Abstract
Metabotropic glutamate (mGlu) receptors provide a mechanism by which the function of NMDA glutamate receptors can be modulated. As NMDA receptor hypofunction is implicated in the etiology of psychiatric disorders, including schizophrenia, the pharmacological regulation of mGlu receptor activity represents a promising therapeutic approach. We examined the effects of the positive allosteric mGlu5 receptor modulator 3- cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB), alone and in combination with the NMDA receptor antagonist MK-801, on a task measuring cognitive set-shifting ability. This task measures NMDA receptor-dependent cognitive abilities analogous to those impaired in schizophrenia. Systemic administration of CDPPB (10 & 30 mg/kg i.p) blocked MK-801 (0.1 mg/kg, i.p.)-induced impairments in set-shifting ability. The effect on learning was dose-dependent, with the 30 mg/kg dose having a greater effect than the 10 mg/kg dose across all trials. This ameliorative effect of CDPPB reflected a reduction in MK-801-induced perseverative responding. These results add to the evidence that mGlu5 receptors interact functionally with NMDA receptors to regulate behavior, and suggest that positive modulators of mGlu5 receptors may have therapeutic potential in the treatment of disorders, like schizophrenia, characterized by impairments in cognitive flexibility and memory.
Keywords: Set-shift, Response inhibition, Extradimensional shift, Perseveration, Prefrontal cortex, Schizophrenia, Addiction
1. Introduction
Group I metabotropic glutamate (mGlu) receptors, comprising the mGlu1 and mGlu5 subtypes, are localized largely at the periphery of the post-synaptic active zone, where they regulate ionotropic receptor activity and intracellular processes via G protein-linked second messenger pathways (Baude et al., 1993; Hassel and Dingledine, 2006; Luján et al., 1997). They are thus well positioned to regulate synaptic activity in response to glutamate diffusing beyond the active zone.
mGlu5 receptors are found in brain regions important for cognition, including prefrontal cortex, striatum, and hippocampus (Manahan-Vaughn and Braunewell, 2005; Melendez et al., 2004; Packard et al., 2001). Accordingly, mGlu5 receptor modulation affects multiple behaviors and cognitive abilities in rodents, including appetite, drug discrimination learning, attention, spatial memory, sensorimotor gating, instrumental learning, and extinction (Ayala et al., 2009; Balschun and Wetzel, 1998; Besheer et al., 2009; Bradbury et al., 2005; De Leonibus et al., 2009; Homayoun et al., 2004; Kinney et al., 2003; Kinney et al., 2005; Manahan-Vaughn and Braunewell, 2005; Semenova and Markou, 2007; Uslaner et al., 2009; Xu et al., 2009).
NMDA glutamate receptors are a key synaptic constituent regulated by mGlu5 receptors (Awad et al., 2000; Bleakman et al., 1992; Fagni et al., 2002). mGlu5 receptor inhibition decreases NMDA receptor activity, whereas stimulation increases it (Awad et al., 2000; Doherty et al., 1997). Selective mGlu5 receptor antagonists typically impair cognition and enhance deficits caused by NMDA antagonists (Homayoun et al., 2004; Manahan-Vaughn and Braunewell, 2005). Less is known about the cognitive effects of mGlu5 receptor activation. The positive allosteric mGlu5 receptor modulator 3-cyano-N- (1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) blocks amphetamine-induced hyperlocomotion and sensorimotor gating abnormalities (Kinney et al., 2005), and enhances recognition memory (Uslaner et al., 2009). Moreover, CDPPB reduces abnormal excitatory neural activity in the rat medial prefrontal cortex induced by the NMDA antagonist MK-801 (Lecourtier et al., 2007).
Aberrant NMDA receptor-mediated neurotransmission is implicated in the etiology of psychiatric disorders, such as schizophrenia, and addiction (Moghaddam, 2003; Tamminga, 1998; Vanderschuren and Kalivas, 2000). NMDA antagonists induce schizophrenia-like cognitive deficits in normal humans and laboratory rodents (Aultman and Moghaddam, 2001; Javitt and Zukin, 1991; Jentsch and Taylor, 2001; Krystal et al., 2005; Stefani and Moghaddam, 2005), including impaired cognitive flexibility (Egerton et al., 2005; Stefani and Moghaddam, 2005) and working memory (Aultman and Moghaddam, 2001; Homayoun et al., 2004). Given their effects on glutamatergic neurotransmission, and particularly on NMDA receptor activity, mGlu5 receptor modulators are promising agents for treatment of psychiatric disorders and addiction (Carroll, 2008; Kinney et al., 2005; Marino and Conn, 2002; Moghaddam, 2004).
We tested the ability of CDPPB to block MK-801-induced cognitive deficits on a task measuring cognitive set-shifting ability. Cognitive set-shifting refers the ability to switch between rules in response to environmental feedback (Birrell and Brown, 2000; Ragozzino et al., 1999; Shepp and Eimas, 1964). Our task is analogous to the Wisconsin Card Sort Task used to assess cognitive flexibility in humans (Haut et al., 1996; Milner, 1963). We hypothesized that treatment with CDPPB would reduce schizophrenia-like set-shifting impairments induced by administration of MK-801.
