Abstract
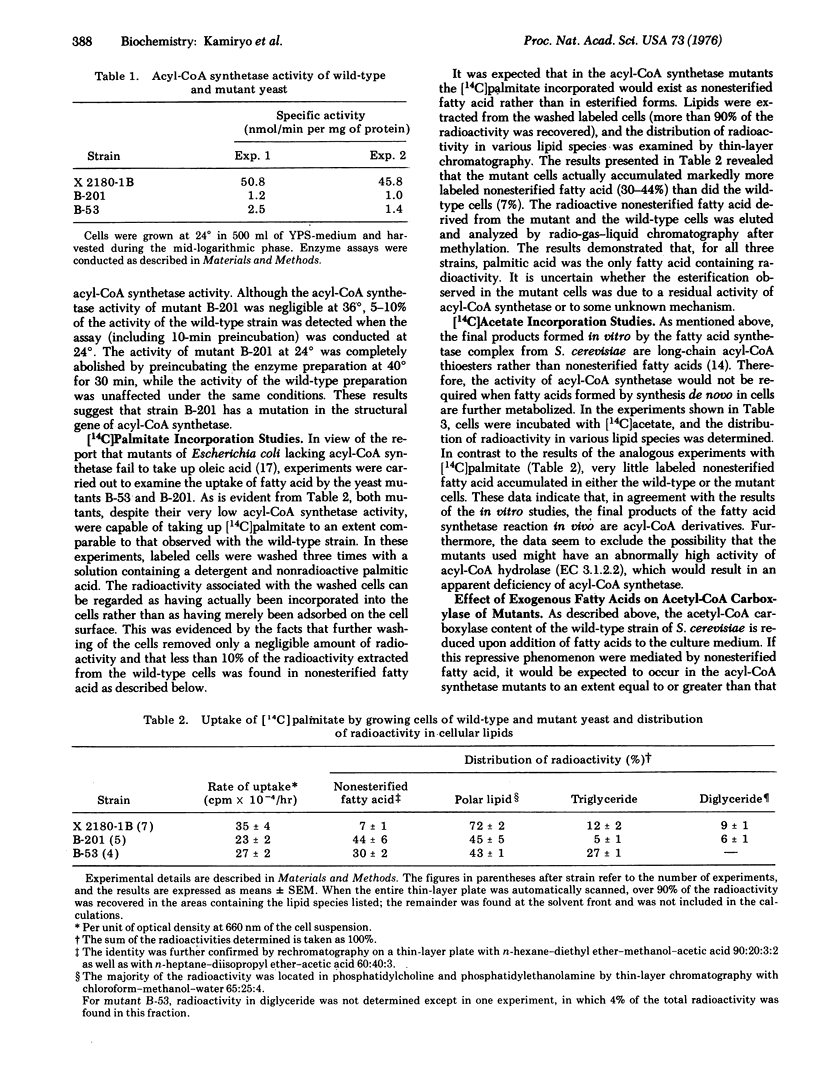
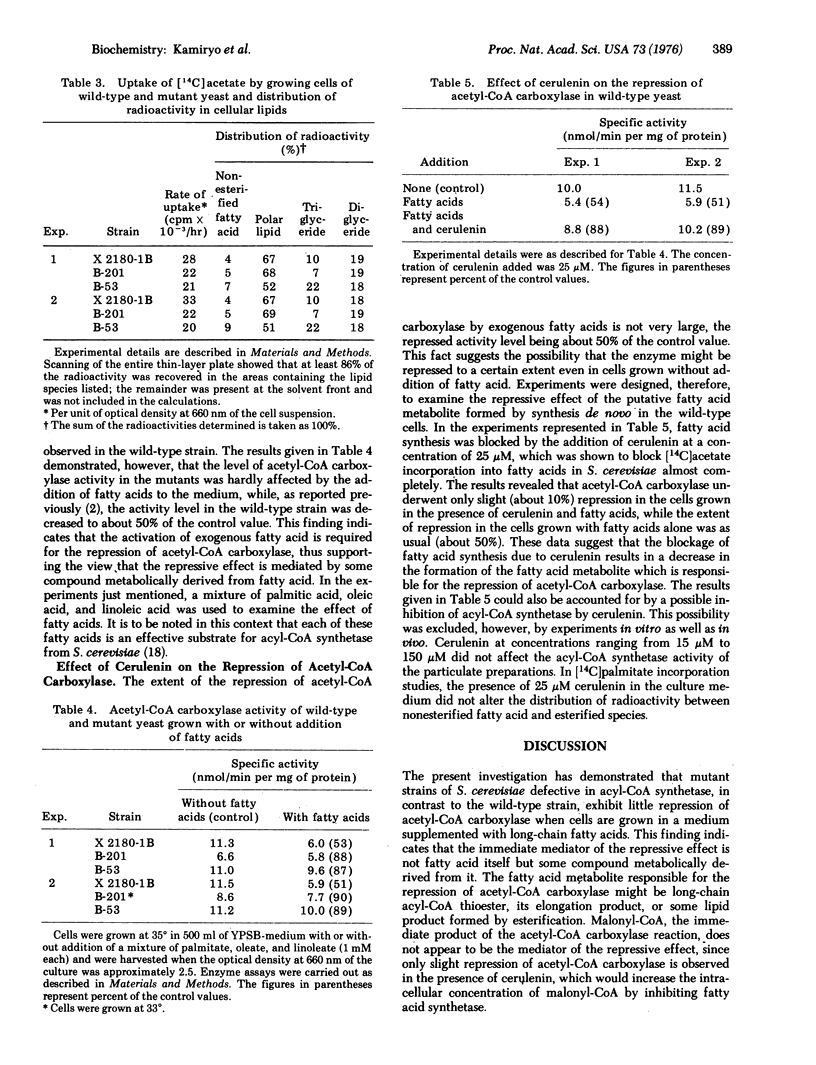
The cellular content of acetyl-CoA carboxylase [acetyl-CoA:carbon-dioxide ligase (ADP-forming), EC 6.4.1.2] in Saccharomyces cerevisiae is reduced by the addition of long-chain fatty acids to the culture medium. Mutant strains of S. cerevisiae defective in acyl-CoA synthetase [acid:CoA ligase (AMP-forming), EC 6.2.1.3] were isolated and used to determine whether fatty acid itself or a metabolite of fatty acid is more directly responsible for the repression of acetyl-CoA carboxylase. Cells of the mutant strains were capable of incorporating fatty acid to an extent comparable to that observed with the wild-type strain, but they accumulated markedly more of the incorporated fatty acid in the nonesterified form than did the wild-type cells. The level of acetyl-CoA carboxylase activity in the mutants, in contrast to that in the wild-type strain, was hardly affected by the addition of fatty acids to the medium. These results indicate that the activation of exogenous fatty acid is required for the repression of acetyl-CoA carboxylase, supporting the view that the repressive effect is mediated by some compound metabolically derived from fatty acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banis R. J., Tove S. B. Solubilization of a long chain fatty acyl-CoA synthetase from chicken adipose tissue microsomes. Biochim Biophys Acta. 1974 May 29;348(2):210–220. doi: 10.1016/0005-2760(74)90232-x. [DOI] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S., Numa S. Partial purification, properties, and subcellulsr distribution of rat liver phosphatidate phosphatase. J Biochem. 1975 Mar;77(3):501–509. doi: 10.1093/oxfordjournals.jbchem.a130751. [DOI] [PubMed] [Google Scholar]
- Jacobs R. A., Sly W. S., Majerus P. W. The regulation of fatty acid biosynthesis in human skin fibroblasts. J Biol Chem. 1973 Feb 25;248(4):1268–1276. [PubMed] [Google Scholar]
- Kamiryo T., Numa S. Reduction of the acetyl coenzyme A carboxylase content of Saccharomyces cerevisiae by exogenous fatty acids. FEBS Lett. 1973 Dec 15;38(1):29–32. doi: 10.1016/0014-5793(73)80505-8. [DOI] [PubMed] [Google Scholar]
