Abstract
The homothallic ascomycete fungus Gibberella zeae is a plant pathogen that is found worldwide, causing Fusarium head blight (FHB) in cereal crops and ear rot of maize. Ascospores formed in fruiting bodies (i.e., perithecia) are hypothesized to be the primary inocula for FHB disease. Perithecium development is a complex cellular differentiation process controlled by many developmentally regulated genes. In this study, we selected a previously reported putative transcription factor containing the Myb DNA-binding domain MYT2 for an in-depth study on sexual development. The deletion of MYT2 resulted in a larger perithecium, while its overexpression resulted in a smaller perithecium when compared to the wild-type strain. These data suggest that MYT2 regulates perithecium size differentiation. MYT2 overexpression affected pleiotropic phenotypes including vegetative growth, conidia production, virulence, and mycotoxin production. Nuclear localization of the MYT2 protein supports its role as a transcriptional regulator. Transcriptional analyses of trichothecene synthetic genes suggest that MYT2 additionally functions as a suppressor for trichothecene production. This is the first study characterizing a transcription factor required for perithecium size differentiation in G. zeae, and it provides a novel angle for understanding sexual development in filamentous fungi.
Introduction
The homothallic ascomycete fungus Gibberella zeae (anamorph: Fusarium graminearum) is a worldwide plant pathogen that causes Fusarium head blight (FHB) in cereal crops and ear rot of maize [1]. This fungal infection leads to severe yield losses and the accumulation of mycotoxins, such as trichothecenes and zearalenone, which are harmful to humans and livestock [2]. G. zeae produces ascospores (sexual spores) and conidia (asexual spores), and both are considered as disease inocula. However, the ascospores formed in fruiting bodies (i.e., perithecia) are proposed to be the primary inocula for FHB. After overwintering as perithecia or perithecia-associated hyphae formed on plant debris, the ascospores are forcibly discharge from mature perithecia during flowering season and are then considered primary inocula [3], [4], [5], [6].
Perithecia are complex multicellular structures that protect sexual spores and ensure their proper discharge [7]. Perithecial morphogenesis can be conveniently divided into three morphologically distinct developmental stages: ascogonial, protoperithecial, and perithecial [8]. Homothallic fungi usually generate female reproductive structures called ascogonia, which further develop into spherical protoperithecia. The tips of ascogenous hyphae contain two nuclei that pair to form the dikaryotic state. This dikaryotic mycelial phase is followed by karyogamy of two haploid nuclei, resulting in a diploid nucleus. The formation of a diploid nucleus is a prerequisite to meiotic division. After meiosis, the four haploid nuclei undergo a postmeiotic mitosis. As a result, every ascus contains eight nuclei, and each nucleus is a starting point for ascospore formation [9], [10]. Although homothallic fungi may not require the mating process, the nuclei are required to pair and form a dikaryon within the ascogenous hyphae [11]. In G. zeae, a previous in-depth microscopic observation of sexual development was unable to identify binucleate hyphae during the initiation of the sexual stage [4]. The binucleate condition was eventually established in the ascogenous hyphae and first observed in the crosiers [4].
The development of perithecia in filamentous ascomycetes is a complex cellular differentiation process that is under polygenic control [12], [13]. The cytology and genetics of ascus development, meiotic silencing by unpaired DNA, and ascospore formation have been studied in considerable detail in Neurospora crassa, Sordaria macrospora, and G. zeae [10], [11], [12], [14], [15], [16]. Some genes identified in these species are now known to be involved in the formation of the perithecia that arise during sexual propagation. Most of these genes take part in signal transduction cascades, transcriptional or posttranscriptional regulation, and primary or secondary metabolism [17]. In S. macrospora, several genes governing the transition from the spherical protoperithecial stage to the flask-shaped perithecial stage have been studied at the molecular level. However, few molecular and biochemical details are known about factors governing this differentiation process [9].
In G. zeae, several genes and pathways are considered to play important roles in perithecial development, including mating type genes [18], [19] and G-protein and MAP-kinase signaling pathways [20], [21], [22], [23]. The release of a genome sequence assembly for G. zeae has allowed the use genome-wide approaches for identifying more genetic elements involved in the sexual reproduction process [24], [25], [26], [27], [28], [29], and forward and reverse genetics-based studies have found many sexual development-related genes [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. With the exception of ROA, null mutants of these genes consistently show defects in the pleiotropic phenotype such as mycelia growth, conidiation, toxin production, virulence, and sexual development.
As a ubiquitous family, proteins containing the Myb DNA-binding domain play diverse roles in eukaryotes, and this domain is typically found in eukaryotic transcription factors. After the identification of the first Myb domain-containing protein, v-Myb of the avian myeloblastosis virus [42], researchers subsequently found several Myb domain-containing proteins, such as c-myb, A-myb, B-myb and their homologs in vertebrates, insects, fungi, and slime molds{Klempnauer, 1982 #8300} [43], [44]. In animals, Myb family members are considered to be oncogenes involved in colon and breast cancer and some human leukemias [45] and to play various roles in the control of cell proliferation, apoptosis, and differentiation [46]. In plants, MYB proteins are a superfamily of transcription factors that regulate networks controlling primary and secondary metabolism, cell fate and identity, developmental processes, and responses to biotic and abiotic stresses [47]. In fungi, even though only a few Myb-related proteins have been reported, the functions of these proteins are various and include G2/M progression and pre-mRNA splicing (cdc5p), termination of rRNA transcription and G1 arrest in response to nitrogen starvation (Reb1), and activation of GCN4-indepentdent HIS4 transcription (BAS1) [48], [49], [50]. The Myb-related gene FlbD was reported in filamentous fungi and is known to control conidiophore development in Aspergillus nidulans [51], [52]. Recently, we found a MYT1 transcription factor containing a Myb domain that is involved in female fertility in G. zeae [41].
Previously, we performed genome-wide functional analyses of whole transcription factor genes in G. zeae [29]. In this study, we selected one gene that previously demonstrated a defect in perithecial development and further characterized its function in G. zeae using a variety of techniques, including gene deletion and overexpression.
Methods
Fungal strains and media
All of the strains used in this study are listed in Table 1. Conidia and mycelia of the wild-type strain Z-3639 [53] and mutants derived from this wild-type strain were stored in 20% glycerol at −70°C. A transgenic strain mat1r carrying both a MAT1–1 deletion and histone H1 tagged with red fluorescence protein (RFP), was used for the co-localization study, as previously described [36]. A minimal medium containing 5 mM agmatine (MMA) was used to evaluate trichothecene production [54]. Yeast malt agar (YMA) was used to induce conidia production as previously described [55]. All of the other media used in this study were prepared as per the Fusarium laboratory manual [1].
Table 1. G. zeae strains used in this study.
Nucleic acid manipulations, primers, and sequencing
Fungal genomic DNA was prepared as previously described [1]. The mycelia or perithecia in different stages were harvested, and total RNA was isolated using the Easy-Spin Total RNA Extraction Kit (Intron Biotech, Seongnam, Korea). Restriction endonuclease digestion, agarose gel electrophoresis, and DNA gel blot hybridization with 32P-labeled probes were performed following standard protocols [56]. PCR primers were synthesized at an oligonucleotide synthesis facility (Bionics, Seoul, Korea) (Table S1) and stored at −20°C at a concentration of 100 μM. General PCR reactions were processed following the manufacturer's instructions (Takara Bio Inc., Otsu, Japan). DNA sequencing was performed by Macrogen Korea (Seoul, Korea), and sequences were compared against the Fusarium Comparative Database at the Broad Institute (http://www.broadinstitute.org/annotation/genome/fusarium_graminearum).
