Abstract
Background
To examine whether prenatal exposure to parental type 1 diabetes, type 2 diabetes, or gestational diabetes is associated with an increased risk of malignant neoplasm or diseases of the circulatory system in the offspring.
Methods/Principal Findings
We conducted a population-based cohort study of 1,781,576 singletons born in Denmark from 1977 to 2008. Children were followed for up to 30 years from the day of birth until the onset of the outcomes under study, death, emigration, or December 31, 2009, whichever came first. We used Cox proportional hazards model to estimate hazard ratios (HR) with 95% confidence intervals (95% CI) for the outcomes under study while adjusting for potential confounders. An increased risk of malignant neoplasm was found in children prenatally exposed to maternal type 2 diabetes (HR = 2.2, 95%CI: 1.5–3.2). An increased risk of diseases of the circulatory system was found in children exposed to maternal type 1 diabetes (HR = 2.2, 95%CI: 1.6–3.0), type 2 diabetes (HR = 1.4, 95%CI: 1.1–1.7), and gestational diabetes (HR = 1.3, 95%CI: 1.1–1.6), but results were attenuated after excluding children with congenital malformations. An increased risk of diseases of the circulatory system was also found in children exposed to paternal type 2 diabetes (HR = 1.5, 95%CI: 1.1–2.2) and the elevated risk remained after excluding children with congenital malformations.
Conclusions
This study suggests that susceptibility to malignant neoplasm is modified partly by fetal programming. Diseases of the circulatory system may be modified by genetic factors, other time-stable family factors, or fetal programming.
Introduction
The number of pregnant women with diabetes is increasing as a result of an increasing prevalence of diabetes, a higher age at the time of birth, and earlier onset of diabetes. [1], [2] The health consequences for their children are only partly known. Children born to mothers with diabetes have increased perinatal morbidity and mortality, [3], [4] and more often have early markers of cardiovascular disease [5]–[7] such as insulin resistance. [6] They have a higher birth weight [8] which has been associated with an increased risk of a variety of cancers.[9]–[12] We hypothesized that mothers with diabetes may have children with an increased susceptibility to malignant neoplasm and disease of the circulatory system. Both genetic factors as well as the diabetic intrauterine environment shape disease susceptibility in the offspring. [13].
We therefore conducted a population-based cohort study of 1.7 million children who were followed for up to 30 years to examine whether children born to mothers or fathers with diabetes are more susceptible to malignant neoplasms or diseases of the circulatory system. If parental diabetes affects the outcomes in the offspring mainly through changes in the intrauterine environment, one would expect only maternal diabetes but not paternal diabetes to be associated with the outcomes. If mainly time stable family conditions including genetic factors play a role, one would expect to see both maternal diabetes and paternal diabetes to be associated with the outcomes.
Methods
Ethics Statement
According to Danish laws, register-based studies do not need to obtain consents from individuals, when personal identifiers have been encrypted and stored by a trusted third part (Statistic Denmark). The data recruitment was approved by the Danish Data Protection Agency (J.nr.2008-41-2680).
Study Design, Participants, Exposure, and Outcomes
We identified 1,927,343 singletons born in Denmark from 1977 to 2008 in the Danish Medical Birth Register which includes all births in Denmark since 1973. [14] All persons living in Denmark are assigned a unique personal identification number (civil registration number), which enables accurate linkage of personal data at the individual level. The Danish Civil Registration System was established in 1968 and provides not only civil registry numbers, but also data on vital status and family structure. [15].
The Danish National Hospital Register holds nationwide data on all admissions to any Danish hospital since 1977 and on all outpatient visits since 1995. The information on diabetes was based on the Danish version of the 8th revision of the International Classification of Diseases (ICD-8) from 1977 to 1993, and the 10th revision (ICD-10) from 1994 onwards. [16] All patients with type 1 diabetes or type 2 diabetes were recoded with the same code (ICD-8: 250) between 1977 and 1986 in the Danish version of ICD-8. From 1987 to 1993, the Danish version of ICD-8 differentiated between type 1 diabetes (ICD-8: 249) and type 2 diabetes (ICD-8∶250). From 1994 to 2008, the ICD-10 differentiated between type 1 diabetes (ICD-10: E10, O240), type 2 diabetes (ICD-10: E11, O241), gestational diabetes (ICD-10: O24·4, O24·9), and unspecified diabetes (ICD-10: E12, E13, E14, O242, and O243). We categorized diabetes as unspecified diabetes if the same person were recorded with two or more different ICD codes of diabetes at the same time. If the same person was recorded with different types of diabetes at different times, we categorized diabetes and starting time according to the first ICD code.