- Kitajima K., Tashiro S., Numa S. Acetyl-coenzyme-A carboxylase in cultured hepatocytes. Effects of exogenous fatty acids on the content, synthesis and degradation of the enzyme. Eur J Biochem. 1975 Jun;54(2):373–383. doi: 10.1111/j.1432-1033.1975.tb04148.x. [DOI] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYNEN F., HOPPER-KESSEL I., EGGERER H. ZUR BIOSYNTHESE DER FETTSAEUREN. 3. DIE FETTSAEURENSYNTHETASE DER HEFE UND DIE BILDUNG ENZYMGEBUNDENER ACETESSIGSAEURE. Biochem Z. 1964 Jul 8;340:95–124. [PubMed] [Google Scholar]
- LYNEN F. Participation of acyl--CoA in carbon chain biosynthesis. J Cell Comp Physiol. 1959 Dec;54:33–49. doi: 10.1002/jcp.1030540406. [DOI] [PubMed] [Google Scholar]
- Numa S., Yamashita S. Regulation of lipogenesis in animal tissues. Curr Top Cell Regul. 1974;8(0):197–246. doi: 10.1016/b978-0-12-152808-9.50012-2. [DOI] [PubMed] [Google Scholar]
- Omura S., Katagiri M., Nakagawa A., Sano Y., Nomura S. Studies on cerulenin. V. Structure of cerulenin. J Antibiot (Tokyo) 1967 Nov;20(6):349–354. [PubMed] [Google Scholar]
- Orme T. W., McIntyre J., Lynen F., Kühn L., Schweizer E. Fatty-acid elongation in a mutant of Saccharomyces cerevisiae deficient in fatty-acid synthetase. Eur J Biochem. 1972 Jan 21;24(3):407–415. doi: 10.1111/j.1432-1033.1972.tb19700.x. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Mead J. F. Long chain fatty acid activation in subcellular preparations from rat liver. J Biol Chem. 1968 Jan 25;243(2):352–361. [PubMed] [Google Scholar]
- Serrano R., Gancedo J. M., Gancedo C. Assay of yeast enzymes in situ. A potential tool in regulation studies. Eur J Biochem. 1973 May 2;34(3):479–482. doi: 10.1111/j.1432-1033.1973.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Nakaya N., Miki Y., Numa S. Separation of 1-acylglycerolphosphate acyltransferase and 1-acylglycerolphosphorylcholine acyltransferase of rat liver microsomes. Proc Natl Acad Sci U S A. 1975 Feb;72(2):600–603. doi: 10.1073/pnas.72.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]