2. Materials and Methods
2.1 Subjects
Male Sprague-Dawley rats (280–310 g on arrival; Harlan, Frederick, MD) were housed two or three to a cage and maintained on a 12/12 h light/dark cycle (lights on at 0700). The rats had unrestricted access to food for a minimum of two weeks following arrival, after which point they were placed on a restricted diet of 15 g of rat chow per day per rat. This diet was adequate to maintain the rats at approximately 85% of their free-feeding weight. The rats had unrestricted access to water for the duration of the experiment. Animal care and experimental procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.2 Set-Shifting Behavior Task
2.2.1 Apparatus
Set-shifting ability was measured in a four-arm ‘plus’ maze constructed of Plexiglas (0.63 cm thick). The maze consisted of a square central platform 14 cm on a side to which were joined four arms. The arms joined the central platform in such a way that no space existed between adjacent arms. The arms were 14.0 cm wide, 40.6 cm long and 20.3 cm high. A food well 1.9 cm in diameter and 0.63 cm deep was located approximately 2.5 cm from the distal end of each arm. The food well was sufficiently deep to hide the food pellet from the view of the rat from the arm entrance. Maze arms varied along two stimulus dimensions, brightness and texture. Arms were painted so as to be either dark (gray; Valspar #335A-4) or light (off-white; Valspar #47583), and were textured either smooth (paint alone) or rough (paint mixed with sand). The center square and door of the maze were painted with gray primer and were distinct in contrast from the maze arms. The maze was placed on a locking rotary platform of our design that permitted the maze to be rotated between trials. The holding cage used for inter-trial intervals was constructed of gray-painted Plexiglas, and measured 35.6 × 35.6 × 35.6 cm.
2.2.2 Habituation
One week following arrival, a handling and habituation period approximately two weeks in duration was begun. During the first week, each rat was handled by the experimenter for 3 min per day. Food reinforcer pellets (dustless precision pellets, purified formula, 45 mg, Bio-Serv, Frenchtown, NJ) were given to the rats in their home cages each day after handling in order to allow the rats to become familiar with the taste and odor of the reinforcers. Food restriction was begun on the second day of handling.
Following the handling phase, the rats began a period of maze habituation lasting approximately one week. During the first phase of maze habituation, all four arms of the maze were open for exploration. On the first day of habituation, cage-mates were placed in the maze together and allowed to roam freely for 5 minutes. Four reinforcer pellets were placed in each food well to condition the rats to receiving the reinforcer in the maze. On the second and third days of habituation the rats were placed in the maze individually, and the maze baited with a single reinforcer pellet per arm. Rats were allowed to explore until all the food was eaten or for a maximum of 5 min. Immediately after the habituation period each day, the rats were placed in the holding cage for 1 min before being returned to their home cages. Rats that did not quickly consume the food pellets were habituated for additional sessions.
The rats next received 1–3 days of habituation to the maze in its test, ‘T,’ configuration (see Stefani and Moghaddam, 2005 for task diagram). For each trial, the arm directly across from the start arm was blocked with a removable, opaque Plexiglas door. The rat was placed at the end of the start arm (the stem of the T) and allowed to explore one of the two ‘choice’ arms and to consume any food reward there. Each rat received 8 trials per day; two starts from each arm. The order of start arms was pseudo-random, and a food reinforcer was not always present at the end of a choice arm. Between trials, the rats were held in the holding cage. The inter-trial interval was approximately 15 s.
Through the above process the rats were habituated to the maze, to receiving a food reward at the end of a choice arm, and to being repeatedly transferred between the holding cage and the maze. The rats were pseudo-randomly rewarded during the habituation period in an attempt to forestall the development of associations between the presence or absence of a reinforcer and arm stimulus attributes.
2.2.3 Set-Shifting Task
Set-shift testing consisted of two sessions, or sets, separated by an inter-set interval of approximately 24 h. During set 1 the rats were trained on either a brightness (dark vs. light maze arms) or texture (rough vs. smooth arms) discrimination task to a criterion performance level. During set 2 the rats were trained on the alternative discrimination strategy for 80 trials, regardless of performance level. Rats were assigned to treatment and training groups pseudo-randomly.
Set 1
The maze was in a T configuration such that, from a start arm a rat had a choice of making a 90° turn into either a dark or light arm, and a smooth or rough arm. A rat was scored as having made an arm entry when its hindquarters passed beyond the inner door rail at the entrance of the maze arm (approximately 2 cm from the juncture of the maze arm and the central square). Rats were trained to a criterion performance level of 8 consecutive entries into a rewarded arm, at which point training was stopped. Rats were trained for a maximum of 120 trials. Rats that did not reach criterion performance on set 1 within 120 trials were excluded from the experiment. Within each sequential block of 8 trials, there were two starts from each of the four arms. The maze was rotated 90° with respect to the testing room every trial to discourage the use of extra-maze cues. The inter-trial interval was approximately 15 s. During the inter-trial intervals the rats were placed in the holding cage, which contained fresh bedding material.
