Abstract
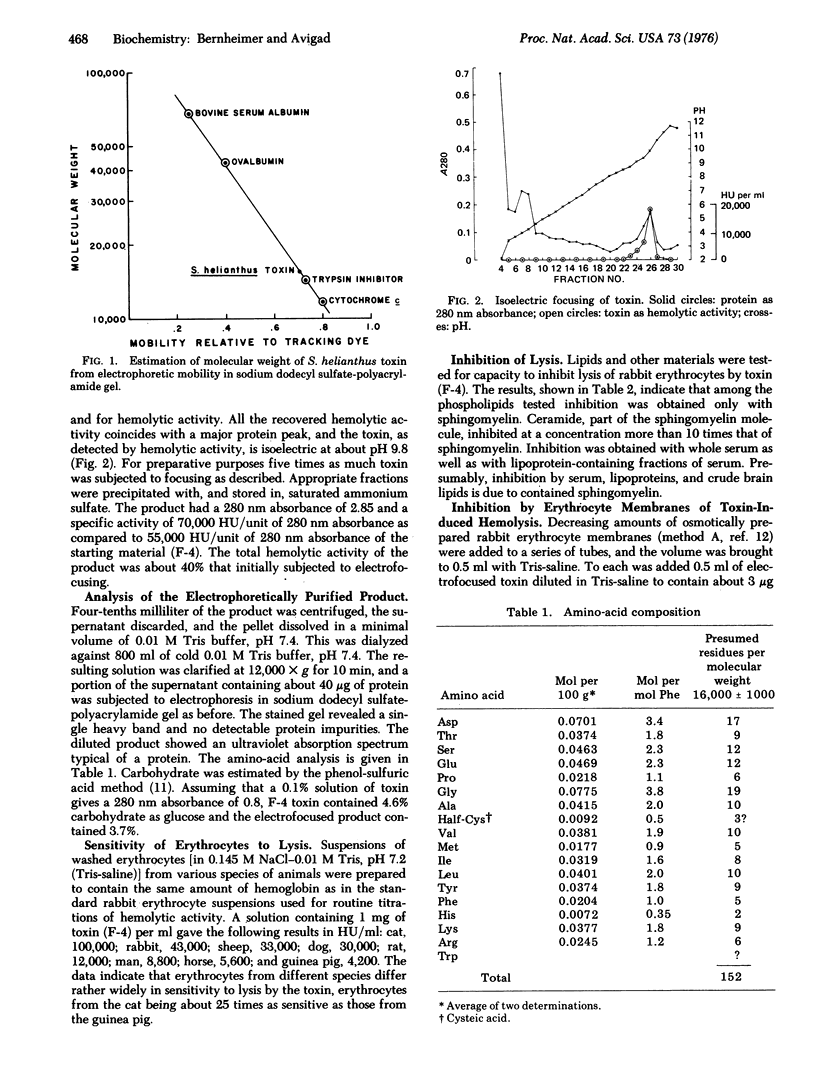
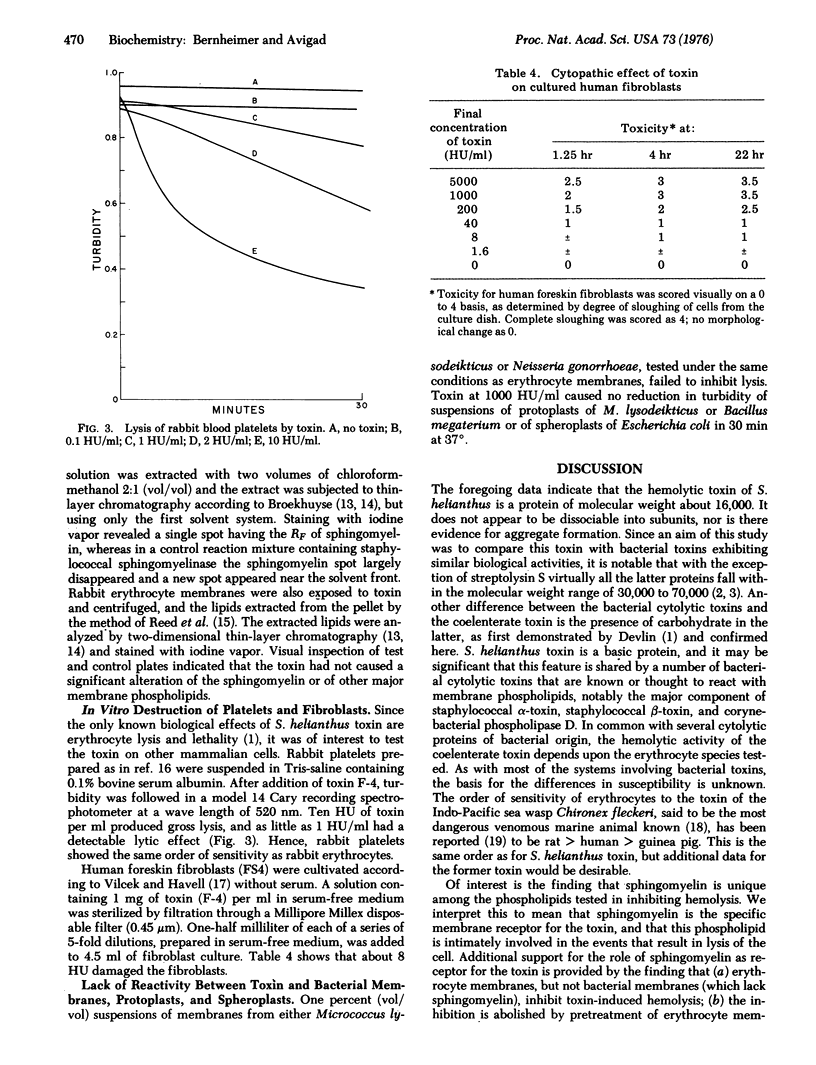
Stoichactis helianthus toxin, a protein derived presumably from the nematocysts, was purified to homogeneity. It has a molecular weight of about 16,000, an isoelectric pH of 9.8, and it contains approximately 3.7% carbohydrate. It is powerfully hemolytic for erythrocytes derived from a variety of animal species, those of the cat being the most sensitive and those of the guinea pig the most resistant. The toxin is lytic also for rabbit blood platelets, and it destroys cultured fibroblasts but is inactive for several kinds of bacterial protoplasts and spheroplasts. The hemolytic activity is specifically inhibited by sphingomyelin, and it is proposed that this phospholipid is the constituent of the membrane which functions as receptor for the toxin. Supporting evidence includes the findings that enzymes known to destroy sphingomyelin (a) prevent erythrocyte membranes from inhibiting hemolysis, and (b) render erythrocytes resistant to lysis by the toxin. The mechanism underlying hemolysis may involve translocation of membrane sphingomyelin by virtue of a specific affinity of the coelenterate protein for this phospholipid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIMER A. W., SCHWARTZ L. L. EFFECT OF STAPHYLOCOCCAL AND OTHER BACTERIAL TOXINS ON PLATELETS IN VITRO. J Pathol Bacteriol. 1965 Jan;89:209–223. doi: 10.1002/path.1700890121. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S., Avigad G. Interactions between aerolysin, erythrocytes, and erythrocyte membranes. Infect Immun. 1975 Jun;11(6):1312–1319. doi: 10.1128/iai.11.6.1312-1319.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S., Kim K. S. Staphylococcal sphingomyelinase (beta-hemolysin). Ann N Y Acad Sci. 1974 Jul 31;236(0):292–306. doi: 10.1111/j.1749-6632.1974.tb41499.x. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S. Partial characterization of aerolysin, a lytic exotoxin from Aeromonas hydrophila. Infect Immun. 1974 Jun;9(6):1016–1021. doi: 10.1128/iai.9.6.1016-1021.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse R. M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim Biophys Acta. 1968 Mar 4;152(2):307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M. Quantitative two-dimensional thin-layer chromatography of blood phospholipids. Clin Chim Acta. 1969 Mar;23(3):457–461. doi: 10.1016/0009-8981(69)90349-0. [DOI] [PubMed] [Google Scholar]
