Abstract
Throwing tumors a left hook punch: The oncoprotein MDM2 negatively regulates the activity and stability of the tumor suppressor protein p53, and is an important molecular target for anticancer therapy. Mirror image phage display identifies a high-affinity D-peptide ligand of MDM2 that can be developed into a potent and protease-resistant p53 activator with potential antitumor activity.
Keywords: p53, tumor suppressor, MDM2, D-peptides, drug discovery
Peptide inhibition of protein-protein interactions is a promising venue for the development of novel classes of therapeutic compounds[1]. Compared with small molecule inhibitors, peptides are capable of binding and antagonizing target proteins often with high affinity and unsurpassed specificity[2]. Despite significant progress in peptidomimetic chemistry and drug delivery, however, two major technical hurdles still hinder the thriving of peptide therapeutics – poor in vivo stability and membrane permeability. Here, we report the design, aided by mirror image phage display (MIPD)[3] and native chemical ligation (NCL)[4] of a potent D-peptide ligand, termed DPMI-β (TAWYANFEKLLR), of MDM2 – the oncogenic E3 ubiquitin ligase that negatively regulates the activity and stability of the tumor suppressor protein p53. Structural and functional studies indicate DPMI-β competes with p53 for MDM2 binding. Since inhibitors of the p53-MDM2 interaction activate the p53 signaling pathway and induce p53-dependent killing of tumor cells both in vitro and in vivo[5], the proteolytically stable DPMI-β and its derivatives, when coupled with a clinically viable delivery modality, may be of important therapeutic value in tumor eradication.
MDM2 binds the N-terminal transactivation domain of p53 to suppress p53-mediated growth inhibitory and apoptotic responses and to target p53 into the ubiquitin-proteasome pathway for degradation[6]. MDM2 recognizes a minimum of eight amino acid residues of p53, i.e., F19S20D21L22W23K24L25L26 [7], of which Phe19, Trp23 and Leu26 are the most critical residues for recognition[8]. By screening a phage-expressed duodecimal peptide library against a chemically synthesized, site-specifically biotinylated p53-binding domain of MDM2 (25–109MDM2), we previously identified a potent L-peptide ligand termed PMI (TSFAEYWNLLSP) that bound MDM2 identically to p53 but at a significantly higher affinity (KdPMI-MDM2 = 3.2 nM)[9]. However, as L-peptides are susceptible to proteolytic degradation in vivo with poor bioavailability, PMI is of limited therapeutic value. To tackle peptide susceptibility to proteolysis, Kim and colleagues pioneered MIPD – an elegant combinatorial technique that enables identification of proteolysis-resistant D-peptide ligands of a native protein through phage library screening against the (chemically synthesized) D-enantiomer of the L-target[3]. A broad application of MIPD in peptide drug discovery has been made possible by NCL – a powerful synthetic methodology for chemical protein synthesis developed by Kent and co-workers[4]. Screening the phage library against the D-enantiomer of 25–109MDM2 led to the identification of an L-peptide ligand of the D-protein - TNWYANLEKLLR (Figure S1). The D-enantiomer of this phage-selected peptide, termed DPMI-α, competed with p53 for MDM2 binding at an affinity of 219 nM (Table 1 and Figure S2) – 68-fold weaker than PMI but 2-fold stronger than 17–28p53 of the same length.
Table 1.
Amino acid sequences of PMI, 17–28p53, DPMI-α, DPMI-α analogs, and DPMI-β and their dissociation equilibrium constants (Kd) for synthetic 25–109MDM2. Each Kd value is the mean of three independent measurements.
