Abstract
There are declines with age in speed of processing, working memory, inhibitory function, and long-term memory, as well as decreases in brain structure size and white matter integrity. In the face of these decreases, functional imaging studies have demonstrated, somewhat surprisingly, reliable increases in prefrontal activation. To account for these joint phenomena, we propose the scaffolding theory of aging and cognition (STAC). STAC provides an integrative view of the aging mind, suggesting that pervasive increased frontal activation with age is a marker of an adaptive brain that engages in compensatory scaffolding in response to the challenges posed by declining neural structures and function. Scaffolding is a normal process present across the lifespan that involves use and development of complementary, alternative neural circuits to achieve a particular cognitive goal. Scaffolding is protective of cognitive function in the aging brain, and available evidence suggests that the ability to use this mechanism is strengthened by cognitive engagement, exercise, and low levels of default network engagement.
Keywords: default network, dedifferentiation, hippocampus, compensation, cognitive reserve, frontal activation
INTRODUCTION
All highly developed nations in the world are experiencing substantial increases in the proportion of elderly adults in the population due to falling birth rates combined with increased longevity. By 2050, there will be many more older adults in wealthy, developed countries (26%) than children under 15 (about 16% of total population) ( J. E. Cohen 2003). The aging of the population represents both an opportunity and threat for society. The opportunity comes from the tremendous reserve of human capital and experience represented by older citizens; the threat emerges from the disconcerting fact that at this time, adults aged 85 and older have a dementia rate (typically in the form of Alzheimer’s disease) of nearly 50% (Hebert et al. 2003), with a very high cost to affected individuals and families, as well as to limited medical resources. At present, it is fair to say that neurocognitive frailty is the biggest threat to successful aging in our society.
Fortunately, as our aging population has grown, so has our knowledge about the aging mind. For the past 25 years, our understanding of the behavioral changes that occur in cognition with age has increased tremendously, and in the past 10 years, the advent of neuroimaging tools has ushered a truly stunning increase in what we know about the aging mind. Neuroimaging techniques such as structural and functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) allow us to see how both brain structure and function change with age. In the present review, we integrate the vast body of behavioral research in cognitive aging with recent data revealed by imaging techniques. We argue that the unprecedented opportunity to look into the operation of the mind afforded by neuroimaging indicates the brain and cognitive system to be more dynamic and adaptive then was ever previously suspected. We propose that the extant behavioral and brain data can best be understood within a new model: the scaffolding theory of aging and cognition (STAC).
After reviewing the range of data examining brain and behavioral function with age, we believe that the corpus of these data suggests that the brain is a dynamic organism seeking to maintain homeostatic cognitive function. With age, the number of dopaminergic receptors declines; many brain structures show volumetric shrinkage; white matter becomes less dense; and brains of even very highly functioning individuals are frequently characterized by destructive neurofibrillary plaques and tangles. We argue that the brain responds to these neural insults by engaging in continuous functional reorganization and functional repairs that result in self-generated support of cognitive function. We term this homeostatic, adaptive model of aging the scaffolding theory of aging and cognition. Encarta defines scaffolding as “a supporting framework.” In the context we are using this term, scaffolding is a process that results in changes in brain function through strengthening of existing connections, formation of new connections, and disuse of connections that have become weak or faulty.
We begin our review with a brief and broad overview of major behavioral theories of cognitive aging and then discuss what has been learned about the structure of the aging brain. Because our notion of scaffolding has developed largely from the results of functional imaging studies of aging, we then review the findings regarding frontal, mediotemporal, and ventral visual function from the neuroimaging and aging literature. In the next section, we present the STAC model and critical assumptions that describe how the brain continues to change and reorganize with age, along with key predictions of the model. We close with a summary of what issues associated with STAC are speculative or unresolved, and we propose directions for new research.
BEHAVIORAL MECHANISMS OF COGNITIVE AGING
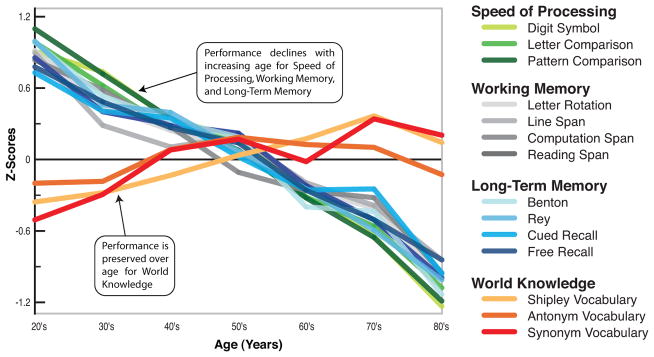
We have an extensive amount of knowledge about how cognition changes with age (Park & Schwarz 2000). As individuals age, many aspects of information processing become less efficient, including speed of processing, working memory capacity, inhibitory function, and long-term memory. At the same time, other aspects of cognitive function such as implicit memory and knowledge storage, are protected and relatively resistant to cognitive aging. A summary of typical findings from many different studies of cognitive aging appears in Figure 1, which depicts results from a lifespan sample of adults aged 20–89 who were tested extensively (Park et al. 2002). Park et al. (2002) collected three measures of perceptual speed, two measures of visuospatial working memory, and two measures of verbal working memory. In addition, subjects completed recall tests of both verbal and visuospatial recall, as well as three different vocabulary tests. The results clearly show that there are gradual age-related declines in the cognitive mechanisms of speed, working memory, and long-term memory, beginning in young adulthood. Only verbal ability, which is an estimate of accrued knowledge rather than a cognitive mechanism, is protected from age differences. Because these data are cross-sectional, it is possible that the observed differences are due to cohort effects or some other confound. However, data from the Victoria Longitudinal Study (Hultsch et al. 1999) show strikingly similar findings for speed of processing, working memory, list recall, and vocabulary, suggesting that the results are also found in longitudinal data and are not primarily due to cohort effects.
Figure 1.
Cross-sectional aging data adapted from Park et al. (2002) showing behavioral performance on measures of speed of processing, working memory, long-term memory, and world knowledge. Almost all measures of cognitive function show decline with age, except world knowledge, which may even show some improvement.
A major challenge for cognitive aging researchers has been to understand the causes of age-related declines in cognitive function. The general approach has been to isolate specific mechanisms and then determine whether the mechanism can account for the vast majority of age-related variance on a diverse set of cognitive measures. One dominant construct in the cognitive aging literature has been that of speed of processing. Salthouse (1991, 1996) has cogently argued that perceptual speed (measured by the rate at which individuals can make “same/difference” judgments about simple shapes, dot matrices, or strings of letters or digits) is a fundamental, cognitive primitive that accounts for nearly all of the age-related variance on a broad range of cognitive tasks. Salthouse (1991, 1996) has marshaled considerable evidence for this argument in an impressive research program that spans thousands of subjects and many different and creative methodological approaches.
A second important approach to understanding cognitive aging comes from evidence that working memory function decreases with age and, along with speed, mediates age-related variance on a broad array of cognitive behaviors (Park et al. 1996, 2002; Wingfield et al. 1988). Indeed, working memory, which encompasses both the short-term maintenance and active manipulative processing of information, figures prominently in a third view of cognitive aging that emphasize declines in executive control processes, namely inhibitory function (Hasher & Zacks 1988, Hasher et al. 2007). According to this view, age-related deficits in cognition stem from the inefficiency of inhibitory processes that normally control the contents of consciousness (i.e., working memory). Older adults show working memory deficiencies and slowing due to selection of irrelevant information into the contents of working memory, along with inefficient deletion of working memory contents that are no longer relevant to task performance. Inhibitory dysfunction with age is a source of general attentional dysregulation and accounts for age-related deficits in other cognitive domains such as task switching, response competition, and response suppression.
Further theorizing about aging has emphasized the distinction between automatic and effortful/controlled processing (Hasher & Zacks 1979), especially in reference to age-related changes in mechanisms specific to memory. Jacoby and colleagues ( Jennings & Jacoby 1993) have demonstrated that some memory differences with age are due to declines in controlled, but not automatic, processes resulting in poor explicit memory but relatively good memory for gist or versions of stimuli that feel familiar. Indeed, there is considerable support for the idea that in the absence of environmental support or explicit instruction, older adults engage less in controlled processes such as binding operations and elaborative processing that facilitates explicit recollective memory (Daniels et al. 2006). Congruent with this position, Johnson and colleagues (Chalfonte & Johnson 1996, Hashtroudi et al. 1989) have shown that age-related memory declines are characterized by poor memory for source and other contextual details associated with target information. Consequently, aging memory relies heavily on gist, making it highly susceptible to distortions and misremembering, as demonstrated by the impressive research program of Schacter and colleagues (Dodson & Schacter 2002, Koutstaal & Schachter 1997, Norman & Schacter 1997).