Rapid amplification of cDNA ends (RACE)-PCR
We determined the MYT2 open reading frame (ORF) using rapid amplification of cDNA ends (RACE)-PCR. The cDNA library used as template was constructed in a previous study [36]. Three fragments located around the MYT2 ORF were amplified with MYT2-seq1/MYT2-seq2, pPRN3-N-For/MYT2-seq2, and pPRN3-N-Rev/MYT2-seq1 primers and then directly sequenced.
Genetic manipulations and fungal transformations
We applied a slightly modified double-joint (DJ) PCR strategy [57] to construct fusion PCR products for complementation and overexpression. To complement the MYT2 deletion mutant (Δmyt2), the MYT2 ORF was fused with green fluorescent protein (GFP) by DJ PCR as previously described [36]. In brief, the MYT2 ORF with its own promoter was fused with GFP-hyg, carrying the GFP gene and the hygromycin resistance gene cassette (hyg) amplified from pIGPAPA [58], and the 3′ flanking region of the MYT2 gene. Using this PCR product as a template, a final fusion construct was amplified with the nested primer pair MYT2–5N/MYT2–3N. Finally, we transformed the fusion construct into the myt2 mutant strain.
To overexpress MYT2, we generated a fusion construct containing the 5′ flanking regions of MYT2, the MYT2 ORF, and the gen-PEF1α-carrying elongation factor 1α promoter (PEF1α) from Fusarium verticillioides as previously described [37]. The gen-PEF1α sequence was amplified from pSKGEN [37] with primers Neo-for new and eGFP-P1. The 5′ flanking regions of MYT2 and the MYT2 ORF were amplified by primer pairs MYT2–5F/MYT2–5R OE and MYT2–3F OE/MYT2–3R OE, respectively. Three amplicons were then fused by a secondary round of DJ PCR. Using this fusion fragment as a template, a final PCR product was amplified with the nested primers MYT1–5N and MYT1–3N OE. Finally, this fusion construct was transformed into the wild-type strain.
Quantitative real-time (qRT)-PCR
To obtain the MYT2 expression profile in different G. zeae strains, we extracted the total RNA of each strain from vegetative cultures at 5 d after inoculation and sexual cultures at 3, 5, and 7 d after sexual induction, respectively. We then synthesized the first strand of cDNA from the total RNA with SuperScriptIII reverse transcriptase (Invitrogen, Carlsbad, CA, USA). qRT-PCR was performed using SYBR Green Super mix (Bio-Rad, Hercules, CA, USA) and a 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) with the MYT2-realtime-F/MYT2- realtime-R primers (Table S1). For normalization, the cyclophilin gene (CyP1; locus ID: FGSG_07439.3) was used as an endogenous control [59]. The PCR was repeated three times with two replicates per run. The relative transcript level of MYT2 in each strain was calculated as previously described [60]. Briefly, gene expression was calibrated using the formula 2-ΔΔC T method. The threshold cycle (CT) value of CyP1 was subtracted from that of MYT2 to obtain a ΔCT value. The ΔCT value of MYT2 expression in the wild-type strain at the 5-d vegetative stage was subtracted from the ΔCT value of each sample to obtain a ΔΔCT value. The MYT2 expression level relative to the calibrator was obtained as 2-ΔΔC T. Significant differences (p<0.05) of 2-ΔΔC T were examined statistically among the mean values of the samples based on Tukey's test.
To confirm whether MYT2 regulates the toxin synthesis-related genes, Tri5 and Tri6, we incubated the conidia of wild-type, myt2, and MYT2OE strains in MMA media for 5 d and isolated total RNA from these cultures. We synthesized first-strand cDNA and performed qRT-PCR for the Tri5 and Tri6 genes as described above.
Sexual crosses
Each strain was incubated on carrot agar [1] at 25°C for 5 d. Mycelia grown on carrot agar were mock fertilized with 700 μl of 2.5% Tween 60 solution to induce fertilization and were then incubated under a near-UV light (wavelength: 365 nm, HKiv Import & Export Co., Ltd., Xiamen, China) at 25°C. Seven days after induction, the perithecia from each strain were dissected in a drop of 15% glycerol. The cell size of perithecia and asci rosettes within the perithecia were observed under a DE/Axio Image A1 microscope (Carl Zeiss, Oberkochen, Germany). Nine days after sexual induction, we collected ascospores discharged from the perithecia of each strain and measured the number of septa and the length and width of the ascospores using the same microscope.
We also counted the number of ascospores per perithecia for each strain as previously described [34]. Each strain was inoculated on carrot agar and was mock fertilized. The circular agar block (14.5 mm in diameter) of each strain was downwards fixed on the lid of a 24-well plastic plate (SPL Lifesciences, Pocheon, Korea) 7 d after sexual induction, but before the ascospores were discharged from the perithecia, and incubated at 25°C for another 7 d. Ascospores within the perithecia can be completely discharged onto the plastic plate. All discharged ascospores were collected from the 24-well plastic plate through 14 d after sexual induction with 500 μl of sterile distilled water and were counted with a haemacytometer (Superior Co., Germany). After counting the number of perithecium on each block, the total ascospore number per perithecium was obtained. The experiments were performed three times with three replicates.
Conidia production, morphology, and germination
After a 72-h incubation in 50 ml of complete media (CM) at 25°C on a rotary shaker (150 rpm), the mycelia of each strain were harvested and washed twice with sterile distilled water. To induce conidiation, the mycelia were spread onto YMA plates and incubated at 25°C under near-UV light. After 48 h, the conidia that had formed on the YMA were collected with sterile distilled water, filtered through cheese cloth, washed with sterile distilled water, and centrifuged (5000 rpm, 25°C, 5 min). A 1-ml conidia suspension (1×105 conidia ml−1) of each strain was inoculated into 50 ml of CMC and then incubated at 25°C on a rotary shaker (150 rpm). The number of conidia produced after a 3-d incubation in CMC media was counted to measure conidia production by each strain.
To observe conidial morphology, the conidia produced by each strain on YMA were harvested and stained with Calcofluor white stock solution (10 mg ml–1; Sigma, 18909). Microscopic observation was performed with a DE/Axio Imager A1 microscope (Carl Zeiss) using the filter set 49 (excitation 356; emission 445/50), the number of septa was counted, and the length and width of the conidia were measured.
The conidia germination rate was counted as previously described [60]. A 1-ml conidia suspension (1×107 conidia ml−1) harvested from YMA medium was inoculated into 20 ml of CM or minimal medium (MM). The number of germinated conidia per 200 conidia was counted after incubation at 0, 2, 4, 6, and 8 h. The experiment was performed twice with three replicates for each point.
Virulence test and trichothecene analysis
To test the virulence of each strain on wheat head, the point inoculation method was performed as previously described [33]. The conidia of each strain were harvested from CMC and adjusted to 105 conidia ml−1. Thereafter, 10 μl of each conidial suspension was injected into a center spikelet of the wheat head (cultivar Eunpamil) at mid-anthesis. The wheat plants were then incubated in a humidity chamber for 3 d and transferred to a greenhouse. The number of spikelets showing disease symptoms was counted 14 d after inoculation as previously described [36]. The experiment was performed with five replicate inoculations per strain, and two independent mutant strains were used for the experiment.