| Name | Sequence | Kd ± S.D. (nM) |
ΔΔG (kcal/mol) |
|---|---|---|---|
| PMI | TSFAEYWNLLSP | 3.2* | N.A. |
| 17–28p53 | ETFSDLWKLLPE | 452* | N.A. |
| DPMI-α | TNWYANLEKLLR | 219 ± 11 | 0 |
| N2A-DPMI-α | TAWYANLEKLLR | 134 ± 6 | −0.29 |
| W3A-DPMI-α | TNAYANLEKLLR | 197 ± 10 µM | 3.96 |
| Y4A-DPMI-α | TNWAANLEKLLR | 9.4 ± 0.6 µM | 2.19 |
| W3A/Y4A-DPMI-α | TNAAANLEKLLR | 558 ± 30 µM | 4.57 |
| N6A-DPMI-α | TNWYAALEKLLR | 730 ± 40 | 0.70 |
| L7A-DPMI-α | TNWYANAEKLLR | 108 ± 6 µM | 3.61 |
| E8A-DPMI-α | TNWYANLAKLLR | 2.3 ± 0.1 µM | 1.37 |
| L10A-DPMI-α | TNWYANLEKALR | 982 ± 52 | 0.87 |
| L11A-DPMI-α | TNWYANLEKLAR | 10.7 ± 0.5 µM | 2.27 |
| L7F-DPMI-α | TNWYANFEKLLR | 59.8 ± 5.9 | −0.76 |
| L7W-DPMI-α | TNWYANWEKLLR | 352 ± 24 | 0.28 |
| DPMI-β | TAWYANFEKLLR | 34.5 ± 0.6 | −1.08 |
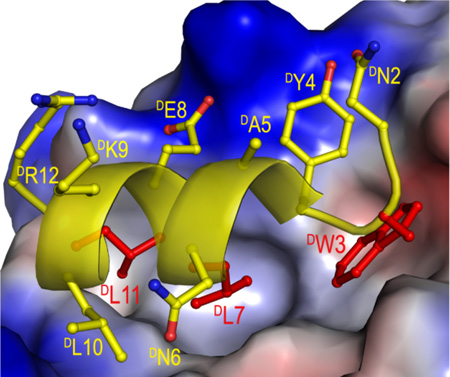
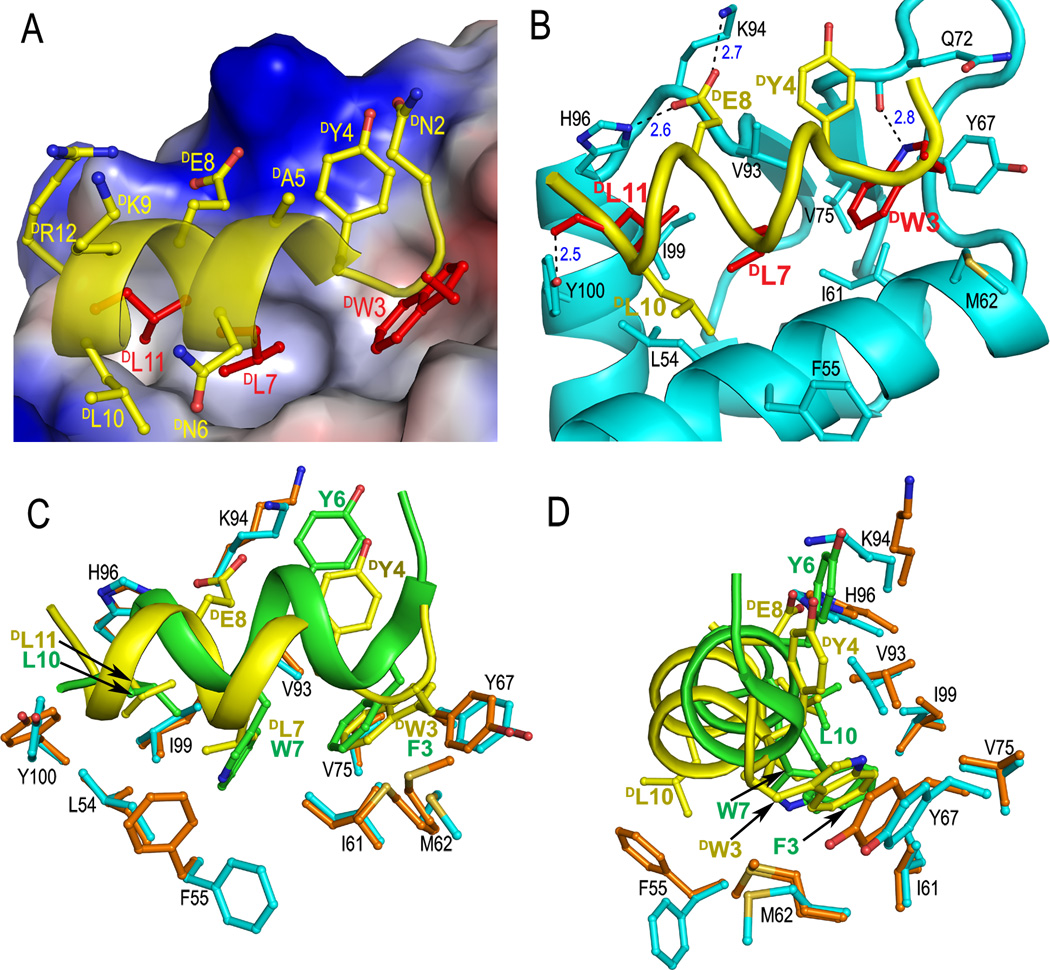
To decipher the structural basis of D-peptide inhibition of the p53-MDM2 interaction, we determined the co-crystal structure of DPMI-α and synthetic 25–109MDM2 at 2.4 Å resolution (Table S1 and Figure S3). As shown in Figure 1A, DPMI-α adopts an amphipathic left-handed helical conformation in the complex, docking the hydrophobic side chains of DTrp3, DLeu7 and DLeu11 into the p53-binding cavity of MDM2. These three D-residues collectively contribute almost 60% of the total buried surface area (BSA) of DPMI-α in the complex. DTyr4 forms π-cation interactions with Lys94 and stacks against His73 and Val93 of MDM2, while DLeu10 stabilizes the peptide-protein complex by making hydrophobic contacts with Leu54 and Phe55 (Figure 1B). Together, DTyr4 and DLeu10 account for roughly 25% of the total BSA. Despite the dominance of hydrophobic force in DPMI-α and MDM2 recognition, electrostatic interactions also play an important role. Four H-bonds form between DPMI-α and MDM2, involving DTrp3 Nε1-Gln72 O, DGlu8 Oε1-Lys94 Nζ, DGlu8 Oε2-His96 Nε2, and DLeu11 O-Ty100 Oη.