We also extracted information on diabetes from the Danish National Diabetes Register that aims at including persons with diabetes who were only treated outside the hospital. The Danish National Diabetes Register holds information on diabetes in the Danish population from 1 January 1995 to 1 January 2007 and it is based on existing registers including the Danish National Hospital Register, the National Health Insurance Service Registry, and the Register of Medicinal Product Statistics. [1], [17] The Danish National Diabetes Register, however, does not differentiate between type 1 diabetes and type 2 diabetes and the register does not include gestational diabetes. [1], [17] We assume that persons with diabetes entirely treated outside the hospital were more likely to have type 2 diabetes, thus any additional diabetes from the Danish National Diabetes Register were categorized as type 2 diabetes.
Children were classified as prenatally exposed to maternal (or paternal) diabetes if they were born after the time when their mothers (or fathers) were recorded with diabetes. Firstly, exposed children born from 1987 to 1993 were categorized as exposed to type 1 or type 2 diabetes at the time of birth. Secondly, Exposed children born from 1994 to 2008 were categorized as exposed to type 1, type 2, or gestational diabetes at the time of birth. Finally, any types of diabetes were grouped in a single category and all exposed children born from 1977 to 2008 and were categorized as exposed to maternal (or paternal) diabetes regardless of types of diabetes.
Information on the outcomes of interest was obtained from the Danish National Hospital Register. Malignant neoplasm were identified as hospitalizations with ICD-8 (140–209) and ICD-10 (C00–C97), diseases of the circulatory system were identified as hospitalizations with ICD-8 (390–458) and ICD-10 (I00–I99), and congenital malformations were identified as hospitalizations with ICD-8 (740-759) and ICD-10 (Q00–Q99).
Information on gestational age, birth weight, maternal age at birth, and parity was obtained from the Danish Medical Birth Registry. [14] Information on maternal education and marital status at the time of birth from 1980 through 2007 was obtained from Statistics Denmark. We used data from 1980 to substitute missing values on maternal education and marital status from 1977 to 1979 and data from 2007 to substitute missing values in 2008, which were not yet available at the time of the study. Some of other missing values on maternal education (or marital status) were replaced by available information in the closest preceding or following five (or three years), whichever came first.
Statistical Analysis
The children were followed from the day of birth until the first hospitalization or first outpatient visit for the outcomes under study, death, emigration, or December 31, 2009, whichever came first.
Since some children were born to the same mothers, we used robust inference for the Cox proportional hazards model to estimate hazard ratios (HRs) with 95% confidence interval (95% CI) for hospitalization due to malignant neoplasm, diseases of the circulatory system, or congenital malformation for children exposed to maternal or paternal diabetes compared to the cohort of unexposed children. In Model 1, we adjusted for maternal age (five years interval), parity (1, 2, 3+), sex of children (boy, girl), maternal education (low, middle, and high), maternal marital status (yes, no), and calendar year (1987–1990, 1991–1993, 1994–1998, 1999–2003, and 2004–2008). In Model 2, we adjusted for the same variables but excluding children with any congenital malformations. In Model 3, we not only excluded children with any congenital malformations but also extended the adjustment to include gestational age in weeks, birth weight, and birth weight squared as continuous variables.
The statistical analyses were done using Stata11 (StataCorp, College station, TX, USA).