- Devlin J. P. Isolation and partial purification of hemolytic toxin from sea anemone, Stoichactis helianthus. J Pharm Sci. 1974 Sep;63(9):1478–1480. doi: 10.1002/jps.2600630936. [DOI] [PubMed] [Google Scholar]
- Ferlan I., Lebez D. Equinatoxin, a lethal protein from Actinia equina--I. Purification and characterization. Toxicon. 1974 Jan;12(1):57–61. doi: 10.1016/0041-0101(74)90099-3. [DOI] [PubMed] [Google Scholar]
- Hessinger D. A., Lenhoff H. M. Degradation of red cell membrane phospholipids by sea anemone nematocyst venom. Toxicon. 1974 Aug;12(4):379–383. doi: 10.1016/0041-0101(74)90005-1. [DOI] [PubMed] [Google Scholar]
- Keen T. E., Crone H. D. The hemolytic properties of extracts of tentacles from the cnidarian Chironex fleckeri. Toxicon. 1969 Jun;7(1):55–63. doi: 10.1016/0041-0101(69)90164-0. [DOI] [PubMed] [Google Scholar]
- Keen T. E. Interaction of the hemolysin of Chironex fleckeri tentacle extracts with lipid monolayers. Toxicon. 1973 Apr;11(3):293–299. doi: 10.1016/0041-0101(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Norton T. R., Kashiwagi M. Purification of a potent antitumor agent from a Tahitian sea anemone and methods of administration. Studies with Ehrlich ascites tumor in mice. J Pharm Sci. 1972 Nov;61(11):1814–1817. doi: 10.1002/jps.2600611127. [DOI] [PubMed] [Google Scholar]
- REED C. F., SWISHER S. N., MARINETTI G. V., ENEN E. G. Studies of the lipids of the erythrocyte. I. Quantitative analysis of the lipids of normal human red blood cells. J Lab Clin Med. 1960 Aug;56:281–289. [PubMed] [Google Scholar]
- Soucková A., Soucek A. Inhibition of the hemolytic action of and lysins of Staphylococcus pyogenes by Corynebacterium hemolyticum, C. ovis and C. ulcerans. Toxicon. 1972 Aug;10(5):501–509. doi: 10.1016/0041-0101(72)90176-6. [DOI] [PubMed] [Google Scholar]
- VAN HEYNINGEN W. E., MILLER P. A. The fixation of tetanus toxin by ganglioside. J Gen Microbiol. 1961 Jan;24:107–119. doi: 10.1099/00221287-24-1-107. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Wadström T., Vesterberg K., Svensson H., Malmgren B. Studies on extracellular PROTEINS FROM Staphylococcus aureus. I. Separation and characterization of enzymes and toxins by isoelectric focusing. Biochim Biophys Acta. 1967 Apr 11;133(3):435–445. doi: 10.1016/0005-2795(67)90547-8. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A. Stabilization of interferon messenger RNA activity by treatment of cells with metabolic inhibitors and lowering of the incubation temperature. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3909–3913. doi: 10.1073/pnas.70.12.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