Set 2
As during set 1, from each of the four start arms a rat had a choice of making a 90° turn into an arm that was either rough or smooth, and light or dark. The rats received 80 trials, regardless of choice accuracy during those trials. The number of trials required to reach the criterion of 8 consecutive correct arm entries used for set 1 was recorded, as was the time required to complete all 80 trials. The sequence of arm starts was identical to that used for set 1.
2.3 Drugs
MK-801 (Sigma, St. Louis, MO) was prepared at a stock concentration of 1.0 mg/ml in sterile physiological saline and frozen in aliquots at −20° C. On the day of the experiment, aliquots were thawed and diluted in sterile physiological saline solution to a final concentration of 0.1 mg/ml. CDPPB was synthesized in-house according to the method of Lindsley et al. (Lindsley et al., 2004). On the day of the experiment, CDPPB was dissolved in a vehicle solution containing 10% dimethylsulfoxide and 90% polyethylene glycol 400 (v/v). Doses of CDPPB were chosen based on those used by Kinney et al. (2005).
Drugs were administered via intraperitoneal (i.p.) injection at volumes of 1.0 ml/kg, resulting in doses of 0.1 mg/kg for MK-801 and either 10 or 30 mg/kg for CDPPB. The first injection was given 40 min prior to the start of behavior testing and the second injection 20 min later. Rats were housed in their home cages during the injection process.
2.4 Data Analysis
Between-group comparisons of behavior measures were made by one-way or two-way analysis of variance (ANOVA), with Tukey-adjusted post hoc testing. Where ceiling effects potentially influenced the normality of the data, as in the cases of the trials to criterion and trial block 10 scores during set 2, the non-parametric Kruskal-Wallis and Mann-Whitney U tests were used for multiple comparisons and post hoc tests, respectively. Performance across trial blocks for set 2 was assessed by calculating ‘percent correct’ scores for ten consecutive blocks of eight trials each, and comparing these scores using a two-way, mixed measures ANOVA, with drug treatment as the between-subjects measure and trial block as the within-subjects measure.
Perseverative responding on set 2 was evaluated by comparing the percent correct scores from each of two start arm classes, termed ‘perseveration arms’ and ‘reinforcement arms,’ within each block of eight trials. For a given rat, the perseveration arms were defined as the two start arms from which, during set 2, responding according to the stimulus-reward contingency that had been correct during set 1 would result in an incorrect, non-rewarded response. The reinforcement arms were defined as the two start arms from which responding, during set 2, according to the set 1 discrimination rule would result in a (spuriously) correct, rewarded response (see Stefani et al., 2003; Stefani and Moghaddam, 2005, for illustrations and discussion of start arm classes). As with the overall performance across trial blocks, treatment effects on perseveration and reinforcement arm start performance were analyzed by comparing percent correct scores using two-way, mixed measures ANOVAs, with treatment group as the between-subjects measure and performance across trial blocks from either perseveration or reinforcement arm starts as the within-subjects measure. Differences between perseveration and reinforcement arm start performance for each treatment group were analyzed using paired t-tests for planned trial block comparisons. The alpha for all tests was 0.05.
3. Results
3.1 Set 1 (Acquisition of first discrimination rule)
No drug treatment was administered before training on set 1. Evaluated as a single group (n = 40), the rats required 52 ± 2 trials (mean ± S. E. M.) to reach the criterion of 8 consecutive correct arm entries. The mean time per trial (including the 15 s inter-trial interval) was 0.51 ± 0.01 min. An analysis of rats grouped according to their eventual set 2 treatments found no significant between-group performance differences for either the trials to criterion (F4,35 = 0.78, P = 0.55) or time per trial (F4,35 = 0.76, P = 0.56) measures.
3.2 Set 2 (Acquisition of second discrimination rule, and extradimensional shift)
Acquisition of the set 2 discrimination rule was analyzed using two related measures. First, a simple measure of learning was evaluated by examining the number of trials required to reach the criterion of 8 consecutive correct arm entries, as was done for set 1. Second, learning curves reflecting performance across the 80 trials comprising set 2 were evaluated for overall performance (all trials), and for start arm classes (trials from perseveration and reinforcement arm starts). Both measures showed that treatment with MK-801 significantly impaired the rule shift from set 1 to set 2. Furthermore, this impairment was attenuated by subsequent administration of CDPPB, although the attenuation did not persist robustly across trials for rats treated with the lower, 10 mg/kg, dose of CDPPB.