Results
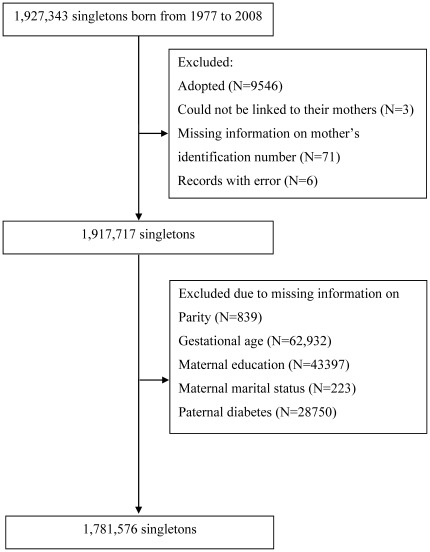
In the final study population, children with missing values on parity, gestational age, birth weight, maternal education, parental marital status, or paternal diabetes were excluded (Figure 1) and 1,781,576 children remained. In the study population, 1,734 (0.1%), 12,401 (0.7%), and 11,507 (0.7%) singletons were prenatally exposed to maternal type 1 diabetes, type 2 diabetes, and gestational diabetes, respectively (Table 1). Pregnant women with type 2 diabetes or gestational diabetes tended to be older than pregnant women without diabetes. A higher proportion of children exposed to maternal diabetes were born preterm (Table 1). Mean birth weight was higher in children exposed to maternal type 1 diabetes (3574 g), type 2 diabetes (3568 g), and gestational diabetes (3583 g) compared to that of children born to mothers without diabetes (3486 g). Children were followed for 15 years on average and up to 30 years.
Figure 1. Selection of the study population.
Table 1. Characteristics of the study population.
| Exposure | Unexposed | TYPE 1 DIABETES | TYPE 2 DIABETES | GESTATIONAL DIABETES | ||||
| N | % | N | % | N | % | N | % | |
| 1,755,712 | 98.6 | 1,743 | 0.1 | 12,401 | 0.7 | 11,507 | 0.7 | |
| Gender | ||||||||
| Boy | 901,560 | 51.4 | 912 | 52.3 | 6,354 | 51.2 | 5,992 | 52.1 |
| Girl | 854,152 | 48.7 | 831 | 47.7 | 6,047 | 48.8 | 5,515 | 47.9 |
| Maternal age | ||||||||
| <20 | 43,122 | 2.5 | 29 | 1.7 | 168 | 1.4 | 50 | 0.4 |
| 20–24 | 340,376 | 19.4 | 243 | 13.9 | 1,792 | 14.5 | 777 | 6.8 |
| 25–29 | 671,642 | 38.3 | 673 | 38.6 | 4,166 | 33.6 | 3,008 | 26.1 |
| 30–34 | 502,165 | 28.6 | 553 | 31.7 | 4,000 | 32.3 | 4,283 | 37.2 |
| 35–39 | 172,677 | 9.8 | 214 | 12.3 | 1,834 | 14.8 | 2,676 | 23.3 |
| > = 40 | 25,730 | 1.5 | 31 | 1.8 | 441 | 3.6 | 713 | 6.2 |
| Parity | ||||||||
| 1 | 805,992 | 45.9 | 808 | 46.4 | 4,298 | 34.7 | 4,093 | 35.6 |
| 2 | 671,117 | 38.2 | 651 | 37.4 | 5,040 | 40.6 | 4,239 | 36.8 |
| 3+ | 278,603 | 15.9 | 284 | 16.3 | 3,063 | 24.7 | 3,175 | 27.6 |
| Gestational week | ||||||||
| <32 | 15,570 | 0.9 | 68 | 3.9 | 263 | 2.1 | 141 | 1.2 |
| 33–36 | 62,296 | 3.6 | 449 | 25.8 | 1,549 | 12.5 | 862 | 7.5 |
| 37 | 68,865 | 3.9 | 522 | 30.0 | 1,874 | 15.1 | 1,286 | 11.2 |
| 38 | 180,318 | 10.3 | 466 | 26.7 | 2,116 | 17.1 | 2,748 | 23.9 |
| 39 | 343,743 | 19.6 | 164 | 9.4 | 2,078 | 16.8 | 3,023 | 26.3 |
| 40 | 620,869 | 35.4 | 53 | 3.0 | 2,582 | 20.8 | 2,672 | 23.2 |
| 41 | 319,005 | 18.2 | 17 | 1.0 | 1,355 | 10.9 | 607 | 5.3 |
| > = 42 | 145,046 | 8.3 | 4 | 0.2 | 584 | 4.7 | 168 | 1.5 |
| Maternal education | ||||||||
| Low | 638,282 | 36.4 | 541 | 31.0 | 4,707 | 38.0 | 4,273 | 37.1 |
| Middle | 560,558 | 31.9 | 626 | 35.9 | 4,092 | 33.0 | 3,488 | 30.3 |
| High | 556,872 | 31.7 | 576 | 33.1 | 3,602 | 29.1 | 3,746 | 32.6 |
| Marital status | ||||||||
| Married | 1,081,838 | 61.6 | 981 | 56.3 | 7,930 | 64.0 | 7,538 | 65.5 |
| Others | 673,874 | 38.4 | 762 | 43.7 | 4,471 | 36.1 | 3,969 | 34.5 |
| Calendar year | ||||||||
| 1977–1981 | 220,649 | 12.6 | 0 | 0.0 | 1,127 | 9.1 | 0 | 0.0 |
| 1982–1986 | 243,977 | 13.9 | 0 | 0.0 | 1,585 | 12.8 | 0 | 0.0 |