The effortful-automatic distinction dovetails nicely with other recent ideas about cognitive control processes and their decline with age (Braver & Barch 2002). Cognitive control operations guide thought and action in accord with task goals, especially when bottom-up, automatic, or prepotent stimulus-response associations must be overridden. Age-related declines in cognitive control make older adults more susceptible to the influence of automatic, bottom-up processes.
A different and increasingly influential idea about the sources of cognitive decline was put forward by Baltes & Lindenberger (1997, Lindenberger & Baltes 1994). Lindenberger & Baltes (1994) presented compelling evidence demonstrating that measures of audition and visual acuity are important predictors of performance on a broad array of cognitive tasks in an older adult sample. In a later study, Baltes & Lindenberger (1997) found that for younger adults (age 60 or less), there was no relationship between sensory function and measures of cognition, indicating that abilities that are independent in young adults become interrelated in old age. Baltes & Lindenberger (1997) argued, “the age-associated link between sensory and intellectual functioning may reflect brain aging” and further suggested, “visual and auditory systems evolve as powerful regulators of intellectual performance in old and very old age … [due to] a common process of aging-induced dedifferentiation.” Whereas young adults selectively engage specific mechanisms for different tasks, dedifferentiation in older adults leads to declining specialization with age. Dedifferentiation can be thought of mechanistically as a decrease in neural specificity and thus a broadening of neural tuning curves such that a given region that responds selectively in young adults will respond to a wider array of inputs in old adults. The concept of dedifferentiation is particularly important because it makes relatively specific predictions about neural function based on behavioral data and provides a strong link between behavioral-based and brain-based theories of cognitive aging.
In summary, age-related cognitive declines may be best understood in terms of a range of mechanisms including speed, working memory, inhibition, and cognitive control (Moscovitch & Winocur 1992, West 1996) that show varying degrees of vulnerability in different individuals. Although these mechanisms can all be categorized as executive processes, other sources of decline, such as dedifferentiation of cognitive function, must also be considered. Given the broad spectrum of cognitive changes with age, it is unlikely that any single process or unitary mechanism can fully explain age-related deficits across all individuals. As we argue below with respect to the STAC model, behavioral performance in older adults must ultimately be understood in terms of combined influence of age-related neurocognitive declines and age-related compensatory processes, many of which are also executive in nature. In the next section, we consider neurological evidence that provides additional constraints and new directions for theorizing about cognitive aging.
AGING AND THE STRUCTURE OF THE BRAIN
Volumetric Data
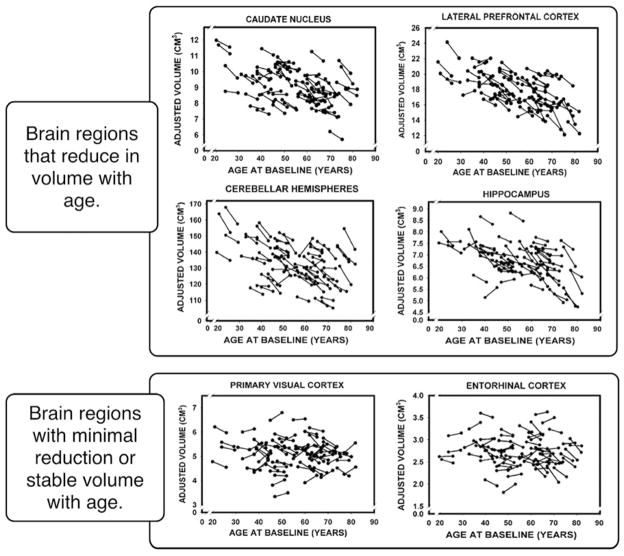
When neuroimaging tools became available to researchers, one of the first approaches to studying aging involved using structural MRI to determine age differences in the size of brain structures, typically measured by the volume of the structure. There is a surprising amount of convergence between the behavioral data on aging and cognition and the structural data from the brain. Figure 2 presents volumetric measures of a number of brain structures across the lifespan in a cross-sectional study (Raz et al. 2005). A particularly unique aspect of this study is that subjects were measured both initially and five years later, so the “spaghetti graph” shows individual measurements for each subject at both of these intervals. Figure 2 indicates that the greatest shrinkage across the lifespan occurs in the caudate, cerebellum, hippocampus, and prefrontal areas. There is minimal shrinkage in the entorhinal cortex, and the visual cortex volume remains stable across the lifespan (Raz 2000, Raz et al. 2004). Resnick and colleagues (2003) reported evidence for decline in gray and white matter over a period as short as two years in very healthy older adults over age 59, with frontal and parietal cortex showing greater decreases than temporal and occipital (see also Good et al. 2001).
Figure 2.
Cross-sectional and longitudinal aging brain volumes across various brain regions (adapted from Raz et al. 2005). Each pair of line-connected dots represents an individual subject’s first and second measurement. The caudate, hippocampal, cerebellar, and frontal regions all show both cross-sectional and longitudinal reduction in volume with age. The entorhinal, parietal, temporal, and occipital regions are relatively preserved with age.
Other measures relating brain structure to aging tell a similar story. Salat et al. (2004) examined measures of thinning in the cortical mantle in a lifespan sample of adults and reported that global thinning was apparent by middle age, with regionally specific thinning occurring in areas similar to those reported above. A particularly intriguing finding was their report of significant age-related atrophy in the calcarine cortex, an area near primary visual cortex, as other studies have suggested that visual cortex is largely preserved with age. The Salat et al. (2004) finding of both frontal and visual cortical thinning with age may provide the neurobiological link for the evidence reported by Baltes & Lindenberger (1997) that declining visual function in old age is closely linked to declining performance on cognitive tasks with significant frontal components.
White Matter
Besides thinning and volumetric shrinkage, studies also have measured the characteristics of white matter, which is composed of axonal bundles beneath the cortical structures of the brain. Diffusion tensor imaging measures the rate and direction at which water diffuses through white matter and provides an index of the density or structural integrity of the white matter. Head et al. (2004) note an anterior-to-posterior gradient across the brain for white matter integrity with age, such that the greatest structural deficiency in white matter is observed in anterior (frontal) regions of the brain. Another measure of white matter health is the number of white matter hyperintensities (WMHs) present in a structural scan. The hyperintensities represent an abnormality of signal from white matter, which likely results from demyelination, trauma, inflammatory disease, or other neural insults. Wen & Sachdev (2004) measured the number of WMHs in a large sample of adults 60–64 years of age. Subjects had an average of 19.1 WMHs, with the largest proportion of tissue affected in frontal and occipital areas. The WMH data suggest, as did the Salat et al. (2004) data on cortical thinning, that visual areas do show negative age-associated effects in structural measures. All subjects in the Wen & Sachdev (2004) study had some small WMHs, and at least half had severe WMHs. Overall, the white matter data, in agreement with volumetric data, present a clear picture of age-related decrease, with particular susceptibility in the frontal areas. The white matter changes are often considered a likely candidate to be the basis for age-related slowing of behavior, as the decreased integrity of the white matter tracts points to a less efficient system for information transmission with age.
Relationship of Structural Measures to Cognition
There is some evidence that age-related changes in volume of specific brain structures have direct consequences for cognitive function. Rodrigue & Raz (2004) reported that shrinkage of entorhinal cortex over a five-year period in an older adult sample predicted poor memory performance. Rosen et al. (2003) found evidence that both hippocampal and entorhinal volume were related to memory performance, although Tisserand et al. (2000) failed to find relationships between hippocampal volume and cognitive performance after controlling for age. The role of frontal volume in predicting cognitive function is not as straightforward as one might expect. Curiously, Salat et al. (2002) noted that increased orbital frontal volume correlated with declining working memory in older adults, and Gunning-Dixon & Raz (2003) found no relationship between prefrontal volume and working memory performance in a lifespan sample. Gunning-Dixon & Raz (2003) did find the expected relationship between prefrontal volume, age, and performance on the Wisconsin Card-Sorting Task—that is, that greater volume predicted better performance, but they failed to find a relationship between prefrontal volume and working memory. Gunning-Dixon & Raz (2003) also found that WMHs predicted performance on the Wisconsin-Card-Sorting Task but did not predict working memory performance. Wolfson et al. (2005) reported that mobility-impaired older subjects, over a 20-month period, showed a fivefold increase in WMHs relative to an unimpaired control group. The magnitude of the increase predicted some changes in mobility, outlining the importance of the accrual of these lesions for everyday functional abilities.