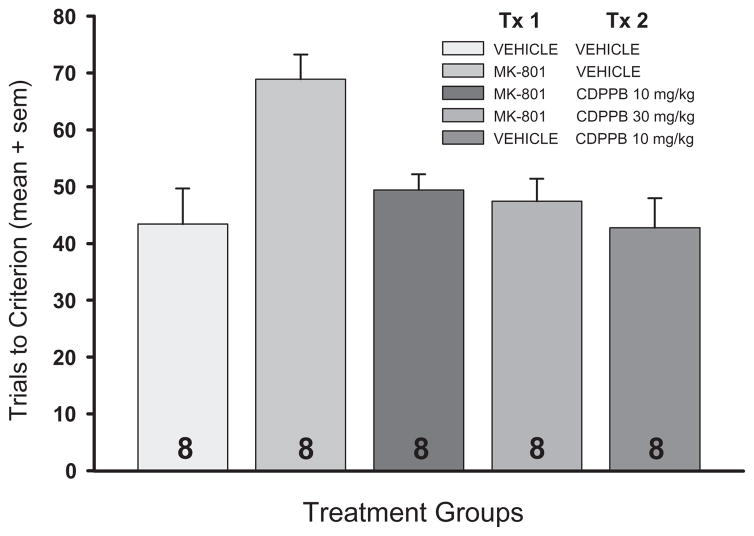
Figure 1 illustrates the significant main effect of drug treatment on the trials required to reach criterion during set 2 (Kruskal-Wallis χ2 = 13.76, P = 0.008). Rats that received i.p. injections of MK-801 + vehicle (MK-801/Vehicle) required significantly more trials to reach criterion than did rats that received injections of the vehicle solutions alone (Mann-Whitney U test, P = 0.008). When CDPPB, at either the 10 or 30 mg/kg dose, was administered subsequent to MK-801, the performance impairment was completely blocked (P’s = 0.004 and 0.008 vs. MK-801/Vehicle, for 10 and 30 mg/kg CDPPB, respectively). Performance of rats given injections of CDPPB, in combination with MK-801 or vehicle, did not differ from vehicle-injected controls (P’s > 0.05).
Figure 1. Set-Shifting Task: Set 2 Trials to Criterion.
Systemic administration of the N-methyl-D-aspartate receptor antagonist MK-801 (0.1 mg/kg, i.p.) prior to set 2 significantly increased the number of trials required to reach the performance criterion of 8 consecutive correct arm entries, relative to vehicle-injected control rats (Vehicle/Vehicle) or rats given injections of the mGlu5 receptor positive allosteric activator CDPPB in combination with vehicle (Vehicle/CDPPB). Treatment with CDPPB (10 or 30 mg/kg, i.p.) 20 min following treatment with MK-801 blocked the performance deficits associated with MK-801 administration. Data are shown as means + S. E. M. The value within each bar represents the number of subjects in that group. *P < 0.05 vs. all other treatment groups.
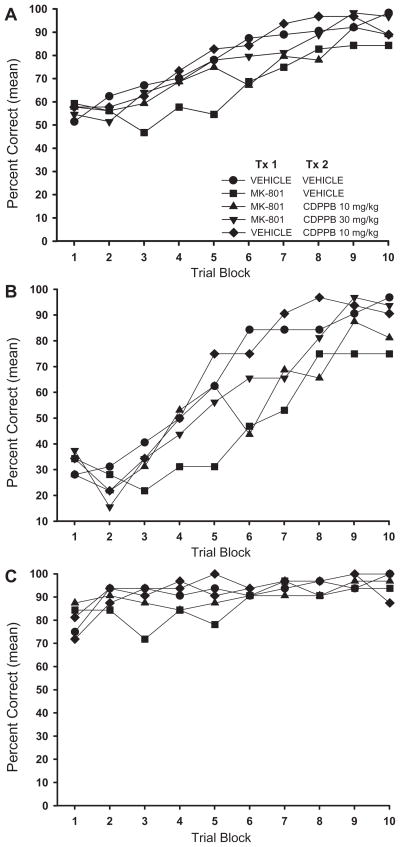
Analysis of the learning curves for overall performance showed that all treatment groups began set 2 performing with near-chance accuracy (50% correct), and improved significantly across trial blocks (Fig. 2A). There were significant main effects of treatment (F4,35 = 4.80, P = 0.003) and trial block (F9,315 = 50.53, P < 0.001). Rats given injections of MK-801 acquired the set 2 discrimination rule significantly more slowly than did the rats given 2 vehicle injections (Vehicle/Vehicle) or vehicle followed by 10 mg/kg of CDPPB (Vehicle/CDPPB 10) (P’s = 0.010 and 0.005, respectively). Administration of CDPPB subsequent to MK-801 partially attenuated the impairing effects of MK-801 alone, resulting in learning rates not significantly different from either the control or MK-801/Vehicle group (P’s > 0.05). However, the attenuating effect of the 30 mg/kg dose of CDPPB approached significance (Tukey-adjusted P = 0.064) and was significant using a less stringent, unadjusted post hoc test (Fisher’s LSD, P = 0.009). There were no treatment-dependent differences in overall percent correct scores at the beginning of set 1 (Fig. 2A, trial block 1; F4,35 = 0.365, P = 0.832). At the conclusion of set 2, treatment-dependent differences in percent correct scores neared significance (Kruskal-Wallis χ2 = 9.16, P = 0.057), with the MK-801/Vehicle group differing most from Vehicle/Vehicle and MK-801/CDPPB 30 groups. There was no interaction between the drug treatment and trial block factors (F36,315 = 1.33, P = 0.106), indicating that despite the MK-801-induced performance deficits, the general pattern of learning was similar between groups.