| 1987–1990 | 223,008 | 12.7 | 96 | 5.5 | 1,287 | 10.4 | 0 | 0.0 |
| 1991–1993 | 183,333 | 10.4 | 155 | 8.9 | 890 | 7.2 | 3 | 0.0 |
| 1994–1998 | 306,180 | 17.4 | 339 | 19.5 | 2,125 | 17.1 | 2,363 | 20.5 |
| 1999–2003 | 294,896 | 16.8 | 510 | 29.3 | 2,727 | 22.0 | 3,202 | 27.8 |
| 2004–2008 | 283,669 | 16.2 | 643 | 36.9 | 2,660 | 21.5 | 5,939 | 51.6 |
| Paternal diabetes | ||||||||
| Unexposed | 1,745,515 | 99.4 | 1,726 | 99.0 | 12,264 | 98.9 | 11,314 | 98.3 |
| TYPE 1 DIABETES | 2,971 | 0.2 | 3 | 0.2 | 27 | 0.2 | 46 | 0.4 |
| TYPE 2 DIABETES | 7,039 | 0.4 | 14 | 0.8 | 108 | 0.9 | 139 | 1.2 |
The prevalence of congenital malformation was increased in singletons exposed to maternal type 1 diabetes, maternal type 2 diabetes, and gestational diabetes when compared to unexposed singletons among singletons born from 1987 to 2008 (Table 2). This increased prevalence was neither seen in singletons exposed to paternal type 1 diabetes nor in singletons exposed to paternal type 2 diabetes (Table 3). The risks did not change much, when analyses were done among all singletons born from 1977 to 2008 and any types of maternal or paternal diabetes were grouped into a single category (Table 2 and 3).
Table 2. Hazard ratios (HRs) for different health outcomes according to types of maternal diabetes.
| All singletons | Singletons with congenital malformations excluded | ||||||||
| Person-years (PY) | Number of cases | IR/103 PY | Crude HRs | Model 1a | Number of cases | IR/103 PY | Model 2b | Model 3c | |
| Maternal diabetes from 1987 to 2008 | |||||||||
| Malignant neoplasm | |||||||||
| Unexposed | 16,835,352 | 3,318 | 0.20 | 2,707 | 0.18 | ||||
| Type 1 diabetes | 16,619 | 4 | 0.24 | 1.2 | 1.3 (0.5–3.5) | 3 | 0.22 | 1.3 (0.4–4.2) | 1.2 (0.4–3.9) |
| Type 2 diabetes | 68,091 | 27 | 0.40 | 2·0 | 2.2 (1.5–3.2) | 19 | 0.31 | 1.9 (1.2–3.1) | 1.9 (1.2–3.0) |
| Gestational diabetes | 85,308 | 10 | 0.12 | 0.6 | 0.7 (0.4–1.3) | 8 | 0.10 | 0.7 (0.3–1.4) | 0.7 (0.3–1.3) |
| Diseases of the circulatory system | |||||||||
| Unexposed | 16,743,165 | 19,235 | 1.15 | 14,107 | 0.93 | ||||
| Type 1 diabetes | 16,377 | 37 | 2.26 | 2.3 | 2.2 (1.6–3.0) | 15 | 1.09 | 1.4 (0.8–2.3) | 1.2 (0.7–2.1) |
| Type 2 diabetes | 67,590 | 98 | 1.45 | 1.5 | 1.4 (1.1–1.7) | 60 | 0.99 | 1.3 (1.0–1.6) | 1.2 (0.9–1.6) |
| Gestational diabetes | 84,803 | 113 | 1.33 | 1.5 | 1·3 (1.1–1.6) | 70 | 0.92 | 1.3 (1.0–1.6) | 1.2 (1.0–1.6) |
| Congenital malformation | |||||||||
| Unexposed | 15,655,126 | 121,737 | 7.78 | ||||||
| Type 1 diabetes | 14,455 | 251 | 17.36 | 1.8 | 1.7 (1·5–2·0) | ||||
| Type 2 diabetes | 62,765 | 680 | 10.83 | 1.2 | 1.6 (1·1–1·3) | ||||
| Gestational diabetes | 78,523 | 1,125 | 14.33 | 1.2 | 1.2 (1·2–1·3) | ||||
| Any maternal diabetes (1977–2008) | |||||||||
| Malignant neoplasm 2 | |||||||||
| Unexposed | 28,751,460 | 7,224 | 0.25 | 5,935 | 0.23 | ||||
| All diabetes | 283,573 | 90 | 0.32 | 1.4 | 1.5 (1.2–1·8) | 65 | 0.26 | 1.4 (1.1–1.7) | 1.3 (1.0–1.7) |
| Diseases of the circulatory system | |||||||||
| Unexposed | 28,556,453 | 41,143 | 1.44 | 31,994 | 1.25 | ||||