The trichothecene analysis was performed as previously described [36]. Briefly, cultures grown in MMA were filtered with cheese cloth and extracted with ethyl acetate. We then concentrated the extracts to dryness. Derivatization of each dry extract was performed with Sylon BZT (BSA + TMCS + TMSI at a 3∶2∶3 ratio, respectively; Supelco, Bellefonte, PA, USA), and the derivatized products were analyzed using a Shimadzu QP-5000 gas chromatograph mass spectrometer (GC-MS, Shimadzu, Kyoto, Japan) with a selected ion-monitoring mode as previously described [61]. We quantified the total trichothecene concentration based on the biomasses produced by each strain in MMA. The experiment was repeated three times.
MYT2-GFP localization
We constructed a strain carrying both MYT2-GFP and hH1-RFP-gen to observe co-localization of MYT2 with nuclei using an outcross between the mat1r strain [36] and the MYT2com strain. After fertilizing the mat1r strain with the MYT2com strain, we performed a single-spore isolation. Ascospores carrying both MYT2-GFP and hH1-RFP-gen were selected using antibiotic resistance and confirmed by PCR assays. We observed localization of the fluorescence signal in cultures grown from CM, MM, carrot agar, and CMC. Microscopic observation was performed with a DE/Axio Imager A1 microscope (Carl Zeiss) using the filter set 38HE (excitation 470/40; emission 525/50) for GFP and the filter set 15 (excitation 546/12; emission 590) for RFP.
Results
MYT2 identification
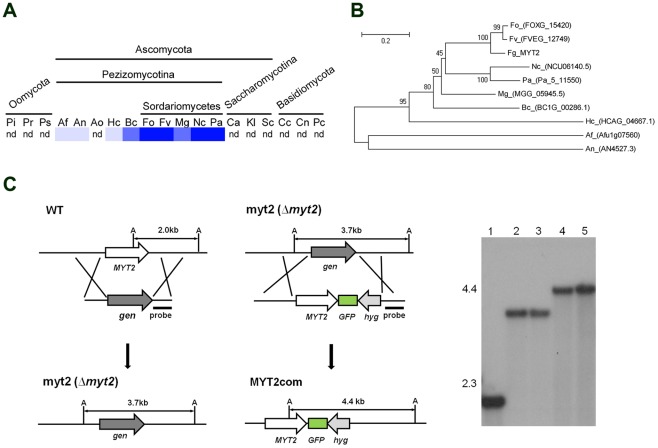
We performed a RACE-PCR and determined that the transcription and splicing of FGSG_07546.3 in vivo were the same as the deduced ORF in the database. We designated FGSG_07546.3 as Myb DNA-binding domain-containing transcription factor 2 (MYT2), which encodes a 323-amino acid polypeptide containing the Myb DNA-binding domain. With the exception of the Myb DNA-binding domain, no other known motif exists in MYT2. MYT2 has no orthologs in the species of the phyla Oomycota and Basidiomycota, but it is conserved in species of the subphylum Pezizomycotina of the Ascomycota, particularly in Sordariomycetes (Figure 1A and B). However, none of the MYT2 orthologs have been functionally characterized in other fungi.
Figure 1. Distribution of MYT2 homologs in fungi and genetic complementation.
(A) Distribution of MYT2 in representative fungal species. The distribution image was constructed using the BLASTMatrix tool that is available on the Comparative Fungal Genomics Platform (http://cfgp.riceblast.snu.ac.kr/) [72]. (B) Phylogenetic tree of MYT2 homologs in several fungal species. The alignment was performed with ClustalW, and the MEGA program, version 4.0, was used to perform a 1,000-bootstrap phylogenetic analysis using the neighbor-joining method. Pi, Phytophthora infestans; Pr, P. ramorum; Ps, P. sojae; Af, Aspergillus fumigatus; An, A. nidulans; Ao, A. oryzae; Hc, Histoplasma capsulatum; Bc, Botrytis cinerea; Fo, Fusarium oxysporum; Fv, F. verticillioides; Mg, Magnaporthe grisea; Nc, Neurospora crassa; Pa, Podospora anserine; Ca, Candida albicans; Kl, Kluyveromyces lactis; Sc, Saccharomyces cerevisiae; Cc, Coprinus cinereus; Cn, Cryptococcus neoformans; Pc, Phanerochaete chrysosporium; nd, not detected. (C) Targeted deletion and complementation of MYT2. WT, G. zeae wild-type strain Z-3639; myt2, MYT2 deletion mutant; MYT2com, myt2-derived strain complemented with MYT2-GFP; A, AvaI; gen, geneticin resistance gene cassette; hyg, hygromycin B resistance gene cassette. Lane 1, Z-3639; lanes 2 and 3, MYT2 mutants; lanes 4 and 5, MYT2com mutants. The sizes of the DNA standards (kb) are indicated to the left of the blot.
Complementation and overexpression
A construct carrying MYT2-GFP was introduced into the previously generated MYT2 deletion mutant [29] for genetic complementation with GFP tagging as previously described [36] to generate the MYT2com strain. Deletion and complementation were confirmed by Southern hybridizations (Figure 1C).
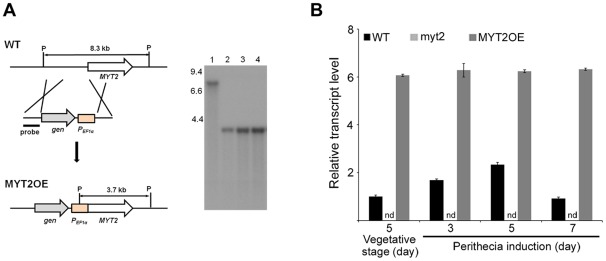
We also generated a MYT2-overexpression strain (MYT2OE) in which MYT2 expression was controlled by inserting the EF1α promoter (Figure 2A). Southern hybridizations were performed to confirm all genetic manipulations (Figure 2A). The MYT2 transcript level in each strain was confirmed by qRT-PCR. In the wild-type strain, the MYT2 expression level was significantly up-regulated at 3 d after sexual induction, increased until 5 d, and decreased again at 7 d. During both the vegetative growth and sexual development stages, the MYT2 expression level in the myt2 strain was undetectable, but it was constitutively up-regulated in the MYT2OE strain by approximately fivefold compared to the wild-type strain (Figure 2B).
Figure 2. MYT2 overexpression.
(A) The MYT2 promoter region was replaced with the EF1α promoter. The left and right panels show the strategy of MYT2OE strain construction and Southern hybridization, respectively. In the blot, lane 1 and lanes 2–4 represent the wild-type strain and the MYT2-overexpressed mutants, respectively. (B) Expression of MYT2 in the wild-type, MYT2 deletion, and MYT2 overexpression strains. MYT2 transcript accumulation was analyzed by quantitative real time-PCR (qRT-PCR) during the vegetative and sexual induction stages. WT, wild-type strain Z-3639; MYT2OE, transgenic strain where the MYT2 promoter region was replaced with the EF1α promoter; P, PstI. The sizes of DNA standards (kb) are indicated to the left of the blot.
