Abstract
Liao ning virus (LNV) is related to Banna virus, a known human-pathogen present in south-east Asia. Both viruses belong to the genus Seadornavirus, family Reoviridae. LNV causes lethal haemorrhage in experimentally infected mice. Twenty seven isolates of LNV were made from mosquitoes collected in different locations within the Xinjiang province of north-western China during 2005. These mosquitoes were caught in the accommodation of human patients with febrile manifestations, or in animal barns where sheep represent the main livestock species. The regions where LNV was isolated are affected by seasonal encephalitis, but are free of Japanese encephalitis (JE). Genome segment 10 (Seg-10) (encoding cell-attachment and serotype-determining protein VP10) and Seg-12 (encoding non-structural protein VP12) were sequenced for multiple LNV isolates. Phylogenetic analyses showed a less homogenous Seg-10 gene pool, as compared to segment 12. However, all of these isolates appear to belong to LNV type-1. These data suggest a relatively recent introduction of LNV into Xinjiang province, with substitution rates for LNV Seg-10 and Seg-12, respectively, of 2.29×10−4 and 1.57×10−4 substitutions/nt/year. These substitution rates are similar to those estimated for other dsRNA viruses. Our data indicate that the history of LNV is characterized by a lack of demographic fluctuations. However, a decline in the LNV population in the late 1980s - early 1990s, was indicated by data for both Seg-10 and Seg-12. Data also suggest a beginning of an expansion in the late 1990s as inferred from Seg-12 skyline plot.
Introduction
The genus Seadornavirus encompasses mosquito-borne 12 segmented dsRNA viruses that have been isolated in South-east Asia. The genus is classified within family Reoviridae, subfamily Sedoreovirinae [1], and contains 3 recognised species, Banna virus (BAV), Kadipiro virus (KDV) and Liao ning virus (LNV) [2]. Seadornaviruses are transmitted by Anopheles, Culex and Aedes mosquitoes. Banna virus, which is the type species, has been isolated from mosquitoes in Indonesia (particularly in Java) [3], Vietnam [4] and China [5]. Structural analysis of outer-capsid proteins of BAV showed an ancestral relationship to the rotaviruses, which are non-vectored enteric dsRNA viruses also belonging to the family Reoviridae [6].
Banna virus was first isolated from cerebrospinal fluids (CSF) (2 isolates) and sera (25 isolates) of patients with encephalitis in the Yunnan province (Xishuang Banna prefecture) of southern China in 1987. It was also isolated in 1992 from patients with fever and flu-like manifestation [7] in the Xinjiang province. Numerous further isolates were obtained from human patients suffering from encephalitis [5], [8], [9]. BAV was also isolated from rodents, cattle and pigs, and from sera and CSF of humans with encephalitis and febrile illness in several other provinces of China (Beijing, Gansu, Hainan, Henan, Shanshi) and is therefore implicated as pathogenic in humans [5], [7], [10]. In contrast Kadipiro virus has only been isolated from mosquitoes [3].
Banna virus and Kadipiro virus replicate in both insect cells and in mice, although their replication in mammalian cells is restricted to BSR cells [11], [12], [13]. Liao ning virus, replicates in insect cells, in a wide range of primary and transformed mammalian cells, and in mice, causing lethal haemorrhages after two successive injections [14]. This paper describes a survey of arboviruses in North-east China, which was intended to identify emerging or re-merging viruses circulating in the region, including flaviviruses (such as Japanese encephalitis virus (JEV)), alphaviruses and Banna virus. Although JEV was not isolated during these surveys, LNV was isolated from 27 pools of mosquitoes (containing a total 3276 insects). We describe sequence analyses and phylogenetic comparisons of genome segments 10 and 12 (Seg-10 and Seg-12) from these isolates. Segment 10 encodes the cell attachment outer capsid protein of LNV and defines serotype [14]. Segment 12 of LNV encodes a non-structural protein of unknown function. Previously, sequences of segment 12 obtained from viremic animals were found to be highly diverse [14].
Results
Virus isolation and identification by PCR
Viruses were isolated from 27 pools of mosquitoes caught during July-August 2005 in the Kashi Prefecture of Xinjiang Province in North-west China (table 1). The viruses were all grown in Ades albopictus C6/36 cells, which became fusiform and detached from the culture surface. RNA preparations extracted from the infected cell-cultures were analysed by 1% agarose gel electrophoresis, in each case generating a migration pattern identical to that of the prototype isolate of LNV-1 (LNV-NE9712) (data not shown).