| All diabetes | 280,922 | 484 | 1.72 | 1.5 | 1.4 (1.3–1.5) | 305 | 1.23 | 1.2 (1.1–1.4) | 1.1 (1.0–1.3) |
| Congenital malformation | |||||||||
| Unexposed | 26,628,315 | 171,729 | 6.45 | ||||||
| All diabetes | 256,183 | 2,911 | 11.36 | 1.4 | 1.3 (1·3–1·4) | ||||
Model 1: Hazard ratios (HRs) were adjusted for maternal age (<20, 20–24, 25–29, 30–34, 35–39, and 40+), parity (1, 2, and 3+), sex (boy and girl), maternal education (low, middle, and high), maternal marital status (yes or no), calendar year (1977–1981, 1982–1986, 1987–1990, 1991–1993, 1994–1998, 1999–2003, and 2004–2008).
Model 2: HRs after exclusion of children with congenital malformations and after adjustments for the same variables as that in model 1.
Model 3: HRs after exclusion o children with congenital malformations and after extended adjustments in the Model 1 by including gestational age at birth as a continuous variable, birth weight, and square of the birth weight.
Table 3. Hazard ratios (HRs) for different health outcomes according to types of paternal diabetes.
| All singletons | Singletons with congenital malformation excluded | ||||||||
| Person-years (PY) | Number of cases | IR/103 PY | Crude HRs | Model 1a | Number of cases | IR/103 PY | Model 2b | Model 3c | |
| Paternal diabetes were divided into subtypes (1987–2008) | |||||||||
| Malignant neoplasm | |||||||||
| Unexposed | 17,167,892 | 3,422 | 0.20 | 2,789 | 0.18 | ||||
| Type 1 diabetes | 27,915 | 6 | 0.21 | 1.1 | 1.2 (0.6–2.8) | 5 | 0.20 | 1.3 (0.5–3.0) | 1.3 (0.5–3.0) |
| Type 2 diabetes | 20,123 | 3 | 0.15 | 0.7 | 0.8 (0.3–2.6) | 2 | 0.11 | 0.7 (0.2–2.8) | 0.7 (0.2–2.8) |
| Diseases of the circulatory system | |||||||||
| Unexposed | 17,073,253 | 19,799 | 1.16 | 14,550 | 0.94 | ||||
| Type 1 diabetes | 27,779 | 31 | 1.12 | 1.2 | 1.1 (0.8–1.6) | 24 | 0.94 | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) |
| Type 2 diabetes | 19,969 | 32 | 1.60 | 1.7 | 1.5 (1·1–2·2) | 23 | 1.27 | 1.6 (1.1–2.5) | 1.6 (1.1–2.5) |
| Congenital malformation | |||||||||
| Unexposed | 15,964,140 | 122,947 | 7.70 | ||||||
| Type 1 diabetes | 26,232 | 245 | 9.34 | 1.0 | 1.0 (0·8–1·1) | ||||
| Type 2 diabetes | 18,713 | 228 | 12.18 | 1.2 | 1.1 (1·0–1·3) | ||||
| Any paternal diabetes (1977–2008) | |||||||||
| Malignant neoplasm | |||||||||
| Unexposed | 28,751,460 | 7,224 | 0.25 | 5,935 | 0.23 | ||||
| All diabetes | 131,704 | 29 | 0.22 | 1.0 | 1.0 (0.7–1.5) | 24 | 0.20 | 1.0 (0.7–1·5) | 1.0 (0.7 -1.5) |
| Diseases of the circulatory system | |||||||||
| Unexposed | 28,556,453 | 41,143 | 1.44 | 31,994 | 1.25 | ||||
| All diabetes | 130,683 | 191 | 1.46 | 1.3 | 1.2 (1.0–1.4) | 149 | 1.27 | 1.2 (1.0–1·4) | 1.2 (1.0–1.4) |
| Congenital malformation | |||||||||
| Unexposed | 26,628,315 | 171,729 | 6.45 | ||||||
| All diabees | 122,039 | 973 | 7.97 | 1.1 | 1.0 (1.0–1.1) | ||||
Model 1: Hazard ratios (HRs) were adjusted for maternal age (<20, 20–24, 25–29, 30–34, 35–39, and 40+), parity (1, 2, and 3+), sex (boy and girl), maternal education (low, middle, and high), maternal marital status (yes or no), calendar year (1977–1981, 1982–1986, 1987–1990, 1991–1993, 1994–1998, 1999–2003, and 2004–2008).