Dopaminergic Receptors
In addition to the structural measures described, techniques also exist for measuring the number of dopamine receptors in the brain. Dopaminergic receptors are integral to cognition because they play an important role in regulating attention and in modulating response to contextual stimuli. Li et al. (2001) suggest that the loss of such dopaminergic receptors is responsible for many of the cognitive aging effects described in this review. Support for this argument comes from computational modeling (Li et al. 2001) as well as imaging work. Wong et al. (1997) and Yang et al. (2003) used radioligands (a radioactive substance that, when injected, binds to dopamine and allows imaging of areas where receptors are located) to measure dopaminergic receptors, and found strong relationships among age, the number of receptors, and cognition. Backman et al. (2000) reported that literally all age-related variance on perceptual speed and episodic memory tasks was attenuated when dopamine receptor binding was statistically controlled. All of these data suggest that dopaminergic receptors play an important role in at least some aspects of cognitive aging. Whether the receptors are the major, or even sole, factor accounting for normal cognitive aging awaits further research.
At this point, we have painted a clear and relatively consistent picture of cognitive function and neural structures across the lifespan. Generally, cognitive function declines in parallel across the lifespan with decreasing brain volume, dopamine receptors, and white matter integrity. At the same time, direct relationships between declining structural measures of the brain and cognitive function are not always observed (Salat et al. 2002, Tisserand et al. 2000), and when they are observed, they are of a modest magnitude. White matter hyperintensities appear to have more significance than total brain volume, but knowledge about brain structure does not fully explain age-related variance in cognitive function.
FUNCTIONAL IMAGING AND VIEWS OF THE AGING MIND
Prefrontal Bilaterality
Functional imaging techniques (fMRI or PET, with most recent studies involving fMRI) allow cognitive neuroscientists to see the brain in action by measuring increases in blood flow and oxygenation in specific brain structures. The changes in blood flow are time-locked to the mental activities in which individuals are engaging so that one can see how brain activity changes as task demands change, with a resolution of about two seconds. Although this is much slower than the rates of many mental and neural processes, it is still the case that functional imaging research has been hugely informative in developing a more complex view of the aging mind than existed prior to the advent of this technique.
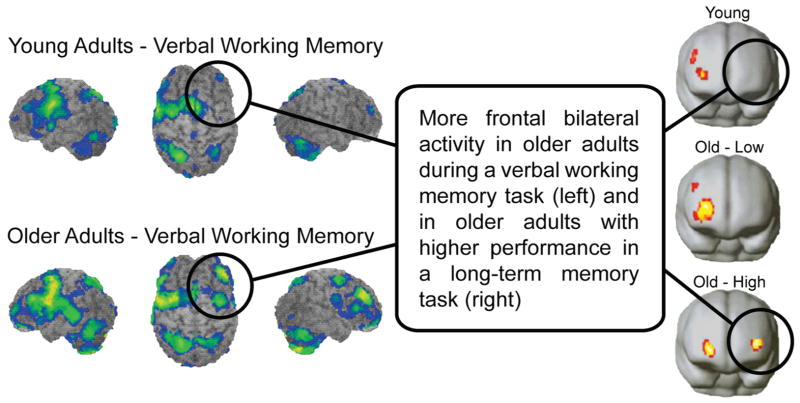
One of the most noteworthy features of functional data is the startling discontinuity that exists between patterns of neural activation in young and old adults. The behavioral and structural data suggest that one might expect to see declining neural activity with age, congruent with the declines in neural structures and cognitive behaviors. Quite surprisingly, initial studies, which focused on verbal working memory (Reuter-Lorenz et al. 2000) and verbal long-term memory (Cabeza et al. 1997, Grady et al. 1999), showed evidence for focal, left prefrontal activity in young adults, whereas older adults showed activation in both left and right pre-frontal areas, as shown in Figure 3. This bilateral activation seemed to suggest that the old brain was working harder and engaging in more distributed, compensatory processing to perform a task that was focal (unilateral) in young adults. A great deal of discussion immediately followed as to whether this appealing explanation was actually correct (Cabeza 2002, Park et al. 2001, Reuter-Lorenz 2002). Perhaps the additional activation in the contralateral hemisphere reflected inefficient operation of inhibitory mechanisms, and this activation was actually interference and a major cause of some of the behavioral deficits observed in cognitive aging.
Figure 3.
Frontal bilaterality is increased with age. (Left side) Left lateralized frontal engagement in young adults during a verbal working memory task; in older adults, an additional right frontal engagement is observed (adapted from Reuter-Lorenz et al. 2000). (Right side) Right lateralized engagement in young adults and low-performing older adults during a long-term memory task, and bilateral frontal engagement in high-performing older adults (adapted from Cabeza et al. 2002).
Since these initial observations and discussions occurred, much more has been learned about the role of additional bilateral, and in many instances, prefrontal, activity in the aging brain. There is now a corpus of evidence suggesting that additional contralateral recruitment is indeed functional and supportive of cognition in older adults. The first finding is the tendency for older adults to engage both the left and right hemispheres, which has been associated with higher performance in older adults in comparison with younger adults (Cabeza et al. 2002, Reuter-Lorenz et al. 2001, Rypma & D’Esposito 2001). Likewise, in a category-learning task, older adults showed greater parietal activation bilaterally than did young adults, and this bilateral activity was associated with higher performance (Fera et al. 2005). Divided visual field experiments manipulating unilateral versus bilateral hemispheric engagement in task performance provided converging evidence that older adults showed unique benefits from engaging both hemispheres (Cherry et al. 2005, Reuter-Lorenz et al. 1999).
A second key finding is that overactivation of prefrontal regions has been specifically linked to improved memory in older adults. In particular, a study of subsequent memory effects of picture encoding in young and older adults (Gutchess et al. 2005) showed heightened parahippocampal activation in young compared to old for pictures that were later correctly remembered. In contrast, older adults showed heightened middle frontal activation for items that were remembered. Thus, increased frontal activation in older adults was uniquely associated with remembering. Moreover, for older adults, high levels of inferior frontal activation were associated with low levels of parahippocampal activation, suggesting that higher frontal activity may be compensatory for decreased mediotemporal activations. Morcom et al. (2003) also showed evidence for more frontal bilaterality in older adults compared to young when encoding subsequently remembered words. In a later study, Cabeza et al. (2004) confirmed a pattern of increased frontal bilaterality and decreased hippocampal activation in older adults that was shared across tasks of attention, working memory, and long-term memory, demonstrating the global nature of this pattern.
A third line of evidence favoring a compensatory interpretation of bilaterality with age comes from a longitudinal study by Persson et al. (2006). They demonstrated that older individuals who showed the most shrinkage in hippocampal volume over a ten-year period had poorer memories but also showed the greatest additional activation in right prefrontal cortex (Persson et al. 2006). Hence, individuals who endured the greatest neural insults and memory decline also showed the greatest extra pre-frontal activation.
Studies that used repetitive transcranial magnetic stimulation (rTMS) in young and older adults have provided especially compelling evidence in favor of contralateral activity enhancing cognitive function in older adults. rTMS is a technique that transiently disrupts neural function by applying repetitive magnetic stimulation to a specific area of the brain, creating highly focal and temporary “lesions.” In a seminal study conducted by Rossi et al. (2004), young and older adults studied pictures while rTMS was applied to the subjects’ left or right dorsolateral prefrontal cortex (DLPFC); the subjects then made recognition judgments while rTMS was again applied to the left or right DLPFC. One of the most interesting finding from this work was that young adults’ memory retrieval accuracy was more significantly affected when the rTMS was applied to the left compared to the right hemisphere. In contrast, older adults’ retrieval was equally affected by the rTMS, whether it was applied to left or right, suggesting that the activations in both hemispheres were useful for performing the recognition task in older adults. In another line of work, memory improvement was demonstrated in older adults using rTMS in a “stimulating mode” to prime or increase the activation of the underlying prefrontal circuitry (Sole-Padulles et al. 2006). fMRI confirmed that older adults who showed greater increases in prefrontal activation post-rTMS also showed the greatest memory improvement. In summary, converging evidence from a range of studies using different approaches suggests that the additional age-associated neural activation, especially in prefrontal areas, appears to be functional and to enhance task performance. Many researchers and models have described these activation increases as “compensatory” and suggestive of an adaptive brain that functionally reorganizes and responds to neural aging, a view of aging that would have been highly unlikely from the behavioral data alone.