Sexual development
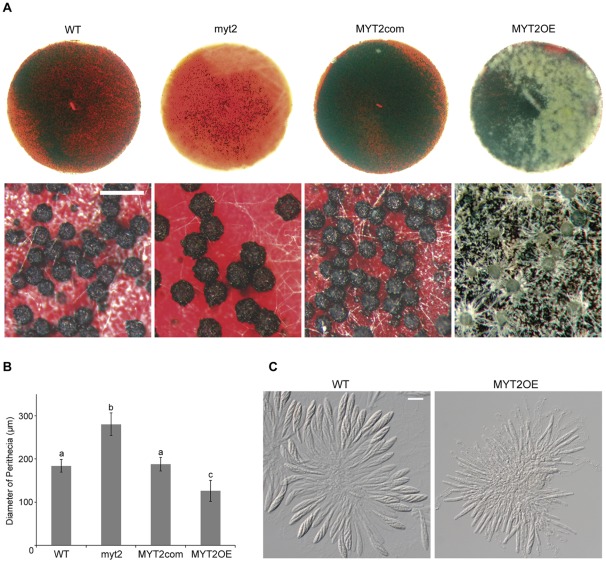
The previously described mutant phenotypes of MYT2 deletion mutants include defects in perithecia formation with normal ascospore formation [29]. Further in-depth examinations were performed in this study. The selfing of the MYT2 deletion strain (myt2) resulted in larger perithecia but approximately 7% of the number of perithecia produced by the wild-type and complemented strains (Figure 3A). The average perithecium diameter produced by myt2 was approximately 280 μm, which was nearly 1.5-fold larger than those produced by the wild-type and complemented strains (p<0.05). By contrast, the average perithecium diameter produced by the MYT2OE strain was approximately 0.7-fold smaller than those produced by the wild-type strain (p<0.05) (Figure 3B).
Figure 3. Self-fertility and asci rosettes of the G. zeae strains.
(A) Perithecia of the G. zeae strains. Five-day old carrot agar culture was mock-fertilized to induce sexual reproduction and incubated for an additional 7 d. The upper and lower panels show the photographs of perithecia formed on a whole carrot agar plate and the photographs taken with a dissecting microscope, respectively. Scale bar = 200 µm. (B) Diameter of the perithecia of the G. zeae strains. The diameters of 300 perithecia were measured for each strain using a dissecting microscope. Values with different letters are significantly different (p<0.05) based on Tukey's test. (C) Asci rosettes of wild-type and MYT2 overexpression strains. Perithecia were dissected seven days after sexual induction. Scale bar = 20 µm. WT, G. zeae wild-type strain Z-3639; myt2, MYT2 deletion mutant; MYT2com, myt2-derived strain complemented with MYT2; MYT2OE, transgenic strain that has the EF1α promoter in place of the MYT2 promoter region.
The cell size of perithecia formed by wild-type and mutant strains was not significantly different (data not shown). Both the myt2 and MYT2com strains formed mature ascospores inside the asci that were similar to the wild-type strain 7 d after sexual induction. Although the MYT2OE mutant showed delayed ascospore maturation, it normally matured and discharged after 3–5 d later (Figure 3C). Ascospores produced by the myt2 mutant were wider than the wild-type strain, and the length and number of septa of the ascospores produced by the MYT2OE mutant were reduced compared to the wild-type strain (Table 2). Thus, myt2 mutants produce larger ascospores, while the MYT2OE mutant produces smaller ascospores.
Table 2. Production and morphology of conidia and ascospores.
| Strain | Conidiation (number/ml)a | Conidiab | Ascosporesc | ||||
| Length | Width | Septa | Length | Width | Septa | ||
| WT | 1.7×106A | 48A | 4.8A | 4.5A | 23A | 5.0A | 2.4A |
| myt2 | 1.7×106A | 49A | 4.9A | 4.5A | 22A | 5.3B | 2.5A |
| MYT2com | 1.8×106A | 48A | 4.9A | 4.5A | 22A | 4.9A | 2.4A |
| MYT2OE | 0.7×105B | 39B | 4.7B | 3.9B | 20B | 4.9A | 1.4B |
Conidiation was measured by counting the number of conidia produced after a 3-d incubation in CMC.
Macroconidia were produced on YMA. A total of 100 macroconidia were observed for each examination.
Ascospores were collected from culture plate lids 10 d after sexual induction. A total of 300 ascospores were observed for each examination.
All experiments were repeated three times with three replicates each. Values within a column with different letters are significantly different (p<0.01) based on Tukey's test.
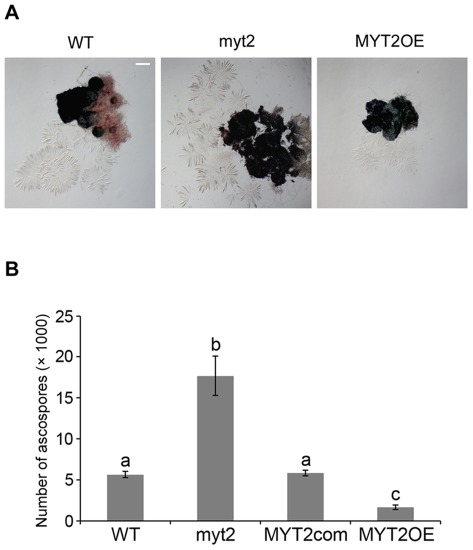
The myt2 and MYT2OE strains contained more and less asci rosettes compared to the wild-type strain, respectively (Figure 4A). The average ascospore number per perithecium in the myt2 mutant was approximately 3-fold greater than the wild-type strain (p<0.05), while the number for the MYT2OE mutant was approximately 0.3-fold less (p<0.05) (Figure 4B). This result was similar to the volume ratio of the perithecia from each strain (1∶3.5∶0.4 for the wild-type strain, the deletion mutant, and the overexpression mutant, respectively), which was calculated based on the diameter assuming the perithecium was a complete globular-shaped structure.
Figure 4. Asci rosettes and ascospores per perithecium of the G. zeae strains.
Each strain was inoculated on carrot agar and was mock fertilized. (A) The perithecia from each strain were softly squeezed with cover slides to exude whole asci rosettes. The picture of each strain is representative of more than 20 repetitions. (B) All discharged ascospores were collected from the culture plate through 14 days after sexual induction. The number of ascospores per perithecia was obtained by dividing the number of perithecia by the number of discharged ascospores. Values with different letters are significantly different (p<0.05) based on Tukey's test. WT, G. zeae wild-type strain Z-3639; myt2, MYT2 deletion mutant; MYT2com, myt2-derived strain complemented with MYT2; MYT2OE, transgenic strain that has the EF1α promoter in place of the MYT2 promoter region.
Conidia production and germination
After a 3-d incubation in CMC media, there was no significant difference in conidial production among the wild-type, myt2, and MYT2com strains. However, the MYT2OE strain only produced 4% of the number of conidia produced by the wild-type strain (p<0.05) (Table 2). Similar to the ascospore morphology, the length, width, and number of septa of the MYT2OE mutant conidia were significantly reduced compared to the other strains (p<0.05) (Table 2). No significant difference was detected among the wild-type, myt2, MYT2com, and MYT2OE strains for conidial germination at 6 h after incubation in CM or MM (Table 3).