Model 2: HRs after exclusion of children with congenital malformations and after adjustments for the same variables as that in model 1.
Model 3: HRs after exclusion of excluding children with congenital malformations and after extended adjustments in the Model 1 by including gestational age at birth as a continuous variable, birth weight, and square of the birth weight.
An increased risk of malignant neoplasm was found in singletons exposed to maternal type 2 diabetes compared to unexposed singletons and the risk remained high after excluding singletons with congenital malformations. The HRs were attenuated a little when analyses were done among all singletons exposed to any types of maternal diabetes (Table 2). A detailed list of ICD codes and number of malignant neoplasm in children exposed to maternal (and paternal) diabetes are available in Table S1.
An increased risk for disease of the circulatory system was found in singletons exposed to maternal type 1 diabetes, maternal type 2 diabetes, and gestational diabetes but these HRs were attenuated when children with congenital malformations were excluded (Table 2). Similar pattern was found when analyses were done among all singletons and any types of maternal or paternal diabetes were grouped into a single category (Table 2). An increased risk for diseases of the circulatory system was also found in singletons exposed to paternal type 2 diabetes. The risks remained high even after excluding children with congenital malformations, but were attenuated when analyses were done among all singletons and any types of maternal or paternal diabetes were grouped into a single category (Table 3). A detailed list of ICD codes and number of disease of the circulatory system in children exposed to maternal (and paternal) diabetes are available in Table S2.
Discussion
An increased risk for malignant neoplasm was found in children prenatally exposed to maternal type 2 diabetes, and the increased risk remained after excluding children with congenital malformations. An increased risk of disease of the circulatory system was found not only in children exposed to maternal diabetes but also in children exposed to paternal type 2 diabetes.
A higher prevalence of congenital malformations was found in children prenatally exposed to maternal diabetes, which is in line with previous studies. [18]–[23] An increased risk of congenital malformation, however, was not seen in children prenatally exposed to paternal diabetes, which indicates that the observed association between maternal diabetes and congenital malformations may be due to the changes in intrauterine environment induced by maternal diabetes rather than genetic factors.
We found an increased risk of malignant neoplasm in children prenatally exposed to maternal type 2 diabetes and the associations seemed not to be modified by higher birth weight or preterm birth since the results did not change much when we adjusted for these factors. A previous study reported a higher rate of hospitalization due to neoplasms in children born to mothers with diabetes (OR = 1.64, 95%CI: 1.06–2.54) [24] but this was related to maternal insulin dependent diabetes. [25] We did not see an increased risk of malignant neoplasm in children prenatally exposed to paternal diabetes. The results indicate that the risk of malignant neoplasm later in life may to some extent be programmed by a suboptimal intrauterine environment associated with maternal diabetes. The biological mechanism underlying the association is unknown but there are several possible factors that could play a role such as maternal and fetal hyperglycemia [4] and the fetal response to these changes such as hyperinsulinmia. [8] Medical treatments of diabetes during pregnancy may also play a role. Our findings are, however, based on few observations and larger studies with longer follow-up time are wanted. Congenital malformations did not act as a mediator for the observed associations between maternal diabetes and risk of malignant neoplasm since the results did not change much when we excluded children with congenital malformations from the analyses.