Compensation for What?
If one wishes to make the argument that the additional activation in prefrontal areas evidenced by older adults is functional and adaptive, it becomes important to specify for what aspects of neural aging the prefrontal activations are compensating.
Deficient hippocampal activations
One obvious possibility, based on the discussion thus far, is that the compensation is for the structural changes that occur in the brain in terms of both volumetric decrease and white matter integrity. Indeed, Persson et al. (2006) demonstrate important functional increases in pre-frontal activations associated with hippocampal shrinkage and memory loss. There are other candidates, however, for the cause of functional compensation besides structural changes and age-associated neural insults. Park & Gutchess (2005) reviewed functional activations associated with long-term memory and noted the frequency with which decreased hippocampal/parahippocampal activations are observed in older adults in the same context that the increased frontal activations are observed. They argued that enhanced prefrontal activity is due to a declining activation in mediotemporal areas, an argument confirmed by Gutchess et al. (2005) in a study that contrasted remembered pictures with forgotten pictures in old and young adult subjects. The Persson et al. (2006) findings are also congruent with such an interpretation, and a wealth of evidence suggests that both hippocampal structure (Driscoll et al. 2003) and function (Daselaar et al. 2003, Johnson et al. 2004, Park et al. 2003) are deficient with age (although see Dickerson et al. 2004 for evidence that mild cognitive impairment is associated with greater activation in hippocampal/parahippocampal structures—a finding somewhat at odds with the present argument).
A different view of the role of hippocampal function has been suggested by Buckner (2004). He proposes that age-associated frontal shrinkage along with increases in frontal activation are typical of normal aging, whereas declining hippocampal/entorhinal volume and activation is associated with pathological aging and is a marker for subclinical dementia. Both the Buckner (2004) proposition and the frontal compensation for hippocampal deficits argument could be correct, if one considers the possibility that both bilaterality and hippocampal function exist on a continuum. Perhaps within the realm of healthy elderly, one sees a compensatory relationship between the two structures, but shrinkage and dysfunction may reach a critical point as pathology increases, and at that point, the reciprocity no longer exists.
Dedifferentiation as an impetus for compensation
In addition to decreased hippocampal activation, there is growing evidence that ventral visual and sensory cortex may show less activation and neural specificity with age. On the surface, this is a surprising pattern of findings because the volume of these structures is largely preserved with aging. However, clear evidence exists for alterations in these structures with age when one examines the neural specificity of activations to categories of perceptual input. It is well known that young adults show activations that are highly specific to faces in left and right fusiform areas of ventral visual cortex (Kanwisher et al. 1997). They also show category-specific activations to pictures of places, houses, and outdoor scenes in the parahippocampus (Epstein & Kanwisher 1998) and to words and numbers in the left fusiform gyrus and collateral sulcus (Polk et al. 2002, Puce et al. 1996). Based on the Baltes & Lindenberger (1997) evidence that sensory and cognitive function is dedifferentiated with age relative to young adults, one might expect these areas to be less specific in older adults. Park et al. (2004) investigated this hypothesis and discovered that in comparison with young adults, older adults show markedly less neural specificity in the fusiform face area, parahippocampal place area, and the lateral occipital area specialized for letters. The notion that neural tissue that is highly specialized in young adults dedifferentiates or becomes less specific in old was reinforced by the work of Chee et al. (2006), who utilized an adaptation paradigm (Malach et al. 1995) to study neural response in ventral visual cortex. In this paradigm, neural response becomes increasingly less pronounced as a visual stimulus is repeated and one can map which areas are specialized for which functions by varying elements of the stimuli. Chee et al. (2006) demonstrated decreased specificity in older adults for object recognition in the lateral occipital cortex and decreased binding of target to context in the hippocampus, but also showed relatively intact processing of background information in the parahippocampal place area. Payer et al. (2006) also reported evidence for decreased specificity of face and place areas on a working memory task. Given these alterations in ventral visual processing, perhaps some of the increased activation of frontal cortex provides additional compensatory processing to recognize and differentiate categories as we age.
A different approach to the frontal/sensory issue was taken by Cabeza et al. (2004), who noted a preponderance of increased frontal and decreased sensory activation in old compared to young subjects across attentional, working memory, and long-term memory tasks. In a later study, Davis et al. (2007) further confirmed this shift from posterior brain activations to anterior activations, and suggested that the increased frontal activation that occurs with age is in response to deficient ventral visual and sensory activations. Overall, there is growing evidence that the additional work of the frontal sites may be a broad response to decreased efficiency of neural processes in perceptual areas of the brain.
Compensation and the default network
Functional imaging measures changes in blood flow relative to some baseline. During baseline, subjects typically lay quietly in the magnet and fixate on a plus sign against an otherwise blank screen. The default network refers to the sites that are activated during the baseline interval when the brain is supposedly at rest and includes sites across frontal, parietal, mediotemporal, and visual areas such as the posterior cingulate, middle frontal cortex, the lateral parietal region, and the lingual gyrus (Raichle et al. 2001). Perhaps the most notable aspect of the default network is that it is suppressed when the brain shifts to a demanding cognitive task (Greicius et al. 2003). However, a number of authors have found that older adults show significantly less suppression of the default network than young adults (Grady et al. 2006, Persson et al. 2007), and that the failure to suppress is actively related to lower performance on some cognitive tasks (Damoiseaux et al. 2007, Persson et al. 2007). It seems plausible that another cause of increased frontal activity in older adults is a failure to shift out of this relaxation or default state into more active modes of cognitive processing (Reuter-Lorenz & Cappell 2008, Reuter-Lorenz & Lustig 2005).
THE SCAFFOLDING THEORY OF COGNITIVE AGING
In the previous sections, a broad range of behavioral and neural data from the cognitive aging literature has been discussed. Little doubt exists that the extent of cognitive and structural decline is substantial. Nevertheless, people generally function remarkably well even into advanced old age, and do so even in the presence of a great deal of pathology as discovered at autopsy (T. W. Mitchell et al. 2002). The puzzle for cognitive neuroscientists is not so much in explaining age-related decline, but rather in understanding the high level of cognitive success that can be maintained by older adults in the face of such significant neurobiological change.
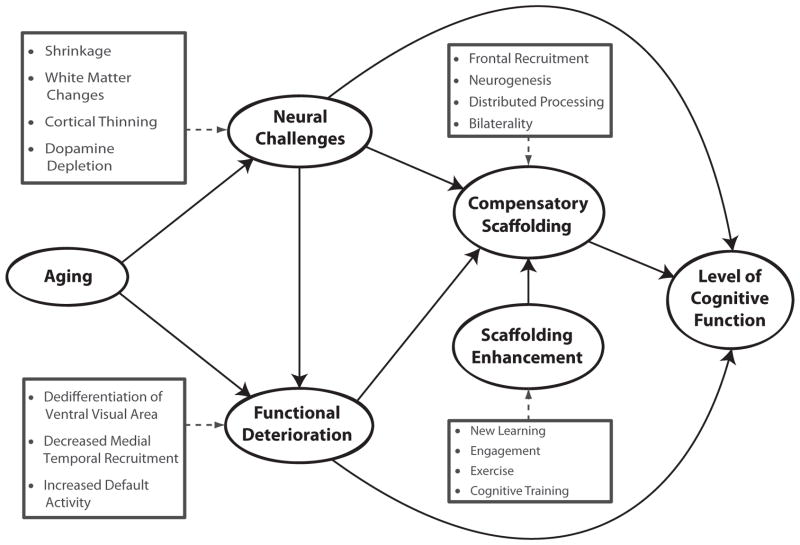
We propose the scaffolding theory of cognitive aging as a testable model for resolving this puzzle (see Figure 4). STAC posits that behavior is maintained at a relatively high level with age, despite neural challenges and functional deterioration, due to the continuous engagement of compensatory scaffolding—the recruitment of additional circuitry that shores up declining structures whose functioning has become noisy, inefficient, or both. The pervasive evidence in the functional brain-imaging literature of greater bilateral activation and overactivation of frontal areas in older adults reflects the engagement of “compensatory scaffolding”—the patterns of brain activation that include both declining networks and the associated compensatory circuitry recruited to meet the task demands. STAC also provides for mechanisms that can bolster compensatory scaffolding. The joint operation of these declining and compensatory forces, as shown in Figure 4, determines cognitive function in late adulthood. Below, we summarize major tenets of STAC, detail predictions from the STAC model, and describe how the STAC model can be distinguished from other views of cognitive aging.
Figure 4.