Table 3. Radial growth and germination rate.
| Strain | Radial growth (mm)a | Germination (%)b | ||
| CMc | MM | CM | MM | |
| WT | 74Ad | 80A | 86A | 48A |
| myt2 | 79B | 84B | 90A | 54A |
| MYT2com | 73A | 82A | 85A | 45A |
| MYT2OE | 61C | 58C | 86A | 42A |
Radial growth was measured after a 5-d incubation.
The germination percentage was measured after a 6-h incubation.
CM, complete medium; MM, minimal medium.
All experiments were repeated three times with three replicates each. Values within a column with different letters are significantly different (p<0.05) based on Tukey's test.
Vegetative growth, virulence, and trichothecene production
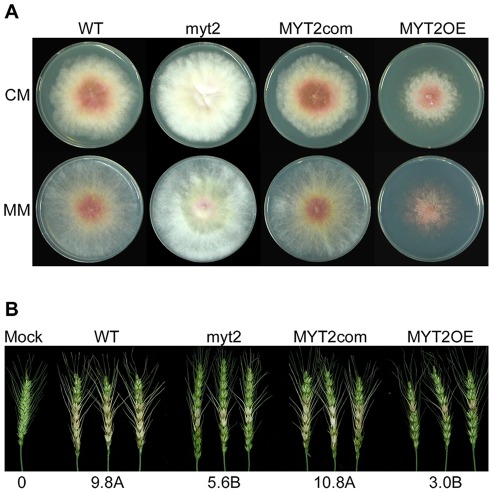
The MYT2 deletion mutant grew faster and produced more aerial mycelia than the wild-type and complemented strains and accumulated a low level of red pigment in both CM and MM. By contrast, the MYT2OE strain had a severe defect in vegetative growth (Figure 5A).
Figure 5. Mycelia growth and wheat head virulence of the MYT1 mutants.
(A) Mycelial growth on complete media (CM) and minimal media (MM) 5 d after inoculation. (B) A center spikelet of each wheat head was injected with 10 µl of conidia suspension. Values with different letters are significantly different (p<0.05) based on Tukey's test. Mock, negative control mock inoculated with 0.01 % Tween 20; WT, G. zeae wild-type strain Z-3639; myt2, MYT2 deletion mutant; MYT2com, myt2-derived strain complemented with MYT2; MYT2OE, transgenic strain that has the EF1α promoter in place of the MYT2 promoter region.
At 14 days after wheat head inoculation, the wild-type and MYT2com strains caused typical head blight symptoms, while both the myt2 and MYT2OE strains showed reduced virulence compared to the wild-type and MYT2com strains (p<0.05). The symptoms merely spread to nearby spikelets on the same wheat heads for the myt2 and MYT2OE strains (Figure 5B).
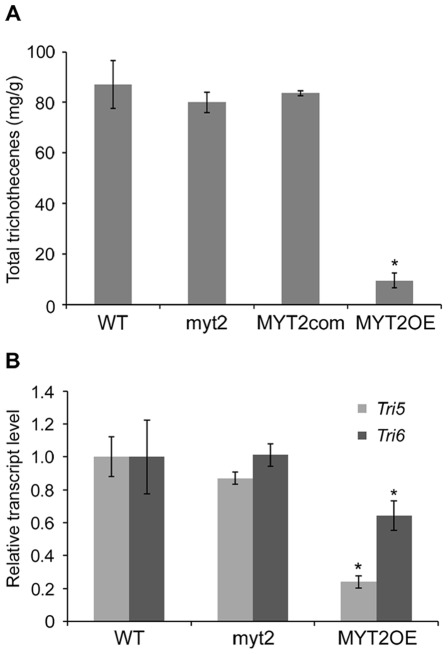
The level of trichothecene synthesized by both the myt2 and MYT2com strains was not significantly different than that of the wild-type strain. However, the MYT2OE mutant produced a significantly reduced level of trichothecene (Figure 6A). Transcription of the trichothecene synthetic genes Tri5 and Tri6 was also significantly reduced in the MYT2OE strain (Figure 6B).
Figure 6. Total trichothecene (deoxynivalenol and 15-acetyldeoxynivalenol) production and transcriptional analyses of trichothecene synthetic genes.
(A) Each strain was grown in minimal medium supplemented with 5 mM agmatine for 7 d. Trichothecenes were analyzed by GC-MS and quantified based on the biomass produced by each strain. Asterisks indicate data that significantly differed (p<0.05) based on Tukey's test (B) Expression of Tri5 and Tri6 in the wild-type, MYT2 deletion, and MYT2 overexpression strains. Gene transcription was analyzed by quantitative real time-PCR (qRT-PCR) 4 d after inoculation in MMA. WT, G. zeae wild-type strain Z-3639; Δmyt2, MYT2 deletion mutant; MYT2com, Δmyt2-derived strain complemented with MYT2; MYT2OE, transgenic strain that has the EF1α promoter inserted in place of the MYT2 promoter region.
MYT2-GFP localization
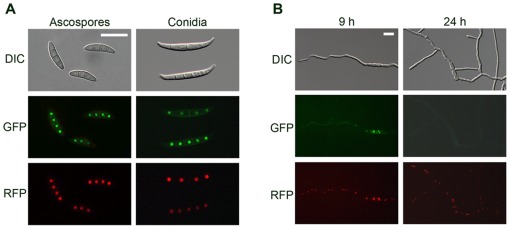
To examine MYT2 localization, the MYT2-GFP fusion construct under the control of its native promoter was transformed into the MYT2 deletion mutant. We selected six MYT2com strains carrying a single MYT2-GFP copy and found a GFP signal in the nuclei of all of the examined strains. To confirm nuclear localization of MYT2-GFP, MYT2comr (Δmyt2::MYT2-GFP-hyg; hH1-RFP-gen) was generated by an outcross between mat1r [36] and MYT2com. MYT2-GFP in the MYT2comr strain co-localized with hH1-RFP and was highly fluorescent in conidia and ascospores (Figure 7A). However, the GFP signals became blurred after germination and were undetectable 24 h later (Figure 7B).
Figure 7. Cellular localization of MYT2.
MYT2 was fused with green fluorescent protein (GFP), and histone H1 was fused with red fluorescent protein (RFP). Co-localization of MYT2-GFP and hH1-RFP in spores (A) and germinated conidia 9 and 24 h after inoculation in complete medium (B). DIC, differential interference contrast Scale bar = 20 µm.
Discussion
The Myb DNA-binding domain is typically found in eukaryotic transcription factors. Previous reports demonstrated that Myb gene family members play diverse roles as transcriptional regulators in multiple cellular processes in animals and plants, including cell proliferation, apoptosis, differentiation, metabolic pathways, cell fate and identity, and stress responses [45], [46], [47], [62], [63], [64], [65]. In fungi, the roles of the transcription factors containing the Myb domain remain largely unknown. However, from the limited studies available, Myb family proteins still show functional diversity and play particularly important roles in cell differentiation and proliferation [49], [50].
In this study, through gene deletion, genetic complementation, and overexpression approaches, we characterized the novel putative transcription factor MYT2, which has functions in various developmental stages including vegetative growth, conidia production, spore morphogenesis, virulence, toxin production, and perithecium development in G. zeae. Interestingly, the deletion of MYT2 resulted in a larger perithecium, while its overexpression resulted in a smaller perithecium when compared to the wild-type strain. Additionally, the ascospores produced by each strain had a relatively consistent perithecial volume. Because MYT2 contains the Myb DNA-binding domain and is localized in nuclei, MYT2 might have an important regulatory role as a transcription factor for the regulation of genes required for cell proliferation and differentiation during perithecium development in G. zeae. Moreover, Sordariomycetes-specific conservation of MYT2 demonstrates a conserved function for perithecial development.