Intrauterine exposure to maternal diabetes is associated with childhood overweight, obesity, metabolic syndrome [26], [27], and type 2 diabetes [28] in the offspring, all of which are risk factors for diseases of the circulatory system. An increased risk of hypertension has been reported in offspring of diabetic mothers in humans [6], [29] and animals. [30] We found an overall increased risk of diseases of the circulatory system, not only in children prenatally exposed to maternal diabetes, but also in children exposed to paternal type 2 diabetes, which suggest that genetic or other time-stable family factors also play a role. However, diseases of the circulatory system in our study population were, to large extent, related to congenital abnormalities rather than age-related cardiovascular diseases, which makes congenital malformations act as a mediator for the observed associations between maternal diabetes and risk of disease of the circulatory system. Longer follow-up time is needed for studies on atherosclerosis, myocardial infarction, stroke, and cardiovascular death. In addition, the endpoints in the current study were hospitalizations due to a category of diseases instead of hospitalizations due to a specific disease. Thus the observed associations between maternal or paternal diabetes and disease of the circulatory system may not apply to specific diseases in the categories.
Although type 1 diabetes and type 2 diabetes have common features in terms of increased level of glucose, triglycerides, and many amino acids in the maternal circulation, their genetic background and ability to modify the intrauterine environment probably differ. [31] Previous studies suggest that changes in long-term health outcomes in the offspring of diabetic mothers are not strongly dependent on the type of maternal diabetes, [28], [32], [33] which is supported by some but not all results in the current study.
We used a large population-based cohort including all children born in Denmark and had up to 30 years of almost complete follow-up. The completeness of the registration of diabetes, malignant neoplasm, and diseases of the circulatory system is high since health services, including antenatal care and hospitalizations, are free of charge for all citizens in Denmark. However, some women have type 2 diabetes without knowing it [34] and their children will remain in the unexposed cohort, which would attenuate the associations slightly. In addition, some misclassification between different types of diabetes is likely, [34] especially between 1987 and 1993. We were able to adjust for a number of variables in the analyses but unfortunately, we had no data on lifestyle factors such as maternal smoking, pre-pregnancy body mass index, or breast-feeding. The observed associations could be confounded by these factors.
Conclusions
This study suggests that susceptibility to malignant neoplasm and congenital malformation may be results of fetal programming induced by maternal diabetes. The risk of disease of circulatory system may be related to genetic factors or other time stable family factors as well as fetal programming. Congenital malformation may act as one of the potential pathways of associations between diabetic intrauterine environment and risk of diseases of the circulatory system in children prenatally exposed to maternal diabetes. We had insufficient follow time to examine risk of atherosclerosis and other aging related cardiovascular diseases. Paternal diabetes had no association with congenital malformation measured as prevalence at birth.
Supporting Information
A detailed list of the ICD codes and number of malignant neoplasm in children exposed to parental type 1 diabetes (T1D), type 2 diabetes (T2D), and gestational diabetes (GD).
(DOCX)
A detailed list of the ICD codes and number of disease of circulatory system in children exposed to parental type 1 diabetes (T1D), type 2 diabetes (T2D), and gestational diabetes (GD).