Figure 1.
Co-crystal structure of DPMI-α and 25–109MDM2. (A) Close-up view of the interface of the DPMI-α-MDM2 complex. The side chains of DTrp3, DLeu7, and DLeu11 of DPMI-α are colored in red, the rest in yellow. The electrostatic potential at the molecular surface of MDM2 is displayed as negative in red, positive in blue, and apolar in white. (B) A ribbon and stick representation of the binding interface. Only the side chains involved in direct interactions between DPMI-α (yellow) and MDM2 (cyan) are shown in sticks. The dash lines depict inter-molecular H-bonds. (C) DPMI-α(yellow) and PMI (green) from their respective complexes with (superimposed) MDM2. Residues lining the hydrophobic cavity of MDM2 are shown as orange sticks in the PMI complex and cyan sticks in the DPMI-α complex. (D) The side view of (C) after a 90° rotation.
To verify the structural findings, we performed a DAla scanning mutational analysis of DPMI-α at selective positions, and quantified the binding affinity of DPMI-α analogs for MDM2 using a surface plasmon resonance-based competiton assay[9, 10]. The Kd values are tabulated in Table 1 and individual binding curves presented in Figure S2. Four critical hydrophobic residues were identified, DTrp3, DTyr4, DLeu7, and DLeu11, contributing 4.0, 2.2, 3.6, 2.3 kcal/mol, respectively, to DPMI-α binding to MDM2. A double mutation W3A/Y4A weakened the binding affinity of DPMI-α for MDM2 by over 2500-fold (4.6 kcal/mol). Interestingly, the N2A mutation improved DPMI-α activity by nearly a factor of 2, whereas the N6A mutation decreased the binding affinity by roughly 3-fold. Overall, the mutational data are concordant with the DPMI-α-MDM2 structure.