Figure 2. Set-Shift Task: Set 2 Performance Across Trial Blocks.
A. Overall Performance. Rats in all treatment groups began set 2 performing at near chance levels and significantly improved their performance across trial blocks. Treatment with MK-801 significantly impaired acquisition of the set 2 discrimination rule relative to vehicle-injected controls (Vehicle/Vehicle). Administration of CDPPB subsequent to MK-801 attenuated the MK-801-induced performance impairments. The effect was dose-dependent, with more consistent attenuation observed in the group given 30 mg/kg of CDPPB (MK-801/CDPPB 30) than that given 10 mg/kg (MK-801/CDPPB 10). B. Perseveration Arm Performance. At the outset of testing, rats in all treatment groups made significantly more perseveration arm start errors than reinforcement arm start errors, indicating retention and use of the set 1 discrimination rule. At trial block 1, there were no significant between-group differences in percent correct scores from perseveration arm starts. As training progressed, rats treated with MK-801 made significantly more errors from perseveration arm starts than did rats in the Vehicle/Vehicle or Vehicle/CDPPB 10 groups. At the end of set 2, rats treated with 30 mg/kg CDPPB (MK-801/CDPPB 30) performed significantly better than did rats given MK-801 alone, and did not differ from Vehicle/Vehicle rats. Performance of rats given 10 mg/kg CDPPB in conjunction with MK-801 was intermediate between MK-801/Vehicle and MK-801/CDPPB 30 groups. C. Reinforcement Arm Performance. There were no significant effects of treatment on performance from reinforcement arm starts. Treatment with MK-801 was associated with non-significantly poorer reinforcement arm performance, relative to the Vehicle/Vehicle group. Performance from reinforcement arm starts by rats in the MK-801/Vehicle, MK-801/CDPPB 30 and Vehicle/CDPPB 30 groups improved significantly over trial blocks; no significant improvement across trial blocks was seen for the other 2 treatment groups due to ceiling effects. There were no significant between-group differences in percent correct scores from reinforcement arm starts at trial block 1 or 10.
Data are expressed as mean percent correct for each block of 8 trials. Alpha = 0.05. The number of rats per treatment group is the same as noted in Figure 1.
The attenuation of the MK-801-induced set-shifting deficit by CDPPB was observed through the first half of set 2. However, during trial block 6, performance by rats in the MK-801/CDPPB 10 group declined to levels statistically similar to those of rats in the MK-801/Vehicle group, and remained so for the duration of testing. Treatment with 30 mg/kg of CDPPB more reliably attenuated the MK-801-induced deficit during the latter half of the test period, although there was no statistical difference between groups. There were no significant differences between the Vehicle/Vehicle, Vehicle/CDPPB 10, MK-801/CDPPB 10 and the MK-801/CDPPB 30 groups (P’s > 0.05).
The contribution of errors due to perseveration and those due to other factors, such as random responding or turn bias can be ascertained by analyzing performance according to start arm type (see Stefani et al., 2003; Stefani and Moghaddam, 2005 for discussion). A comparison of the data in Figs. 2B and 2C shows that, consistent with previous studies, all groups began set 2 making significantly fewer correct choices from perseveration arm starts than from reinforcement arm starts (paired t-tests, P’s < 0.05), indicating retention and initial use of the set 1 discrimination rule. By trial block 10, all rats were performing equally well, statistically, from perseveration and reinforcement arm starts. However, the differences between perseveration arm and reinforcement arm percent correct scores were greater for the rats in the MK-801/Vehicle and MK-801/CDPPB 10 groups (P’s = 0.08 and 0.05 respectively), with worse performance from perseveration arm starts, indicating perseveration on the set 1 discrimination rule.
Treatment with CDPPB partially attenuated the increases in the magnitude and duration of perseverative responding across trial blocks caused by MK-801 (Fig. 2B). Analysis of perseveration arm starts showed main effects of treatment (F4,35 = 3.54, P = 0.016) and trial block (F9,315 = 49.48, P < 0.001), but no interaction between treatment and trial block factors (F36,315 = 1.21, P = 0.198). Treatment with MK-801 significantly impaired choice accuracy from perseveration arm starts in comparison to Vehicle/Vehicle (P = 0.034) and Vehicle/CDPPB 10 (P = 0.023) groups. As seen for the overall performance scores, treatment with CDPPB did not result in learning curves significantly different from those of Vehicle/Vehicle or MK-801/Vehicle. There were no significant treatment-dependent differences in perseveration arm percent correct scores at trial block 1 (F4,35 = 0.24, P = 0.917). However, at the conclusion of testing, there was a main effect of treatment on perseveration arm start scores (Trial block 10; Kruskal-Wallis χ2 = 9.74, P = 0.045). Rats in the MK-801/Vehicle treatment group had significantly lower percent correct scores that did rats in either the Vehicle/Vehicle or the MK-801/CDPPB 30 groups (Mann-Whitney U test P’s = 0.013 and 0.037, respectively), but not the Vehicle/CDPPB 10 or MK-801/CDPPB 10 groups. There were no other significant between-group differences at trial block 10 (P’s > 0.05).