(DOCX)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was supported by the Danish Cancer Society (grant number DP04127) and the Danish Medical Research Council (FSS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Carstensen B, Kristensen JK, Ottosen P, Borch-Johnsen K. The Danish National Diabetes Register: trends in incidence, prevalence and mortality. Diabetologia. 2008;51:2187–2196. doi: 10.1007/s00125-008-1156-z. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Hawdon JM. Babies born after diabetes in pregnancy: what are the short- and long-term risks and how can we minimise them? Best Pract Res Clin Obstet Gynaecol. 2011;25:91–104. doi: 10.1016/j.bpobgyn.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz R, Teramo KA. Effects of diabetic pregnancy on the fetus and newborn. Semin Perinatol. 2000;24:120–135. doi: 10.1053/sp.2000.6363. [DOI] [PubMed] [Google Scholar]
- 5.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005;90:3225–3229. doi: 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tam WH, Ma RC, Yang X, Li AM, Ko GT, et al. Diabetes Care; 2010. Glucose Intolerance and Cardiometabolic Risk in adolescent exposed to maternal Gestational Diabetes–a 15-year follow-up study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz R. Hyperinsulinemia and macrosomia. N Engl J Med. 1990;323:340–342. doi: 10.1056/NEJM199008023230512. [DOI] [PubMed] [Google Scholar]
- 9.Mellemkjaer L, Olsen ML, Sorensen HT, Thulstrup AM, Olsen J, et al. Birth weight and risk of early-onset breast cancer (Denmark). Cancer Causes Control. 2003;14:61–64. doi: 10.1023/a:1022570305704. [DOI] [PubMed] [Google Scholar]
- 10.Ross JA. High birthweight and cancer: evidence and implications. Cancer Epidemiol Biomarkers Prev. 2006;15:1–2. doi: 10.1158/1055-9965.EPI-05-0923. [DOI] [PubMed] [Google Scholar]
- 11.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 12.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8:1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Niu T, Manson JE, Kwiatkowski DJ, Liu S. Are variants in the CAPN10 gene related to risk of type 2 diabetes? A quantitative assessment of population and family-based association studies. Am J Hum Genet. 2004;74:208–222. doi: 10.1086/381400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45:320–323. [PubMed] [Google Scholar]
- 15.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 16.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 17.Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The National Diabetes Register. Scand J Public Health. 2011;39:58–61. doi: 10.1177/1403494811404278. [DOI] [PubMed] [Google Scholar]
- 18.Damm P, Molsted-Pedersen L. Significant decrease in congenital malformations in newborn infants of an unselected population of diabetic women. Am J Obstet Gynecol. 1989;161:1163–1167. doi: 10.1016/0002-9378(89)90656-x. [DOI] [PubMed] [Google Scholar]
- 19.Aberg A, Westbom L, Kallen B. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum Dev. 2001;61:85–95. doi: 10.1016/s0378-3782(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 20.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen Jørgen. Baltimore: The Williams & Wilkins Company; 1977. The Pregnant Diabetic and Her Newborn.195 [Google Scholar]
- 22.Reece EA, Homko CJ. Prepregnancy care and the prevention of fetal malformations in the pregnancy complicated by diabetes. Clin Obstet Gynecol. 2007;50:990–997. doi: 10.1097/GRF.0b013e31815a634b. [DOI] [PubMed] [Google Scholar]
- 23.Reece EA. Diabetes-induced birth defects: what do we know? What can we do? Curr Diab Rep. 2012;12:24–32. doi: 10.1007/s11892-011-0251-6. [DOI] [PubMed] [Google Scholar]
- 24.Aberg A, Westbom L. Association between maternal pre-existing or gestational diabetes and health problems in children. Acta Paediatr. 2001;90:746–750. [PubMed] [Google Scholar]
- 25.Westbom L, Aberg A, Kallen B. Childhood malignancy and maternal diabetes or other auto-immune disease during pregnancy. Br J Cancer. 2002;86:1078–1080. doi: 10.1038/sj.bjc.6600192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009;94:2464–2470. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- 27.Moore TR. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am J Obstet Gynecol. 2010;202:643–649. doi: 10.1016/j.ajog.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 28.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31:340–346. doi: 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- 29.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, et al. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22:215–220. doi: 10.1038/ajh.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YW, Chenier I, Tran S, Scotcher M, Chang SY, et al. Maternal diabetes programs hypertension and kidney injury in offspring. Pediatr Nephrol. 2010;25:1319–1329. doi: 10.1007/s00467-010-1506-1. [DOI] [PubMed] [Google Scholar]
- 31.Buchanan TA. Derek LeRoith, Jerrold M, Simeon ITaylor., editors. Intermediary Metabolism During Pregnancy: Implications for Diabetes Mellitus. Diabetes Mellitus: A Fundamental and Clinical Text. 2004. pp. 1237–1250.
- 32.Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med. 2009;14:119–124. doi: 10.1016/j.siny.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(Suppl 2):B142–B149. [PubMed] [Google Scholar]
- 34.Glumer C, Jorgensen T, Borch-Johnsen K. Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care. 2003;26:2335–2340. doi: 10.2337/diacare.26.8.2335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A detailed list of the ICD codes and number of malignant neoplasm in children exposed to parental type 1 diabetes (T1D), type 2 diabetes (T2D), and gestational diabetes (GD).
(DOCX)
A detailed list of the ICD codes and number of disease of circulatory system in children exposed to parental type 1 diabetes (T1D), type 2 diabetes (T2D), and gestational diabetes (GD).
(DOCX)