A conceptual model of the scaffolding theory of aging and cognition (STAC).
Scaffolding is a Dynamic, On going Property of an Adaptive Brain
Scaffolding is a process that characterizes neural dynamics across the lifespan. It is not merely the brain’s response to normal aging; it is the brain’s normal response to challenge. The concept of scaffolding has been used to explain the brain’s response to novel skill acquisition in young adults. To acquire a novel skill, an initial set of neural circuits must be engaged and developed that provides the structure for task performance in the early skill-acquisition stages (Petersen et al. 1998; see also Church et al. 2008 for an example of scaffolding in early development). As learning proceeds, performance becomes less effortful and with enough practice, becomes overlearned. By this stage of over-learning (some would call this skilled performance), the circuitry has shifted from a broader, dispersed network to a specific, honed, and optimal circuit of neural regions that are functionally interconnected to mediate efficient processing and storage. Petersen et al. (1998) noted that the regions that provided scaffolding at the early stages of practice continue to be minimally active even as more-specific regions assume task control. This suggests that the initial scaffolding may remain available as secondary or functionally ready circuitry that can be recruited when performance takes place under challenge.
Extending Petersen et al.’s (1998) argument, we suggest that reliance on such secondary networks or scaffolds may be an important aspect of healthy cognitive aging and provide considerable support toward the maintenance of cognitive function as age-associated structural deterioration increases. We presume that scaffolding processes operate with more efficiency in youth, when scaffolding is typically and frequently engaged to meet novel situations and to achieve new learning. With age, scaffolding processes may be invoked to perform familiar tasks and basic cognitive operations as these processes become increasingly challenging with the degredation of existing neural circuitry. We speculate that perhaps the maintenance of language in old age results from the continuous use of language throughout life and a particularly elaborate scaffolding network for this behavior that is both critical and overlearned. We view the recruitment of neural scaffolds as a normal adaptive response of the brain that takes place throughout the lifespan. In response to the challenge neurobiological challenges of aging, new scaffolds can be established, or previously established scaffolds acquired in early development or during new learning can be recruited.
Prefrontal Cortex is a Primary Locus for Scaffolding
Scaffolding can be understood as circuits that provide supplementary, complementary, and, in some cases, alternative ways to achieve a particular behavioral output or cognitive goal. Once it is fully developed, the prefrontal cortex is the most versatile structure in the brain. In the aging brain, scaffolding processes largely reside in this structure. There may be flexibility in other areas as well, and this is an important area for exploration. Not surprisingly, as reviewed above, prefrontal circuits figure prominently in age-related overactivation. In fact, the initial evidence that led to compensatory models of frontal activation (Cabeza 2002) was centered in regions of inferior, lateral, and rostral regions of the prefrontal cortex.
It is important to recognize that some parts of the brain are dedicated to the performance of specific tasks and may not easily play a role in scaffolding, and we hypothesize that scaffolding is frequently erected and engaged to compensate for deficits in these areas. The ventral visual cortex is an example of dedicated circuitry focused on computing the form-based properties of visual input. Such dedicated areas are functionally altered with age, as the dedifferentiation of ventral visual cortex research suggests (Chee et al. 2006, Park et al. 2004, Payer et al. 2006), and it is the poor function of such areas which require the utilization of scaffolding. The hippocampus is another dedicated structure that performs specific functions such as binding and relational processing (N. J. Cohen et al. 1999); both its structure and function decline with age (K. J. Mitchell et al. 2000, Small et al. 2000). Hence, it is likely another neural structure for which scaffolding is required if cognitive performance is to be optimized in the older adult (Park & Gutchess 2005). Accordingly, the pervasive tendency for age-related overactivation of prefrontal sites coupled with underactivation in more-posterior cortices of the occipital and temporal lobes (Davis et al. 2007) is consistent with the notion that pre-frontal circuitry provides scaffolding for age-related inefficiencies of dedicated, hard-wired cortical circuits.
Scaffolding is a Neurocognitive Response to Challenge
Challenges to the neural system can be extrinsic, such as when the brain is confronted with novel, unanticipated, or increased levels of task demand. But challenge can also be intrinsic, such as when the neural circuitry is altered metabolically or structurally. Intrinsic alterations can be transient, as in states of sleep deprivation (Drummond et al. 2004), or continuous, as in the case of biological aging. Because the circuits engaged for scaffolding are not entirely arbitrary, there are similarities in the brain’s response to different forms of challenge. Consider the challenge posed by increasing levels of task demand. Banich (1998) demonstrated that younger adults engage in processing that becomes increasingly bilateral as task complexity increases. Older adults also show patterns of bilateral neurocognitive engagement, but at lower levels of task demand (Reuter-Lorenz et al. 1999). In this way, the brain’s response to extrinsic challenge (higher task demand in young adults) resembles its response to intrinsic challenge (aging). Similarly, in verbal working memory, neurocognitive operations that are sufficient for small loads are inadequate for larger memory loads. Younger adults respond to this challenge by increasing recruitment of right dorsolateral prefrontal cortex (Hillary et al. 2006, Reuter-Lorenz & Cappell 2008). Thus, right DLPFC provides the scaffolding to meet the extrinsically imposed challenge. Likewise, in older adults, right DLPFC activation is evident even at low levels of task demand because biological aging of the brain has resulted in intrinsic conditions that require compensatory scaffolding.
Consistent with the notion that scaffolding occurs in response to challenge are data demonstrating right frontal overactivation in response to other forms of intrinsic challenge posed by disease states such as multiple sclerosis (Hillary et al. 2006) and possibly such interventions as chemotherapy. Scaffolding is not unique to the intrinsic challenge posed by aging. Aging constitutes ongoing and increasing levels of challenges that persist at lower levels of intensity throughout adulthood—continually activating the brain’s adaptive response to its own aging (Reuter-Lorenz & Mikels 2007).
Scaffolded Networks are Less Efficient than Honed Cognitive Networks
As new skills are acquired, performance becomes more efficient and less error prone. At the neural level, this increased efficiency can be explained by the establishment of efficient circuitry that is highly functionally interconnected, which includes honing the neural circuitry through practice and decreasing reliance on the rudimentary, scaffolded stages of skill acquisition. In contrast, with aging, declining neurobiological efficiency of such honed networks leads to increasing reliance on scaffolding, the erection of new scaffolds, or some combination of these compensatory processes. Consequently, performance is less efficient than when it is mediated by the finely honed network in the younger brain. Note, however, that without the compensatory engagement of scaffolded circuits, the older brain would have to rely exclusively on honed networks that are in decline, resulting in poorer performance overall.
The Aged Brain is Less Efficient at Generating Scaffolding, and Pathology May Entirely Limit Scaffolding Operations
Although plasticity is evident throughout the lifespan, age-related declines in neurobiological regeneration will necessarily limit the scaffolding capacity of the aging brain (Burke & Barnes 2006). At the same time, the need for scaffolding is substantial because of reduced efficiency of dedicated circuits. As aging proceeds, the need for compensatory scaffolding exceeds the capacity for plasticity and reorganization, leading to the more frank expression of cognitive loss in the oldest old (Baltes & Mayer 1999). Buckner et al. (2005) utilized five imaging techniques that included visualization of amyloid deposition (a plaque deposited on neurons that is associated with Alzheimer’s disease), and interestingly, high deposits were also associated with high levels of default activity as well as with decreased activation in posterior cortex. Buckner et al. (2005) speculate that increased default activity with aging may enhance amyloid deposition and the promotion of Alzheimer’s disease. Lustig et al. (2003) have shown an association between Alzheimer’s disease and default activity. In our view, Alzheimer’s disease represents the conjoint influence of severe pathological challenge and sustained disintegration of the reparative process due to an attack on cellular health that limits the ability to engage in scaffolding. Ultimately, neural pathology penetrates the scaffolding, leading to selective and eventually total collapse of the scaffolding and the structure it is protecting.
Individual Variability and the Factors that Promote Scaffolding
The causes of cognitive aging are multifactorial, and individuals will vary in both the magnitude of decline and the amount of protective scaffolding that can be engaged. Greater degrees of structural or functional aging could occur due to genetic susceptibility (e.g., hetero-or homozygosity for APOE4) to some diseases (e.g., hypertension), adverse experiences (e.g., chemotherapy), or advanced age. Individual differences could also result from the readiness and efficiency of scaffolding. More effective scaffolding could be the result of higher levels of physical fitness, cognitive stimulation, or other factors that promote scaffolding activity (see next section). Preservation of cognitive function with age could thus occur due to a slow rate of cognitive aging or particularly efficient scaffolding mechanisms. Presumably, individuals who have exceptionally high cognitive ability in old age would be those with low genetic susceptibility to biological aging and with highly effective scaffolding.