Analysis of reinforcement arm starts showed no significant main effect of treatment (Fig. 2C; F4,35 = 1.05, P = 0.394). There was a significant effect of trial block (F9,315 = 5.45, P < 0.001), but no interaction between the two factors (F36,315 = 1.13, P = 0.289). Performance from reinforcement arm starts by rats in the MK-801/Vehicle, MK- 801/CDPPB 30, and Vehicle/CDPPB 30 groups improved significantly over trial blocks, whereas no significant improvement across trial blocks was seen for the other 2 treatment groups due to ceiling effects. There were no significant treatment-dependent differences in percent correct scores at trial block 1 (F4,35 = 1.13, P = 0.289) or at trial block 10 (Kruskal-Wallis χ2 = 6.56, P = 0.161).
There was a main effect of treatment on the time required to complete the 80 trials comprising set 2, although there were no significant post hoc pair-wise group differences (F4,35 = 2.78, P = 0.042). MK-801, alone or in combination with CDPPB, tended to decrease the magnitude of the time measures, indicating a small effect towards faster trial run times, whereas CDPPB alone tended to increase the magnitude of the time variables, indicative of slower run times. Times required to complete all 80 trials were, in min (mean ± S. E. M.): Vehicle/Vehicle, 36.0 ± 0.6; MK-801/Vehicle, 34.9 ± 0.5; Vehicle/CDPPB 10, 36.9 ± 0.6; MK-801/CDPPB 10, 35.1 ± 0.7; and MK-801/CDPPB 30, 34.6 ± 0.5. The mean time per trial values for each treatment group were, in min (mean ± S. E. M.): Vehicle/Vehicle, 0.45 ± 0.01; MK-801/Vehicle, 0.44 ± 0.01; Vehicle/CDPPB 10, 0.46 ± 0.01; MK-801/CDPPB 10, 0.44 ± 0.01; and MK-801/CDPPB 30, 0.43 ± 0.00.
4. Discussion
We report that the mGlu5 receptor positive allosteric modulator CDPPB attenuates the impairing effects of NMDA glutamate receptor blockade on performance of a behavioral task assessing cognitive set-shifting ability. This task requires an extradimensional rule shift; that is, a shift in response strategies that depends on the ability of the animal to redirect attention to a qualitatively different perceptual category, in the present case the texture or brightness of the maze arms, while simultaneously suppressing a previously learned response rule. In rats, extradimensional shifting ability depends on glutamatergic and dopaminergic neurotransmission within the medial prefrontal cortex (Egerton et al., 2005; Floresco et al., 2005; Ragozzino et al., 2002; Stefani and Moghaddam, 2005; Stefani and Moghaddam, 2006) and is sensitive to manipulations which impair the functioning of the medial prefrontal cortex (Birrell and Brown, 2000; Ragozzino et al., 1999; Stefani et al., 2003). Shifting is impaired by NMDA receptor antagonists such as phencyclidine and MK-801 (Egerton et al., 2005; Stefani et al., 2003; Stefani and Moghaddam, 2005), administered systemically or intracranially.
Accordingly, in the present experiments MK-801 robustly impaired task performance, increasing the trials required to make the extradimensional rule shift between sets 1 and 2. CDPPB dose-dependently blocked MK-801-induced set-shifting deficits. CDPPB, when administered alone, neither enhanced nor impaired set-shift task performance, relative to vehicle-injected controls. Consistent with our previous observations that NMDA receptor blockade, subsequent to either systemic or intra-cranial injections into the medial prefrontal cortex, significantly impairs extra-dimensional set-shifting by increasing perseverative responding (Stefani et al., 2003; Stefani and Moghaddam, 2005), MK-801 significantly impaired the shift from the first discrimination rule to the second by increasing perseverative responding. That is, during set 2, rats persisted in responding according to the rule previously learned during set 1, although doing so was counterproductive to maximizing reward. Administration of CDPPB specifically attenuated this MK-801-induced increase in perseveration, initially restoring overall performance to control levels.