MYT2 seems to be a negative regulator for perithecial size differentiation in G. zeae. The perithecial size difference shown in the MYT2 deletion and overexpression mutants suggests that the MYT2 expression level is negatively related to perithecium size. The MYT2 transcriptional profile during sexual development in the wild-type strain also supports its function as a negative regulator of perithecium size. The MYT2 expression level was the highest at 5 d after sexual induction when the perithecial wall had mostly matured and perithecial cell wall proliferation needed to be stopped. Many previously characterized genes related to sexual development are highly expressed from the beginning of sexual induction and increase expression as the perithecia mature, much like MYT2 [25], [66]. Because the “giant perithecium” in the MYT2 deletion mutant is a novel mutant phenotype, further characterization of the regulons under MYT2 control may reveal a novel pathway of perithecial development.
Similar to other proteins containing the Myb DNA-binding motif in fungi [48], [49], [50], our results suggest that MYT2 is also related to cell differentiation and proliferation in various developmental stages. The deletion and overexpression of MYT2 resulted in enhanced and reduced vegetative growth, respectively, which is similar to the results seen for perithecial development. Compared to the wild-type strain, the MYT2 deletion mutant produced bigger spores, while the overexpression mutant produced smaller spores (Table 2). These results indicated that MYT2 is a suppressor for cell proliferation in various developmental stages in G. zeae. Decreased MYT2-GFP expression during conidial germination also supports our hypothesis (Figure 7B). Taken together, MYT2 negatively affects cell proliferation during perithecial development.
There could be two kinds of possibilities to regulate the size of perithecium by MYT2. First, MYT2 could stop cells from dividing at certain point during perithecial development to control the numbers of cells. Second, it could arrest the growth of differentiated cells to regulate the size of individual cell. Our observation showed that the cell size of perithecia formed by myt2 selfing were not different from that of wild-type strain, suggesting that MYT2 is involved in the former case. Several previous works also support that the fungal Myb-domain containing transcription factors regulate cell division. For example, cdc5p of Schizosaccharomyces pombe was found to be essential for G2/M progression [67], and Reb1 of S. pombe is required for fertility. Reb1was originally found to be involved in the termination of ribosomal RNA (rRNA) transcription through binding to 3′ end of the rDNA-coding region [68]. The binding of Reb1 also blocks DNA replication, giving rise to two natural rDNA replication fork barriers (RFBs) [69]. Recently, it was reported that Reb1 binds to a upstream of ste9 +, resulting in ste9 + up-regulation and G1arrest in response to nitrogen starvation [48].
Overexpression of MYT2 influenced most of the observed phenotypes in the fungus including vegetative growth, sexual development, trichothecene production, and virulence. As a suppressor for cell proliferation, excessive expression of MYT2 might negatively affect conidia production, although MYT2 deletion failed to cause a mutant phenotype in conidiation. In trichothecene production, however, we quantified total trichothecenes based on biomass to reduce the effects of decreased mycelial growth on the result. Markedly reduced transcript accumulations of the genes involved in trichothecene production in the MYT2 overexpression mutant demonstrated that MYT2 additionally functions as a transcriptional repressor for these genes, either directly or indirectly (Figure 6).
The MYT2OE mutant also demonstrated a defect in wheat head virulence. We suspected that a reduction in vegetative growth and trichothecene production, of the MYT2OE mutant would be the reason for reduced virulence [70]. However, the MYT2 deletion mutant also showed reduced virulence even though radial growth was increased and trichothecene production was similar to the wild-type strain. G. zeae virulence is frequently altered by changed hyphal characteristics and the absence of secreted enzymes [23], [32], [60], [71]. Because the mycelial colony of the MYT2 deletion mutant differed from the wild-type strain, the MYT2 deletion mutant appears to possess a defect in other biological functions required for virulence.
One of the important steps in the sexual differentiation process is the morphological transition from spherical pre-fruiting bodies (protoperithecia) to flask-like fruiting bodies (perithecia). Much effort has been put forth to understand this developmental stage. Several developmental mutants that arrest after protoperithecia formation were selected and designated as pro series in S. macrospora. The perithecial morphogenesis of another eight sexual developmental mutants blocked at different stages during perithecia formation has recently been described in detail [9]. However, none of these mutants produce larger perithecia than the MYT2 deletion mutant. Therefore, further in-depth studies of the regulatory roles of MYT2 in perithecial morphogenesis will provide a novel angle for understanding sexual development in filamentous fungi.
Supporting Information
Primers used in this study.
(PDF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2012-0000575). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Leslie JF, Summerell BA. Ames, IA: Blackwell Pub; 2006. The Fusarium laboratory manual. [Google Scholar]
- 2.Desjardins AE. Fusarium mycotoxins: chemistry, genetics, and biology. In: AE Desjardins., editor. editor. St. Paul, MN: APS Press; 2006. [Google Scholar]
- 3.Trail F, Xu H, Loranger R, Gadoury D. Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia. 2002;94:181–189. [PubMed] [Google Scholar]
- 4.Trail F, Common R. Perithecial development by Gibberella zeae: a light microscopy study. Mycologia. 2000;92:130–138. [Google Scholar]
- 5.Parry DW, Jenkinson P, Mcleod L. Fusarium ear blight (scab) in small grain cereals-a review. Plant Pathol. 1995;44:207–238. [Google Scholar]
- 6.Sutton JC. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can J Plant Pathol. 1982;4:195–209. [Google Scholar]
- 7.Nowrousian M, Frank S, Koers S, Strauch P, Weitner T, et al. The novel ER membrane protein PRO41 is essential for sexual development in the filamentous fungus Sordaria macrospora. Mol Microbiol. 2007;64:923–937. doi: 10.1111/j.1365-2958.2007.05694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lord KM, Read ND. Perithecium morphogenesis in Sordaria macrospora. Fungal Genet Biol. 2011;48:388–399. doi: 10.1016/j.fgb.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Engh I, Nowrousian M, Kück U. Sordaria macrospora, a model organism to study fungal cellular development. Eur J Cell Biol. 2010;89:864–872. doi: 10.1016/j.ejcb.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Son H, Min K, Lee J, Raju NB, Lee Y-W. Meiotic silencing in the homothallic fungus Gibberella zeae. Fungal Biol. 2011;115:1290–1302. doi: 10.1016/j.funbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Read ND, Beckett A. Ascus and ascospore morphogenesis. Mycol Res. 1996;100:1281–1314. [Google Scholar]
- 12.Raju NB. Genetic control of the sexual cycle in Neurospora. Mycol Res. 1992;96:241–262. [Google Scholar]
- 13.Dyer PS, Ingram DS, Johnstone K. The control of sexual morphogenesis in the ascomycotina. Biol Rev. 1992;67:421–458. [Google Scholar]
- 14.Raju NB. Neurospora as a model fungus for studies in cytogenetics and sexual biology at Stanford. J Biosci. 2009;34:139–159. doi: 10.1007/s12038-009-0015-5. [DOI] [PubMed] [Google Scholar]
- 15.Raju NB. Six decades of Neurospora ascus biology at Stanford. Fungal Biol Rev. 2008;22:26–35. [Google Scholar]
- 16.Zickler D. From early homologue recognition to synaptonemal complex formation. Chromosoma. 2006;115:158–174. doi: 10.1007/s00412-006-0048-6. [DOI] [PubMed] [Google Scholar]
- 17.Pöggeler S, Nowrousian M, Kück U. Kües U, Fischer R, editors. Fruiting-body development in ascomycetes. Growth, Differentiation and Sexuality: Springer Berlin Heidelberg. 2006. pp. 325–355.