Scaffolding is Promoted by Training and Cognitive Activity
A wealth of evidence from experimental studies with nonhuman animals suggests that changes in cortical structures can result from external challenge. In a seminal study, Zhou & Merzenich (2007) demonstrated that adult rats that had degraded auditory cortices early in life were able, with intensive discrimination training in adulthood, to develop normal auditory function. The training resulted in remapping of the auditory cortex through top-down neuromodulation rather than from revitalization of auditory neurons degraded early in life. The STAC model is reminiscent of this finding, as it suggests that top-down frontal function may modulate age-related losses in perceptual function occurring at the level of ventral visual cortex. There is also evidence that the enhancement of brain-derived neurotrophic factor and serotonin increases cognitive performance in older animals (Mattson et al. 2004), and we propose that these substances could play an important role in maintenance of the ability to scaffold or develop mechanisms for an aging brain. Similar to the findings evidenced by the Zhou & Merzenich (2007) data, the mechanism for enhancement of brain-derived neurotrophic factor appears to be external challenge, either in the form of exercise (Cotman & Berchtold 2002, Kramer et al. 2004) or, more speculatively, cognitive challenge. There is also evidence that depression down-regulates these substances (Tsai 2003), which could impair scaffolding abilities and provide some of the basis for decreased cognition with depression.
Studies of aged animals also reveal increased neural volume as a result of cognitive challenge. Kempermann et al. (1998) demonstrated that old rats that had been maintained in complex visual and play environments showed birth of new neurons in the hippocampus in old age, unlike control subjects. Kobayashi et al. (2002) reported improved learning ability in rats exposed to an enriched environment, even in old age, and concluded, “These results show that aged animals still have appreciable plasticity in cognitive function and suggest that environmental stimulation could benefit aging humans as well.” Kempermann et al. (2002) reported a similar effect and demonstrated that short-term exposure of rats to an enriched environment, even in old age, led to a fivefold increase in hippocampal neurogenesis as well as a decrease in age-related atrophy in the dentate gyrus.
Although no direct experimental studies have manipulated the relationship of sustained cognitive challenge to enhanced neural structure in humans, a wealth of studies link sustained cognitive engagement across the lifespan to higher levels of cognitive function as well as to delayed age of onset for dementia. Schooler et al. (1999) reported that individuals who engaged in complex work into late adulthood showed increased intellectual functioning, and in another study, Bosma et al. (2003) reported a similar finding. Other data indicate that high levels of education and occupational attainment protect against the risk of Alzheimer’s disease (Stern et al. 1994). Finally, a number of studies have reported that highly educated people who tend to be involved in more cognitively stimulating activities are more cognitively resilient in the early stages of Alzheimer’s disease (Bennett et al. 2003, Wilson & Bennett 2003, Wilson et al. 2000), even after controlling for other related variables. The scaffolding model not only predicts these effects, it delineates a specific mechanism—the creation of additional neural connections and possibly neural tissue—that controls the observed effects. It is also possible, if not probable, that cognitive training promotes scaffolding and could be an important mechanism for understanding broadly facilitative training effects as opposed to narrow and specific effects.
It should be noted here that the scaffolding model bears some relationship to ideas of cognitive reserve advanced by Stern (2002), who suggests cognitive reserve is “using brain networks that are less susceptible to disruption.” He also discusses compensation and describes it as “using brain structures or networks not normally used by individuals with intact brains in order to compensate for brain damage.” In the Stern (2002) model, cognitive reserve (the use of pathways resistant to disruption) is invoked when the brain is under challenge, with high-ability individuals having more of these pathways. We suggest that individual differences in cognitive reserve may determine the quality, quantity, and/or effectiveness of scaffolding, and in this respect, the two models may be complementary. Therefore, STAC is related to cognitive reserve but differs in some critical ways. The particular strength of STAC is that it considers the engagement and erection of scaffolding a normal and adaptive neural response that is utilized in the face of challenge across the entire lifespan, rather than a process specific to old age. Rudimentary processes provide scaffolding for the establishment and, later on in life, the preservation of more complex processes. In this respect, the engagement of scaffolding need not be a sign of pathology or abnormality. Moreover, according to STAC, the engagement of scaffolding is inherently a compensatory response; the net result may be that performance is less efficient than it would be with intact, finely honed circuitry, but presumably less impaired than if the scaffolds were not engaged. The compensatory function of scaffolding, similar to compensation in the Stern model (use of non-normal pathways), occurs when existing circuitry can no longer meet the task requirements and the brain develops new pathways.
Greenwood (2007) provides a detailed review of relevant biological processes and a nuanced analysis of some imaging results in a paper that proposes a functional plasticity account of aging, wherein cortical thinning initiates reorganization and age-related recruitment changes. The Greenwood perspective on plasticity is in striking agreement with many of the ideas expressed here. However, a key difference is STAC’s conception of scaffolding as a lifelong and continuously adaptive process that utilizes prior and newly formed scaffolds in response to ongoing age-related neurobiological declines.
By its treatment of scaffolding as a lifelong neural response to challenge, STAC provides an explicit model that integrates structural, functional, and experiential data to understand cognitive function with age.
PREDICTIONS OF THE SCAFFOLDING THEORY OF COGNITIVE AGING
We have presented a broad model that integrates a disparate array of findings across many domains of cognitive neuroscience and aging. Below, we consider some testable hypotheses that are logical extensions of STAC.
Scaffolding is a direct response to the magnitude of neural insults that occur with age
Thus, decreases in dopamine receptors, degree of white matter health, and magnitude of shrinkage of neural structures should all predict an increase in scaffolding. It is important to recognize that a curvilinear relationship may exist between degree of scaffolding and neural insults, as a brain can be sufficiently damaged such that scaffolding will no longer be possible.
Scaffolding is not arbitrary
This essentially suggests that brain structures are not interchangeable. For example, Reuter-Lorenz et al. (2001) hypothesized that storage deficits can be compensated for by heightened executive processing, but that the opposite is unlikely. The most likely sites for scaffolding to occur is in contralateral, homologous structures [see recent evidence by Putnam et al. (2008) relating white matter anisotropy to homologous frontal recruitment] or in penumbral areas directly surrounding focal areas for activation (as in stroke patients; Saur et al. 2006, Ward & Cohen 2004). It is important to assess which type of scaffolding is most effective. We hypothesize that subjects who show enhanced bilaterality or enhanced activation in penumbral areas are showing evidence of scaffolding that may be particularly efficient. Scaffolding may also occur by activating areas that are responses to higher levels of challenge in younger adults (Reuter-Lorenz et al. 1999).
Scaffolds, although not arbitrary, may show more site variability with age than does the primary site of activation
As the brain ages, the most efficient site for a scaffolded response may vary depending on which areas of the brain are most healthy within a given subject. For example, a given subject might compensate for diminished function in the fusiform face area by recruiting from the penumbra (see an interesting paper by Baker et al. 2005 on function reorganization of visual cortex with macular degeneration), from a homologous area, or by deployment of additional frontal resources. To the extent that different subjects compensate from different sites, group analyses may be misleading. Specific analytic tools and procedures that allow for the study of individual differences, including functional connectivity and pattern analysis, will be helpful in understanding and identifying functional scaffolding.
Younger brains that use scaffolding characteristic of older adults are at risk for poorer performance and accelerated aging
Reliance on scaffolding is less efficient than using a finely honed network. Middle-aged adults who show extensive scaffolding may be at risk for later pathology and more pronounced age deficits. This pattern of findings is congruent with those reported by Persson et al. (2006) who found a relationship between hippocampal shrinkage and increased right frontal recruitment over 10 years in a longitudinal study. Younger brains that need to rely on scaffolding presumably do so in response to some inefficiency or underlying vulnerability that may be subclinical and potentially measurable behaviorally given sufficient challenge. For example, Smith et al. (2001), using PET and operation span, found that poorly performing young adults showed the same extended activation pattern as that of older adults.
Although we think of scaffolding as primarily functional recruitment, scaffolding can also be instantiated as structural changes in neural sites. For example, the well-known London taxicab driver study (Maguire et al. 2000) demonstrated that cab drivers had larger hippocampi than did controls, suggesting that perhaps the sustained engagement of the structure for wayfinding increased its volume. This argument’s credibility was enhanced by the finding that the more experienced (and older) the driver was, the larger the difference relative to controls. This finding, along with others in the animal literature, suggests that scaffolding could also take the form of structural changes (including neurogenesis) in the brain.