DTrp3, DLeu7 and DLeu11 of DPMI-α are topologically equivalent to Phe3, Trp7, and Leu10 of PMI (or Phe19, Trp23, and Leu26 of p53) despite their opposite handedness (Figures 1C and 1D). Although the overall structures of peptide-bound MDM2 from the PMI-MDM2 and DPMI-α-MDM2 complexes are nearly identical (RMSD(Cα) = 0.56 Å) (Figure S4), superposition of MDM2 reveals notable structural differences at the binding interface between the two complexes. Unlike PMI, the N-terminus of DPMI-α is disordered with DThr1 missing from and DAsn2 less well defined in the electron density map. The D-peptide shifts toward the α2 helix of MDM2, accompanied by a “close-in” movement of residues Val93 to Arg97 lining the opposite edge of the binding pocket. Consequently, Phe55 side chain flips outward to accommodate and interact with DLeu10, while the interacting pattern seen for Tyr6 of PMI is maintained for DTyr4 of DPMI-α. In addition, two new H-bonds involving DGlu8 Oε1 and Oε2 form as indicated earlier. Finally, the side chain of Met62 and the main chain of Tyr67 recess to accommodate DTrp3 in the pocket that is occupied by the Phe residue of PMI or p53.
Importantly, DPMI-α binding to MDM2 did not induce any significant changes to the residues lining the bottom of the p53-binding pocket. As a result, the hydrophobic cavity that accommodates Trp7 of PMI or Trp23 of p53 is only partially filled by the smaller DLeu7 side chain of DPMI-α, suggesting that functional improvement is possible through introduction of a bulkier hydrophobic residue to replace DLeu7. In fact, sequence analysis of all binding clones obtained from MIPD indicates that only Leu, Phe and Trp were selected at position 7 (Figure S1). We therefore mutated DLeu7 to DPhe and DTrp, and characterized L7F-DPMI-α and L7W-DPMI-α with respect to their binding to MDM2 (Table 1 and Figure S2). While the L7W mutation weakened DPMI-α binding (Kd increased to 352 nM), the L7F mutation enhanced the binding affinity of DPMI-α by almost 4-fold (Kd decreased to 59.8 nM). To further improve DPMI-α activity, a double mutation N2A/L7F was introduced, and the resultant D-peptide TAWYANFEKLLR, now termed DPMI-β, bound to MDM2 with a Kd value of 34.5 nM. The predicted ΔΔG value of −1.05 kcal/mol (−0.29–0.76) for DPMI-β relative to DPMI-α is in nearly perfect agreement with the measured binding free energy change of −1.08 kcal/mol due to strongly additive mutational effects. We also quantified DPMI-α and DPMI-β binding to MDMX – a homolog of MDM2 that non-redundantly abrogates the p53 signaling pathway[11]. Despite an almost 8-fold improvement in MDMX binding over DPMI-α, DPMI-β remains a weak ligand of MDMX (Kd = 2.4 µM) (Figure S5).
DPMI-α and DPMI-β are fully resistant to proteolytic degradation (Figure S6). However, neither D-peptide is expected to actively traverse the cell membrane to exert p53-dependent tumor killing activity. Additional work needs to be done to develop delivery vehicles to ensure efficient cellular uptake of these D-peptide activators of p53. Particularly promising in this regard is the hydrocarbon stapling technique developed by Verdine and colleagues, which enables side-chain cross-linked L-α-helical peptides to actively permeabilize cells with enhanced biological activity and proteolytic stability[12]. Several hydrocarbon-stapled L-peptides with various in vitro and/or in vivo antitumor activities have been successfully designed, including a p53-activating peptide[1, 13]. It is conceivable that hydrocarbon stapling of the helical DPMI-α or DPMI-β should result in a cell-penetrating and p53-activating antitumor peptide with enhanced efficacy in vivo due to its full resistance to proteolysis. Notably, various peptidomimetic approaches have been used to design protease-resistant MDM2 antagonists to emulate the activity of the p53 peptide[14]. Of particular interest is the cyclic β-hairpin template developed by Robinson and colleagues[15]. Structured peptide scaffolds have also been used to engineer p53-emulating miniature proteins to antagonize MDM2[10, 16]. However, they are still subject to in vivo degradation by proteases.