- 18.Desjardins AE, Brown DW, Yun SH, Proctor RH, Lee T, et al. Deletion and complementation of the mating type (MAT) locus of the wheat head blight pathogen Gibberella zeae. Appl Environ Microbiol. 2004;70:2437–2444. doi: 10.1128/AEM.70.4.2437-2444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Lee T, Lee Y-W, Yun S-H, Turgeon BG. Shifting fungal reproductive mode by manipulation of mating type genes: obligatory heterothallism of Gibberella zeae. Mol Microbiol. 2003;50:145–152. doi: 10.1046/j.1365-2958.2003.03694.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu H-Y, Seo J-A, Kim J-E, Han K-H, Shim W-B, et al. Functional analyses of heterotrimeric G protein Gα and Gβ subunits in Gibberella zeae. Microbiology. 2008;154:392. doi: 10.1099/mic.0.2007/012260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urban M, Mott E, Farley T, Hammond-Kosack K. The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. Mol Plant Pathol. 2003;4:347–359. doi: 10.1046/j.1364-3703.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 22.Jenczmionka NJ, Maier FJ, Lösch AP, Schäfer W. Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head-blight disease of wheat, are regulated by the MAP kinase gpmk1. Curr Genet. 2003;43:87–95. doi: 10.1007/s00294-003-0379-2. [DOI] [PubMed] [Google Scholar]
- 23.Hou Z, Xue C, Peng Y, Katan T, Kistler HC, et al. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant-Microbe Interact. 2002;15:1119–1127. doi: 10.1094/MPMI.2002.15.11.1119. [DOI] [PubMed] [Google Scholar]
- 24.Hallen HE, Huebner M, Shiu S-H, Güldener U, Trail F. Gene expression shifts during perithecium development in Gibberella zeae (anamorph Fusarium graminearum), with particular emphasis on ion transport proteins. Fungal Genet Biol. 2007;44:1146–1156. doi: 10.1016/j.fgb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Lee S-H, Lee S, Choi D, Lee Y-W, Yun S-H. Identification of the down-regulated genes in a mat1-2-deleted strain of Gibberella zeae, using cDNA subtraction and microarray analysis. Fungal Genet Biol. 2006;43:295–310. doi: 10.1016/j.fgb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Trail F, Xu JR, San Miguel P, Halgren RG, Kistler HC. Analysis of expressed sequence tags from Gibberella zeae (anamorph Fusarium graminearum). Fungal Genet Biol. 2003;38:187–197. doi: 10.1016/s1087-1845(02)00529-7. [DOI] [PubMed] [Google Scholar]
- 27.Güldener U, Seong KY, Boddu J, Cho S, Trail F, et al. Development of a Fusarium graminearum Affymetrix GeneChip for profiling fungal gene expression in vitro and in planta. Fungal Genet Biol. 2006;43:316–325. doi: 10.1016/j.fgb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Qi W, Kwon C, Trail F. Microarray analysis of transcript accumulation during perithecium development in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Mol Genet Genomics. 2006;276:87–100. doi: 10.1007/s00438-006-0125-9. [DOI] [PubMed] [Google Scholar]
- 29.Son H, Seo Y-S, Min K, Park AR, Lee J, et al. A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum. PLoS Pathog. 2011;7:e1002310. doi: 10.1371/journal.ppat.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baldwin TK, Urban M, Brown N, Hammond-Kosack KE. A role for topoisomerase I in Fusarium graminearum and F. culmorum pathogenesis and sporulation. Mol Plant-Microbe Interact. 2010;23:566–577. doi: 10.1094/MPMI-23-5-0566. [DOI] [PubMed] [Google Scholar]
- 31.Han Y-K, Kim M-D, Lee S-H, Yun S-H, Lee Y-W. A novel F-box protein involved in sexual development and pathogenesis in Gibberella zeae. Mol Microbiol. 2007;63:768–779. doi: 10.1111/j.1365-2958.2006.05557.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim J-E, Lee H-J, Lee J, Kim KW, Yun S-H, et al. Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr Genet. 2009;55:449–459. doi: 10.1007/s00294-009-0258-6. [DOI] [PubMed] [Google Scholar]
- 33.Lee S-H, Han Y-K, Yun S-H, Lee Y-W. Roles of the glyoxylate and methylcitrate cycles in sexual development and virulence in the cereal pathogen Gibberella zeae. Eukaryot Cell. 2009;8:1155–1164. doi: 10.1128/EC.00335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min K, Lee J, Kim J-C, Kim SG, Kim YH, et al. A novel gene, ROA, is required for proper morphogenesis and discharge of ascospores in Gibberella zeae. Eukaryot Cell. 2010;9:1495–1503. doi: 10.1128/EC.00083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim W-B, Sagaram US, Choi Y-E, So J, Wilkinson HH, et al. FSR1 is essential for virulence and female fertility in Fusarium verticillioides and F. graminearum. Mol Plant-Microbe Interact. 2006;19:725–733. doi: 10.1094/MPMI-19-0725. [DOI] [PubMed] [Google Scholar]
- 36.Son H, Lee J, Park AR, Lee Y-W. ATP citrate lyase is required for normal sexual and asexual development in Gibberella zeae. Fungal Genet Biol. 2011;48:408–417. doi: 10.1016/j.fgb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Son H, Lee J, Min G, Choi KJ, et al. Functional analyses of two acetyl coenzyme A synthetases in the ascomycete Gibberella zeae. Eukaryot Cell. 2011;10:1043–1052. doi: 10.1128/EC.05071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Liu W, Hou Z, Wang C, Zhou X, et al. A novel transcriptional factor important for pathogenesis and ascosporogenesis in Fusarium graminearum. Mol Plant-Microbe Interact. 2011;24:118–128. doi: 10.1094/MPMI-06-10-0129. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Heyer C, Choi Y-E, Mehrabi R, Xu J-R. The CID1 cyclin C-like gene is important for plant infection in Fusarium graminearum. Fungal Genet Biol. 2010;47:143–151. doi: 10.1016/j.fgb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Son H, Min K, Lee J, Choi GJ, Kim J-C, et al. Differential roles of pyruvate decarboxylase in aerial and embedded mycelia of the ascomycete Gibberella zeae. FEMS Microbiol Lett. 2012;329:123–130. doi: 10.1111/j.1574-6968.2012.02511.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin Y, Son H, Lee J, Min K, Choi GJ, et al. A putative transcription factor MYT1 is required for female fertility in the ascomycete Gibberella zeae. PLoS ONE. 2011;6:e25586. doi: 10.1371/journal.pone.0025586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klempnauer K-H, Gonda TJ, Bishop JM. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: The architecture of a transduced oncogene. Cell. 1982;31:453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- 43.Lipsick JS. One billion years of Myb. Oncogene. 1996;12:223–235. [PubMed] [Google Scholar]
