Pharmacol. 1995;287(1):93–6. doi: 10.1016/0014-2999(95)00625-x. [DOI] [PubMed] [Google Scholar]
- 34.Kubes P, Szruki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88(11):4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams B, Nehrhoff U, Späte A, Linke PC, Schulze A, Baur S, et al. Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc Res. 2002;54(1):95–104. doi: 10.1016/s0008-6363(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 36.Kamisaki Y, Wada K, Bian K, Balabanli B, Davis K, Martin E, et al. An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc Natl Acad Sci USA. 1998;95(20):11584–91. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6(3):138–51. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 38.Borges I, Jr, Moreira EA, Filho DW, de Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm. 2000;45794:1–5. doi: 10.1155/2007/45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahna E, Deniz E, Bay-Karabulut A, Burma O. Melatonin protects myocardium from ischemia-reperfusion injury in hypertensive rats: role of myeloperoxidase activity. Clin Exp Hypertens. 2008;30(7):673–81. doi: 10.1080/10641960802251966. [DOI] [PubMed] [Google Scholar]
- 40.Gómez-Moreno G, Cutando-Soriano A, Arana C, Galindo P, Bolaños J, Acuña-Castroviejo D, et al. Melatonin expression in periodontal disease. J Periodontal Res. 2007;42(6):536–40. doi: 10.1111/j.1600-0765.2007.00978.x. [DOI] [PubMed] [Google Scholar]