In conclusion, by using NCL and MIPD coupled with mutational analysis and rational design, we identified for the first time potent D-peptide ligands of MDM2. X-ray crystallographic studies elucidated the structural basis for high-affinity D-peptide inhibition of the p53-MDM2 interaction, and validated the mode of action of DPMI peptides as a novel class of p53 activators. D-peptide inhibitors are superior to many existing drug candidates in aspects such as potency, specificity, and particularly, in vivo stability. Coupled with a therapeutically viable delivery modality, DPMI-α or DPMI-β and its derivatives may have the potential to be developed into antitumor agents for clinical use.
Experimental Section
Synthesis of D-peptides and D- and L-proteins
All peptides and proteins used in this work were chemically synthesized using the published protocols[4, 17]. MBHA resin was used for the synthesis of D-peptides/proteins, whereas L-peptides/proteins were made on PAM resins. The synthesis of 25–109MDM2, 24–108MDMX and N79K-biotin-L-25–109MDM2 was described previously[9]. Identical procedures were used for the preparation of N79K-biotin-D-25–109MDM2 (Figures S7–S8). All peptides and proteins were purified to homogeneity by reversed-phase HPLC, and their molecular masses were ascertained by electrospray ionization mass spectrometry. Peptide and protein quantification was performed by UV measurements at 280 nm using molar extinction coefficients calculated according to the published algorithm[18].
Mirror image phage display
Screening of the Ph.D.-12™ duodecimal peptide phage library was carried out against 1 µM N79K-biotin-D-25–109MDM2 immobilized on streptavidin-agarose resin as described[9]. Bound phage particles were competitively eluted with 1 mM D-15–29p53, and subsequently amplified in host strain E. coli ER2738. After four rounds of selection, 10 binding clones were randomly picked and sequenced. A second independent screening was performed for confirmation (Figure S1).
Surface plasmon resonance based competition binding assay
The Kd values of D-peptides for MDM2 and MDMX were determined as described[9, 10]. A more detailed description of the assay conditions and the binding curves are presented as supplementary information (Figure S2 and Figure S5). The results tabulated in Table 1 were from three independent measurements.
Crystallization, data collection, structure solution, and refinement
DPMI-α-MDM2 crystals were grown at room temperature using the hanging-drop vapor diffusion method in a buffer containing 0.2 M ammonium sulfate, 0.1 M sodium cacodylate trihydrate, and 30% PEG 8000, pH 6.5. X-ray diffraction data were collected at the X-ray Crystallography Core Facility, University of Maryland at Baltimore. Data integration and scaling, and structure solution and refinement were performed as described[9]. 25–109MDM2 coordinates extracted from the MDM2-PMI complex structure (PDB code: 3EQS)[9] were used as a search model for molecular replacement. Data collection and refinement statistics are summarized in Table S1. Coordinates and structure factors have been deposited in the Protein Data Bank with accession number 3LNJ. Molecular graphics were generated using Pymol (http://pymol.org).
Footnotes
This work was supported in part by a Research Scholar Grant CDD112858 from the American Cancer Society and the National Institutes of Health Grants AI072732 and AI061482 (to W.L.).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Min Liu, Institute of Human Virology, University of Maryland School of Medicine, 725 W. Lombard St., Baltimore, MD 21201 (USA); The First Affiliated Hospital, Xi’an Jiaotong University, School of Medicine (China).
Marzena Pazgier, Institute of Human Virology, University of Maryland School of Medicine, 725 W. Lombard St., Baltimore, MD 21201 (USA).
Changqing Li, Institute of Human Virology, University of Maryland School of Medicine, 725 W. Lombard St., Baltimore, MD 21201 (USA).
Weirong Yuan, Institute of Human Virology, University of Maryland School of Medicine, 725 W. Lombard St., Baltimore, MD 21201 (USA).
Chong Li, Institute of Human Virology, University of Maryland School of Medicine, 725 W. Lombard St., Baltimore, MD 21201 (USA).
Wuyuan Lu, Email: wlu@ihv.umaryland.edu, Institute of Human Virology, University of Maryland School of Medicine, 725 W. Lombard St., Baltimore, MD 21201 (USA).