- 44.Weston K. Myb proteins in life, death and differentiation. Curr Opin Genet Dev. 1998;8:76–81. doi: 10.1016/s0959-437x(98)80065-8. [DOI] [PubMed] [Google Scholar]
- 45.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 46.Oh I-H, Reddy EP. The myb gene family in cell growth, differentiation and apoptosis. Oncogene. 1999;18:17. doi: 10.1038/sj.onc.1202839. [DOI] [PubMed] [Google Scholar]
- 47.Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, et al. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez-Sánchez L, Rodríguez-López M, García Z, Tenorio-Gómez M, Schvartzman JB, et al. The fission yeast rDNA-binding protein Reb1 regulates G1 phase under nutritional stress. J Cell Sci. 2011;124:25–34. doi: 10.1242/jcs.070987. [DOI] [PubMed] [Google Scholar]
- 49.McDonald WH, Ohi R, Smelkova N, Frendewey D, Gould KL. Myb-related fission yeast cdc5p Is a component of a 40S snRNP-containing complex and Is essential for pre-mRNA splicing. Mol Cell Biol. 1999;19:5352–5362. doi: 10.1128/mcb.19.8.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tice-Baldwin K, Fink G, Arndt K. BAS1 has a Myb motif and activates HIS4 transcription only in combination with BAS2. Science. 1989;246:931–935. doi: 10.1126/science.2683089. [DOI] [PubMed] [Google Scholar]
- 51.Shen WC, Wieser J, Adams TH, Ebbole DJ. The Neurospora rca-1 gene complements an Aspergillus flbD sporulation mutant but has no identifiable role in Neurospora sporulation. Genetics. 1998;148:1031–1041. doi: 10.1093/genetics/148.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wieser J, Adams TH. FlbD encodes a Myb-Like DNA-binding protein that coordinates Initiation of Aspergillus nidulans conidiophore development. Genes Dev. 1995;9:491–502. doi: 10.1101/gad.9.4.491. [DOI] [PubMed] [Google Scholar]
- 53.Bowden RL, Leslie JF. Sexual recombination in Gibberella zeae. Phytopathology. 1999;89:182–188. doi: 10.1094/PHYTO.1999.89.2.182. [DOI] [PubMed] [Google Scholar]
- 54.Gardiner DM, Kazan K, Manners JM. Novel genes of Fusarium graminearum that negatively regulate deoxynivalenol production and virulence. Mol Plant-Microbe Interact. 2009;22:1588–1600. doi: 10.1094/MPMI-22-12-1588. [DOI] [PubMed] [Google Scholar]
- 55.Harris SD. Morphogenesis in germinating Fusarium graminearum macroconidia. Mycologia. 2005;97:880–887. doi: 10.3852/mycologia.97.4.880. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook J, Russell DW. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning: a laboratory manual, 2nd ed. [Google Scholar]
- 57.Yu J-H, Hamari Z, Han K-H, Seo J-A, Reyes-Dominguez Y, et al. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol. 2004;41:973–981. doi: 10.1016/j.fgb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Horwitz BA, Sharon A, Lu SW, Ritter V, Sandrock TM, et al. A G protein alpha subunit from Cochliobolus heterostrophus involved in mating and appressorium formation. Fungal Genet Biol. 1999;26:19–32. doi: 10.1006/fgbi.1998.1094. [DOI] [PubMed] [Google Scholar]
- 59.Kwon S-J, Cho S-Y, Lee K-M, Yu J, Son M, et al. Proteomic analysis of fungal host factors differentially expressed by Fusarium graminearum infected with Fusarium graminearum virus-DK21. Virus Res. 2009;144:96–106. doi: 10.1016/j.virusres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Lee S-H, Lee J, Lee S, Park E-H, Kim K-W, et al. GzSNF1 is required for normal sexual and asexual development in the ascomycete Gibberella zeae. Eukaryot Cell. 2009;8:116–127. doi: 10.1128/EC.00176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo J-A, Kim J-C, Lee D-H, Lee Y-W. Variation in 8-ketotrichothecenes and zearalenone production by Fusarium graminearum isolates from corn and barley in Korea. Mycopathologia. 1996;134:31–37. doi: 10.1007/BF00437050. [DOI] [PubMed] [Google Scholar]
- 62.Mizuguchi G, Nakagoshi H, Nagase T, Nomura N, Date T, et al. DNA binding activity and transcriptional activator function of the human B-myb protein compared with c-MYB. J Biol Chem. 1990;265:9280–9284. [PubMed] [Google Scholar]
- 63.Toscani A, Mettus RV, Coupland R, Simpkins H, Litvin J, et al. Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature. 1997;386:713–717. doi: 10.1038/386713a0. [DOI] [PubMed] [Google Scholar]
- 64.Baumann K, Perez-Rodriguez M, Bradley D, Venail J, Bailey P, et al. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development. 2007;134:1691–1701. doi: 10.1242/dev.02836. [DOI] [PubMed] [Google Scholar]
- 65.Duprey SP, Boettiger D. Developmental regulation of c-myb in normal myeloid progenitor cells. Proc Natl Acad Sci USA. 1985;82:6937–6941. doi: 10.1073/pnas.82.20.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, Park C, Kim J-C, Kim J-E, Lee Y-W. Identification and functional characterization of genes involved in the sexual reproduction of the ascomycete fungus Gibberella zeae. Biochem Biophys Res Commun. 2010;401:48–52. doi: 10.1016/j.bbrc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Ohi R, Mccollum D, Hirani B, Denhaese GJ, Zhang X, et al. The Schizosaccharomyces pombe cdc5+ gene encodes an essential protein with homology to c-Myb. EMBO J. 1994;13:471–483. doi: 10.1002/j.1460-2075.1994.tb06282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao A, Guo A, Liu Z, Pape L. Molecular cloning and analysis of Schizosaccharomyces Pombe Reblp: sequence-specific recognition of two sites in the far upstream rDNA intergenic spacer. Nucleic Acids Res. 1997;25:904–910. doi: 10.1093/nar/25.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez-Gorostiaga A, Lopez-Estrano C, Krimer DB, Schvartzman JB, Hernandez P. Transcription termination factor reb1p causes two replication fork barriers at its cognate sites in fission yeast ribosomal DNA in vivo. Mol Cell Biol. 2004;24:398–406. doi: 10.1128/MCB.24.1.398-406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Proctor RH, Hohn TM, McCormick SP. Reduced virulence of Gibberella zeae caused by disruption of a trichthecene toxin biosynthetic gene. Mol Plant-Microbe Interact. 1995;8:593–601. doi: 10.1094/mpmi-8-0593. [DOI] [PubMed] [Google Scholar]
- 71.Hohn TM, Desjardins AE. Isolation and gene disruption of the Tox5 gene encoding trichodiene synthase in Gibberella pulicaris. Mol Plant-Microbe Interact. 1992;5:249–256. doi: 10.1094/mpmi-5-249. [DOI] [PubMed] [Google Scholar]
- 72.Park J, Park B, Jung K, Jang S, Yu K, et al. CFGP: a web-based, comparative fungal genomics platform. Nucleic Acids Res. 2008;36:D562–D571. doi: 10.1093/nar/gkm758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used in this study.
(PDF)