Group I mGlu receptors have been described as ‘tuning the activity of fast-acting ionotropic neurotransmission’ (Ango et al., 2002), and have important modulatory interactions with NMDA receptors (Kew and Kemp, 2005; Pin and Acher, 2002). Our results are consonant with such an interaction between mGlu5 receptors and NMDA receptors within the rat medial prefrontal cortex, although this cannot be concluded with certainty on the basis of experiments using systemic drug treatments alone. In support of this mechanism, however, histological and behavioral studies indicate that mGlu5 receptors are expressed in brain regions associated with performance of tasks requiring executive function and memory, including the prefrontal cortex, striatum, septal region, and hippocampus. (Lecourtier et al., 2007; Melendez et al., 2004; Packard et al., 2001; Page et al., 2005; Romano et al., 1995). Systemically administered CDPPB has been found to block MK-801-induced abnormalities in rat medial prefrontal cortical neural activity, including increased firing rate and decreased burst frequency (Lecourtier et al., 2007). Furthermore, extinction behavior has been reported to be impaired in mGlu5 receptor knock-out mice (Xu et al., 2009). In the current context, perseveration can be interpreted as an inability to extinguish the performance of a learned behavior when it is no longer adaptive. Systemic administration CDPPB also attenuates MK-801-induced deficits in recognition memory, and correspondingly increases phosphorylation of NMDA and AMPA receptors, and the intracellular signaling molecules calcium/calmodulin-dependent kinase II and CREB, in both hippocampus and medial prefrontal cortex (Uslaner et al., 2009).
The efficacy of CDPPB differed depending on the measure of set-shifting performance considered. When only the number of trials required to reach criterion was taken to indicate successful shifting, both the 10 and 30 mg/kg doses of CDPPB restored set-shifting ability to control levels equally well. However, when set 2 performance was examined across the full 80 trials, it became evident that the temporal effects of CDPPB were dose-dependent. The performance of rats treated with the lower dose of CDPPB declined markedly soon after reaching criterion. Significantly, this decline in correct choices was selectively evident for perseveration arm start trials, indicating a relapse into perseverative responding. The rats treated with the higher CDPPB dose showed more sustained performance improvements.
This dose-dependency is unlikely to be due to CDPPB metabolism, as its plasma half-life is reported to be approximately 4 h (Kinney et al., 2005). The duration of set 2 in the current behavior paradigm is approximately 40 min; thus, behavior testing is completed within approximately 60 min following drug administration. It is possible that the modulatory effect exerted by mGlu5 receptors on NMDA receptors is subject to rapid desensitization. Alternatively, because MK-801 is a ‘use-dependent’ NMDA antagonist, requiring the NMDA channel to be in the open, active state for inhibition to occur, it is possible that certain levels of CDPPB facilitate NMDA receptor inhibition by increasing the number of open NMDA receptor channels.
Dose-dependent effects on the performance of other tasks measuring cognitive ability have been reported. Uslaner et al. (2009) found that CDPPB has a dose-dependent effect on recognition memory, assessed using a novel object recognition task. They found that exploration of a novel object was increased by a systemic dose of 10 mg/kg, but not by a dose of 30 mg/kg. Furthermore, recognition memory deficits induced by systemically administered MK-801 were attenuated by 3 mg/kg CDPPB, but not by 10 or 30 mg/kg. The dose of MK-801 used by Uslaner et al. (2009) to induce recognition memory impairments, 0.3 mg/kg, was higher than the 0.1 mg/kg dose used in our studies, and we observed no enhancement of set-shift task performance by CDPPB alone. Nevertheless, together these results suggest that the effects of CDPPB on cognition-dependent behaviors likely will depend on an interaction of the behavior task and drug dose, and may exhibit the inverted-U dose-response relationship frequently observed for putative cognitive enhancers.
The effects of CDPPB are likely due to cognitive, rather than motivational or motoric aspects of set-shift task performance. Rats in all groups readily negotiated the maze and consumed the available reinforcers. There were no pronounced effects of CDPPB, alone or in combination with MK-801, on locomotor activity in the maze. There was a main effect of drug treatment on the time rats required to complete set 2, but no individual treatment group differed significantly from any other. Rats treated with CDPPB alone required slightly more time per trial than did rats in the control group, whereas rats treated with MK-801 completed each trial more rapidly than did controls. CDPPB tended to enhance the effect of MK-801 on locomotion; however, these effects were small, possibly due to the experimenter-controlled nature of the task. The time per trial values observed in this study were similar to those we have recorded in previous experiments using this set-shift paradigm (Stefani and Moghaddam, 2005; Stefani and Moghaddam, 2006).
The widespread distribution of mGlu5 receptors, together with their modulatory influences on NMDA receptors, among other neuronal constituents, suggests that drugs targeting these receptors will affect multiple behaviors apart from those considered cognitive in nature. These include effects on locomotor sensitization following amphetamine or cocaine treatment (Kinney et al., 2003; Kotlinska and Bochenski, 2009), sensorimotor motor gating (Kinney et al., 2005), appetite and energy balance (Bradbury et al., 2005), and drug discrimination (Besheer et al., 2009). Such effects suggest a role for mGlu5 modulators in the treatment of disorders affecting systems mediating reward perception and impulse control, such as addiction.
In summary, enhancing the function of group I, type 5 mGlu receptors with the systemically available positive allosteric activator CDPPB was capable of attenuating cognitive deficits caused by NMDA receptor hypofunction. Our data suggest that mGlu5 receptors and NMDA receptors interact to influence the activity of neural networks which include the prefrontal cortex. The present data show the merits of analyzing set-shift data across trials when possible, rather than relying on the more simple trials to criterion measurement. The present findings, together with other recently published findings (Ayala et al., 2009; Balschun et al., 2006; Kinney et al., 2005; Kotlinska and Bochenski, 2009; Lecourtier et al., 2007; Liu et al., 2008; Uslaner et al., 2009) provide further evidence that mGlu receptors in general, and mGlu5 receptors in particular, are attractive targets for the development of therapeutic agents for the treatment of cognitive disorders.