References
- 1.a) Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Nature. 2009;462:182. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkin MR, Wells JA. Nat Rev Drug Discov. 2004;3:301. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 3.a) Schumacher TN, Mayr LM, Minor DL, Jr, Milhollen MA, Burgess MW, Kim PS. Science. 1996;271:1854. doi: 10.1126/science.271.5257.1854. [DOI] [PubMed] [Google Scholar]; b) Eckert DM, Malashkevich VN, Hong LH, Carr PA, Kim PS. Cell. 1999;99:103. doi: 10.1016/s0092-8674(00)80066-5. [DOI] [PubMed] [Google Scholar]
- 4.a) Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Science. 1994;266:776. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]; b) Kent SB. Chem Soc Rev. 2009;38:338. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]
- 5.a) Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Nat Rev Cancer. 2009;9:862. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]; b) Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J, Bernard D, Zhang J, Lu Y, Gu Q, Shah RB, Pienta KJ, Ling X, Kang S, Guo M, Sun Y, Yang D, Wang S. Proc Natl Acad Sci U S A. 2008;105:3933. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. Science. 2004;303:844. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 6.a) Vousden KH, Lane DP. Nat Rev Mol Cell Biol. 2007;8:275. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]; b) Toledo F, Wahl GM. Nat Rev Cancer. 2006;6:909. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 7.Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]; b) Schon O, Friedler A, Bycroft M, Freund SM, Fersht AR. J Mol Biol. 2002;323:491. doi: 10.1016/s0022-2836(02)00852-5. [DOI] [PubMed] [Google Scholar]
- 8.Bottger A, Bottger V, Garcia-Echeverria C, Chene P, Hochkeppel HK, Sampson W, Ang K, Howard SF, Picksley SM, Lane DP. J Mol Biol. 1997;269:744. doi: 10.1006/jmbi.1997.1078. [DOI] [PubMed] [Google Scholar]
- 9.Pazgier M, Liu M, Zou G, Yuan W, Li C, Li J, Monbo J, Zella D, Tarasov SG, Lu W. Proc Natl Acad Sci U S A. 2009;106:4665. doi: 10.1073/pnas.0900947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Pazgier M, Liu M, Lu WY, Lu W. Angew Chem Int Ed Engl. 2009;48:8712. doi: 10.1002/anie.200904550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marine JC, Dyer MA, Jochemsen AG. J Cell Sci. 2007;120:371. doi: 10.1242/jcs.03362. [DOI] [PubMed] [Google Scholar]
- 12.Schafmeister CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891. [Google Scholar]
- 13.Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. J Am Chem Soc. 2007;129:2456. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray JK, Gellman SH. Biopolymers. 2007;88:657. doi: 10.1002/bip.20741. [DOI] [PubMed] [Google Scholar]
- 15.a) Fasan R, Dias RL, Moehle K, Zerbe O, Vrijbloed JW, Obrecht D, Robinson JA. Angew Chem Int Ed Engl. 2004;43:2109. doi: 10.1002/anie.200353242. [DOI] [PubMed] [Google Scholar]; b) Robinson JA. Acc Chem Res. 2008;41:1278. doi: 10.1021/ar700259k. [DOI] [PubMed] [Google Scholar]
- 16.a) Kritzer JA, Zutshi R, Cheah M, Ran FA, Webman R, Wongjirad TM, Schepartz A. Chembiochem. 2006;7:29. doi: 10.1002/cbic.200500324. [DOI] [PubMed] [Google Scholar]; b) Hu B, Gilkes DM, Chen J. Cancer Res. 2007;67:8810. doi: 10.1158/0008-5472.CAN-07-1140. [DOI] [PubMed] [Google Scholar]; c) Li C, Liu M, Monbo J, Zou G, Yuan W, Zella D, Lu WY, Lu W. J Am Chem Soc. 2008;130:13546. doi: 10.1021/ja8042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnolzer M, Alewood P, Jones A, Alewood D, Kent SB. Int J Pept Protein Res. 1992;40:180. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 18.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. Protein Sci. 1995;4:2411. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]