Compensatory scaffolding can both be created and dissipated by training
Scaffolding is created as an individual acquires a new task or strategy, but paradoxically, training may also result in a decrease in reliance on scaffolding and more reliance on the primary, honed network. We tentatively hypothesize that when older adults are already relying on overactivation relative to young adults for task performance, the target for training should be to decrease activation in these secondary scaffolded areas and improve the efficiency of honed networks. This will lead to a more efficient use of neural resources. Similarly, if older adults show significant under-activation or deterioration of a network (e.g., dedifferentiated ventral visual function), the focus of training should be to establish new scaffolds in penumbral areas to perform a task that is being done inefficiently or not at all.
Creating novel scaffolds through training for task performance is possible but particularly effortful
The rehabilitation literature illustrates the possibility that entirely new neural circuitry can be developed from thousands of repetitions. Are there areas of the brain that are particularly amenable to novel scaffolds, and if so, what types of scaffolding tasks are they best suited to perform (e.g., sensory, motor, or cognitive)? Cognitive neuroscientists should begin to map areas that are particularly amenable to scaffolding of different types. This would yield huge gains in developing tools and techniques for maintaining maximum function of the human mind in late adulthood.
CONCLUSION
The STAC model integrates the impact of biological aging and experience to account for the neural reorganization of function that occurs in late adulthood. STAC invokes a scaffolding mechanism as a basis for understanding neurocognitive aging—a mechanism that has been used in educational, developmental, and rehabilitative contexts to describe how existing strengths can be harnessed to build new skills or recover and sustain capabilities that have been threatened by challenge. STAC therefore places neurocognitive aging within the context of both plasticity and challenge, and by so doing draws potentially informative parallels between the brain’s response to aging, early development, new skill acquisition, and both transient and chronic states of disorder, including sleep deprivation, neurological lesions, and other pathological stressors.
According to STAC, the performance of older adults must be understood in terms of multiple determining factors: those that influence decline and those that influence compensatory scaffolding. The speculations we offer about what these factors may be point to guidelines for successful aging and for the development of interventions that may not only forestall declines, but also promote the potential for effective scaffolding. STAC embraces the brain’s lifelong potential for plasticity and its ability to adapt to its own aging. We recognize that many questions remain unanswered by STAC and that some aspects of the model are quite speculative. Nevertheless, STAC provides a broad integrative framework for understanding the relationship of structural and functional changes in the brain in combination with life experiences to understand levels of cognitive function in late adulthood.
The need to understand how to preserve or enhance cognitive function in late adulthood could not be more urgent. We believe some of the most significant scientific breakthroughs of the twenty-first century will involve behavioral and pharmacological interventions to preserve cognitive function and forestall the onset of dementing disorders. Theoretical models such as STAC that provide a blueprint for investigation of mechanisms associated with preservation and positive change provide clear direction for important questions. Happily, increasingly sophisticated behavioral and imaging techniques for understanding the mind provide methodologies to find answers.
SUMMARY POINTS.
The basic hardware of cognition significantly declines with advanced age, although knowledge and expertise are relatively protected from age-related decline. Neural structure also shows changes. Many brain structures show significant shrinkage, the integrity of the white matter decreases, and dopamine depletion occurs.
In contrast to the age-related declines in cognitive function and brain structure, functional brain activity increases with age, particularly in the frontal cortex. The proposed scaffolding theory of aging and cognition suggests that this increased functional activity is due to compensatory scaffolding—the recruitment of additional circuitry with age that shores up declining structures whose function has become noisy, inefficient, or both.
Prefrontal cortex is the most flexible structure in the brain, and brain scaffolding processes in the aging brain largely reside in this structure.
Scaffolding is the brain’s response to cognitive challenge and is not unique to aging. Aging simply results in more frequent cognitive challenges at lower levels of intensity.
Scaffolded networks that develop with age may be less efficient than the original, direct, and finely honed networks developed at younger ages.
The aged brain is less efficient at generating scaffolding, and significant pathology (as occurs in advanced Alzheimer’s disease) may entirely limit scaffolding operations.
The causes of cognitive aging are multifactorial, and individuals will vary in both the magnitude of decline and the amount of protective scaffolding that can be activated. Presumably, individuals who have exceptionally high cognitive ability in old age would be those with low genetic susceptibility to biological aging and a high level of scaffolding generation.
Scaffolding is promoted by cognitive activity. A wealth of evidence in the animal literature suggests that changes in cortical structures can occur as a result of external challenge, and growing evidence suggests that humans develop scaffolds as a result of stimulating experiences.
FUTURE ISSUES.
If scaffolding is a response to neural insults with age, is there a direct relationship between brain degradation and degree of scaffolding?
Does the occurrence of compensatory scaffolding at a younger age (say in middle age) predict cognitive frailty in later adulthood?
What activities promote brain health? Can we develop a set of lifestyle changes that would be protective of the brain in late adulthood?
How much do specific types of cognitive training change the brain? What type of training is most effective in sustaining cognitive health?
Are certain types or sites for neural scaffolding particularly effective (e.g., bilateral recruitment of frontal areas versus penumbral activation)?
Acknowledgments
This work was supported by a grant from the National Institute on Aging (5R37AG006265–24), whose support is gratefully acknowledged. Additionally, the authors thank Andy Hebrank and Blair Flicker, who provided a great deal of support in manuscript preparation.
Glossary
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- STAC
scaffolding theory of aging and Ccognition
- WMHs
white matter hyperintensities
- rTMS
repetitive transcranial magnetic stimulation
- DLPFC
dorsolateral prefrontal cortex
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
Denise C. Park, Email: denise@utdallas.edu.
Patricia Reuter-Lorenz, Email: parl@umich.edu.
LITERATURE CITED
- Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry. 2000;157(4):635–37. doi: 10.1176/ajp.157.4.635. [DOI] [PubMed] [Google Scholar]
- Baker CI, Peli E, Knouf N, Kanwisher NG. Reorganization of visual processing in macular degeneration. J Neurosci. 2005;25(3):614–18. doi: 10.1523/JNEUROSCI.3476-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. Describes the surprising relationship between sensory function and cognition in very old age, utilizing a large lifespan sample of adults. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Mayer KU. The Berlin Aging Study: Aging from 70 to 100. New York: Cambridge Univ. Press; 1999. [Google Scholar]
- Banich MT. The missing link: the role of interhemispheric interaction in attentional processing. Brain Cogn. 1998;36(2):128–57. doi: 10.1006/brcg.1997.0950. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–15. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- Bosma H, van Boxtel MP, Ponds RW, Houx PJ, Burdorf A, Jolles J. Mental work demands protect against cognitive impairment: MAAS prospective cohort study. Exp Aging Res. 2003;29(1):33–45. doi: 10.1080/03610730303710. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26(7):809–17. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. An excellent review of the relationship between cognitive function and brain structure and activity in late adulthood. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14(4):364–75. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford Univ. Press; 2005. An edited volume that includes review chapters on nearly every salient topic in the emerging field of the cognitive neuroscience of aging. [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Mem Cogn. 1996;24(4):403–16. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Venkatraman V, Tan JC, Gutchess A, et al. Age-related changes in object processing and contextual binding revealed using fMR adaptation. J Cogn Neurosci. 2006;18(4):495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Cherry BJ, Adamson M, Duclos A, Hellige JB. Aging and individual variation in interhemispheric collaboration and hemispheric asymmetry. Aging Neuropsychol Cogn. 2005;12(4):316–39. doi: 10.1080/17444128.2005.10367004. [DOI] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex. 2008 doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JE. Human population: the next half century. Science. 2003;302(5648):1172–75. doi: 10.1126/science.1088665. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Daniels K, Toth J, Jacoby L. The aging of executive functions. In: Bialystok E, Craik FIM, editors. Lifespan Cognition: Mechanisms of Change. New York: Oxford Univ. Press; 2006. pp. 96–111. [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Deep processing activates the medial temporal lobe in young but not in old adults. Neurobiol Aging. 2003;24(7):1005–11. doi: 10.1016/s0197-4580(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior anterior shift in aging. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm155. Presents evidence for differences in activation patterns in posterior brain areas between young and old adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson CS, Schacter DL. Aging and strategic retrieval processes: reducing false memories with a distinctiveness heuristic. Psychol Aging. 2002;17(3):405–15. doi: 10.1037//0882-7974.17.3.405. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13(12):1344–51. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27(3):445–51. [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, et al. Neural mechanisms underlying probabilistic category learning in normal aging. J Neurosci. 2005;25(49):11340–48. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cereb Cortex. 1999;9(8):805–14. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18(2):227–41. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21(6):657–73. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100(1):253–58. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41(14):1929–41. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks RT. Inhibitory mechanisms and the control of attention. In: Conway A, Jarrold C, Kane M, Towse J, editors. Variation in Working Memory. New York: Oxford Univ. Press; 2007. pp. 227–49. [Google Scholar]
- Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol Gen. 1979;108(3):356–88. [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. In: Bower GH, editor. The Psychology of Learning and Motivation. New York: Academic; 1988. pp. 193–225. [Google Scholar]
- Hashtroudi S, Johnson MK, Chrosniak LD. Aging and source monitoring. Psychol Aging. 1989;4(1):106–12. doi: 10.1037//0882-7974.4.1.106. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14(4):410–23. doi: 10.1093/cercor/bhh003. A nice presentation on age differences in white matter integrity with differences in demented and nondemented individuals. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J. Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp. 2006;27(11):837–47. doi: 10.1002/hbm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA, Small BJ. Memory Changes in the Aged. London: Cambridge Univ. Press; 1999. [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: aging, attention, and control. Psychol Aging. 1993;8(2):283–93. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42(7):980–89. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52(2):135–43. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–12. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Ohashi Y, Ando S. Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. J Neurosci Res. 2002;70(3):340–46. doi: 10.1002/jnr.10442. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schachter DL. Gist-based false recognition of pictures in older and younger adults. J Mem Lang. 1997;37(4):555–83. [Google Scholar]
- Kramer AF, Bherer L, Colcombe SJ, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. J Gerontol A Biol Sci Med Sci. 2004;59(9):M940–57. doi: 10.1093/gerona/59.9.m940. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S. Aging cognition: from neuromodulation to representation. Trends Cogn Sci. 2001;5(11):479–86. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9(3):339–55. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100(24):14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97(8):4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92(18):8135–39. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27(10):589–94. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Res Cogn Brain Res. 2000;10(1–2):197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol. 2002;51(2):182–89. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126(Pt. 1):213–29. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The neuropsychology of memory and aging. In: Salthouse TA, Craik FIM, editors. The Handbook of Aging and Cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 315–72. [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: exploring the characteristics of illusory memories. Mem Cogn. 1997;25(6):838–48. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Long-term memory and aging: a cognitive neuroscience perspective. See Cabeza et al 2005. 2005:218–45. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Park DC, Polk TA, Mikels J, Taylor SF, Marshuetz C. Cerebral aging: integration of brain and behavioral models of cognitive function. Dialogues Clin Neurosci. 2001;3:151–65. doi: 10.31887/DCNS.2001.3.3/dcpark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 2004;101(35):13091–95. doi: 10.1073/pnas.0405148101. Details clear evidence for decreased neural specificity in the aging brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Schwarz N, editors. Cognitive Aging: A Primer. Philadelphia, PA: Psychol. Press; 2000. [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL, Frieske D, et al. Mediators of long-term memory performance across the life span. Psychol Aging. 1996;11(4):621–37. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, et al. Working memory for complex scenes: age differences in frontal and hippocampal activations. J Cogn Neurosci. 2003;15(8):1122–34. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Payer D, Marshuetz C, Sutton B, Hebrank A, Welsh RC, Park DC. Decreased neural specialization in old adults on a working memory task. Neuroreport. 2006;17(5):487–91. doi: 10.1097/01.wnr.0000209005.40481.31. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19(6):1021–32. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16(7):907–15. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA. 1998;95(3):853–60. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D’Esposito M, et al. Neural specialization for letter recognition. J Cogn Neurosci. 2002;14(2):145–59. doi: 10.1162/089892902317236803. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci. 1996;16(16):5205–15. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam MC, Wig GS, Grafton ST, Kelley WM, Gazzaniga MS. Structural organization of the corpus callosum predicts the extent and impact of cortical activity in the nondominant hemisphere. J Neurosci. 2008;28(11):2912–18. doi: 10.1523/JNEUROSCI.2295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik F, Salthouse TA, editors. The Handbook of Aging and Cognition. Hillsdale, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. Summarizes structural changes in the aging brain and variables that modify changes in structure. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–38. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6(9):394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell K. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17(3):177–82. A very accessible review of the compensatory role played by age-related increases in brain activation. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12(1):174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15(2):245–51. doi: 10.1016/j.conb.2005.03.016. An overview of how neuroimaging work has changed scientific thinking about the aging mind. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Marshuetz C, Jonides J, Smith EE, Hartley A, Koeppe R. Neurocognitive ageing of storage and executive processes. Eur J Cogn Psychol. 2001;13(1–2):257–78. [Google Scholar]
- Reuter-Lorenz PA, Mikels J. Affective working memory: converging evidence for a new construct. In: Yoshikawa S, editor. Emotional Mind: New Directions in Affective Science. 2008. In press. [Google Scholar]
- Reuter-Lorenz PA, Stanczak L, Miller A. Neural recruitment and cognitive aging: two hemispheres are better than one especially as you age. Psychol Sci. 1999;10:494–500. [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci. 2004;24(4):956–63. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JD, Stoub T, O’Hara R, et al. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behav Neurosci. 2003;117(6):1150–60. doi: 10.1037/0735-7044.117.6.1150. [DOI] [PubMed] [Google Scholar]
- Rossi S, Miniussi C, Pasqualetti P, Babiloni C, Rossini PM, Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24(36):7939–44. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Age-related changes in brain-behaviour relationships: evidence from event-related functional MRI studies. Eur J Cogn Psychol. 2001;13(1–2):235–56. [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cereb Cortex. 2002;12(5):494–505. doi: 10.1093/cercor/12.5.494. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Theoretical Perspectives on Cognitive Aging. Hillsdale, NJ: Elrbaum; 1991. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403–28. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, et al. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt. 6):1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging. 1999;14(3):483–506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, Delapaz R, Stern Y. Evaluating the function of hippocampal subregions with high-resolution MRI in Alzheimer’s disease and aging. Microsc Res Tech. 2000;51(1):101–8. doi: 10.1002/1097-0029(20001001)51:1<101::AID-JEMT11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: effects of performance and aging. Proc Natl Acad Sci USA. 2001;98(4):2095–100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole-Padulles C, Bartres-Faz D, Junque C, Clemente IC, Molinuevo JL, et al. Repetitive transcranial magnetic stimulation effects on brain function and cognition among elders with memory dysfunction. A randomized sham-controlled study. Cereb Cortex. 2006;16(10):1487–93. doi: 10.1093/cercor/bhj083. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–60. [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271(13):1004–10. [PubMed] [Google Scholar]
- Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000;21(4):569–76. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Tsai SJ. Brain-derived neurotrophic factor: a bridge between major depression and Alzheimer’s disease? Med Hypotheses. 2003;61(1):110–13. doi: 10.1016/s0306-9877(03)00141-5. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61(12):1844–48. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22(1):144–54. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120(2):272–92. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA. Cognitive activity and risk of Alzheimer’s disease. Curr Dir Psychol Sci. 2003;12(3):87–91. Comprehensively reviews how various cognitive activity and lifestyle factors affect the risk of being diagnosed with Alzheimer’s disease. [Google Scholar]
- Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer’s disease and their relation to age. Psychol Aging. 2000;15(1):18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Stine EA, Lahar CJ, Aberdeen JS. Does the capacity of working memory change with age? Exp Aging Res. 1988;14(2–3):103–7. doi: 10.1080/03610738808259731. [DOI] [PubMed] [Google Scholar]
- Wolfson L, Wei X, Hall CB, Panzer V, Wakefield D, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232(1–2):23–27. doi: 10.1016/j.jns.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors Theory, validation, and changes during normal aging. J Cereb Blood Flow Metab. 1997;17(3):316–30. doi: 10.1097/00004647-199703000-00009. [DOI] [PubMed] [Google Scholar]
- Yang YK, Chiu NT, Chen CC, Chen M, Yeh TL, Lee IH. Correlation between fine motor activity and striatal dopamine D2 receptor density in patients with schizophrenia and healthy controls. Psychiatry Res. 2003;123(3):191–97. doi: 10.1016/s0925-4927(03)00066-0. [DOI] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Intensive training in adults refines A1 representations degraded in an early postnatal critical period. Proc Natl Acad Sci USA. 2007;104(40):15935–40. doi: 10.1073/pnas.0707348104. [DOI] [PMC free article] [PubMed] [Google Scholar]