Acknowledgments
This work was supported by National Institute of Mental Health grant R01-MH48404. We thank Dr. Gilles Tamagnan for generously providing CDPPB, and Alicia DeFrancesco for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20:323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacologia. 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Shefflere DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones P, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of group I metabotropic glutamate receptors blocks spatial learning in rats. Neurosci Lett. 1998;249:41–44. doi: 10.1016/s0304-3940(98)00388-7. [DOI] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Sallling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D, Rusin KI, Chard PS, Glaum SR, Miller RJ. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol Pharmacol. 1992;42:192–196. [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L, Cosford NDP, Anderson J, Varney MA, Strack AM. Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther. 2005;313:395–402. doi: 10.1124/jpet.104.076406. [DOI] [PubMed] [Google Scholar]
- Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: Structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci. 2008;1141:221–232. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- De Leonibus E, Managò F, Giordani F, Petrosino F, Lopez S, Oliverio A, Amalric M, Mele A. Metabotropic glutamate receptors 5 blockade reverses spatial memory deficits in a mouse model of Parkinson’s disease. Neuropsychopharmacology. 2009;34:729–738. doi: 10.1038/npp.2008.129. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA. Impairment in perceptual attentional set-shifting following PCP administration: A rodent model of set-shifting deficits in schizophrenia. Psychopharmacology. 2005;179:77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- Fagni L, Worley PF, Ango F. Homer as both a scaffold and transduction molecule. Sci STKE %R. 2002:re8. doi: 10.1126/stke.2002.137.re8. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2005;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Haut MW, Cahill J, Cutlip WD, Stevenson JM, Makela EH, Bloomfield SM. On the nature of Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 1996;65:15–22. doi: 10.1016/0165-1781(96)02940-x. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: Effects on working memory, instrumental learning, motor behaviors and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O’Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M. Pretreatment with group I metabotropic glutamate receptor antagonists attenuates lethality induced by acute cocaine overdose and expression of sensitization to hyperlocomotor effect of cocaine in mice. Neurotox Res. 2009 doi: 10.1007/s12640–009–9136–8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EBJ, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, MacDougall L, Abi-Saab W, D’Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine. Arch Gen Psychiatry. 2005;62:985–995. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry. 2007;62:739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Wisnoski DD, Leister WH, O’Brien JA, Lemaire W, Williams DL, Burno M, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Duggan ME, Hartman GD, Conn PJ, Huff JR. Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-Diphenyl-1H-pyrazol-5-yl)benzamides that potentiate receptor function in vivo. J Med Chem. 2004;47:5825–5828. doi: 10.1021/jm049400d. [DOI] [PubMed] [Google Scholar]
- Liu F, Grauer S, Kelley C, Navarra R, Graf R, Zhang G, Atkinson PJ, Popiolek M, Wantuch C, Khawaja X, Smith D, Olsen M, Kouranova E, Lai M, Pruthi F, Pulicicchio C, Day M, Gilbert A, Pausch MH, Brandon NJ, Beyer CE, Comery TA, Logue S, Rosenzweig-Lipson S, Marquis KL. ADX47273 [S-(4-Fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone]: A novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther. 2008;327:827–839. doi: 10.1124/jpet.108.136580. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughn D, Braunewell KH. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex. 2005;15:1703–1713. doi: 10.1093/cercor/bhi047. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Direct and indirect modulation of the N-methyl D-aspartate receptor. Curr Drug Targets CNS Neurol Disord. 2002;1:1–16. doi: 10.2174/1568007023339544. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29:1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card-sorting, the role of the frontal lobes. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Packard MG, Vecchioli SF, Schroeder JP, Gasbarri A. Task-dependent role for dorsal striatum metabotropic glutamate receptors in memory. Learn Mem. 2001;8:96–103. doi: 10.1101/lm.37401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Szeliga P, Gasparini F, Cryan JF. Blockade of the mGlu5 receptor decreases basal and stress-induced cortical norepinephrine in rodents. Psychopharmacology. 2005;179:240–246. doi: 10.1007/s00213-005-2142-5. [DOI] [PubMed] [Google Scholar]
- Pin JP, Acher F. The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr Drug Targets CNS Neurol Disord. 2002;1:297–317. doi: 10.2174/1568007023339328. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori JJY, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Semenova S, Markou A. The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats’ performance in the 5-choice serial reaction time task. Neuropharmacology. 2007;52:863–872. doi: 10.1016/j.neuropharm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepp BE, Eimas PD. Intradimensional and extradimensional shifts in the rat. J Comp Physiol Psychol. 1964;57:357–361. doi: 10.1037/h0043967. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 2006;26:8810–8818. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JSH, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]