Table 1. LNV isolates made from wild-caught Culex mosquitoes collected between July and August 2005.
| Isolate code | Virus isolate | Mosquito | Places collected | Date of collection (night of/month/year) | Number of mosquitoes per pool |
| J1 | 0507JS1 | Culex species | Human accommodation, Qi Village, Jiashi county | 8–9/07/2005 | 82 |
| J2 | 0507JS2 | Culex species | Human accommodation, Qi Village, Jiashi county | 8–9/07/2005 | 88 |
| J4 | 0507JS4 | Culex species | Stock,, Qi Village, Jiashi county | 8–9/07/2005 | 93 |
| J24 | 0507JS24 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J27 | 0507JS27 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J29 | 0507JS29 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J32 | 0507JS32 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J35 | 0507JS35 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J44 | 0507JS44 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J54 | 0507JS54 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J55 | 0507JS55 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J59 | 0507JS59 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| J60 | 0507JS60 | Culex species | Stock, Tierimu Town, Jiashi County | 11–12/07/2005 | 100 |
| B5 | 0507BS5 | Culex species | Stock, Boshikeranmu village | 7–8/07/2005 | 201 |
| B6 | 0507BS6 | Culex species | Stock, Boshikeranmu village | 7–8/07/2005 | 200 |
| B7 | 0507BS7 | Culex species | Stock, Boshikeranmu village | 7–8/07/2005 | 233 |
| B8 | 0507BS8 | Culex species | Stock, Boshikeranmu village | 7–8/07/2005 | 200 |
| B9 | 0507BS9 | Culex species | Human accommodation, Boshikeranmu village | 7–8/07/2005 | 179 |
| B10 | 0507BS10 | Culex species | Stock, Boshikeranmu village | 7–8/07/2005 | 200 |
| J′13 | 0508JS13 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
| J′18 | 0508JS18 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
| J′36 | 0508JS36 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
| J′37 | 0508JS37 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
| J′41 | 0508JS41 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
| J′44 | 0508JS44 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
| J′46 | 0508JS46 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
| J′49 | 0508JS49 | Culex species | Stock, Tierimu Town, Jiashi County | 15–16/08/2005 | 100 |
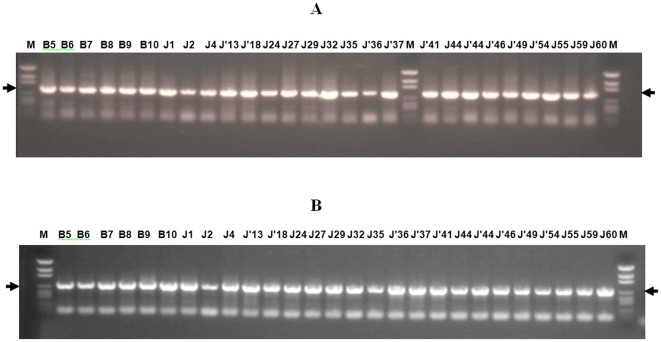
RT-PCRs, using primers designed to target Seg-10 (903 bp-long) and Seg-12 (760 bp-long) of the LNV genome, generated cDNAs of the expected sizes (845 bp for Seg-10 and 675 bp for Seg-12) from all 27 virus isolates (Figure 1). However, reactions containing generic flavivirus primers, alphavirus primers, or primers designed to amplify BAV Seg-9 (Table 2), all failed to generate PCR amplicons (positive-controls included RNA from Middleburg virus [genus Alphavirus], yellow fever virus 17D [genus Flavivirus] and Banna virus [BAV-Ch] - data not shown).
Figure 1. PCR amplicons from segments 10 and 12 of LNV isolates from Xinjiang province.
A: amplicon obtained using primers LNV10s/LNV10r1 (products size 845 bp), B: amplicon obtained using primers LNV12S/LNV10R (products size 675 bp).the PCR products were run on 1% agarose gel in TAE buffer and subsequently stained with ethidium bromide.
Table 2. List of primers used in PCR amplifications.
| virus | Primer designation | Position/orientation | Reference sequence accession number | Sequence | Reference |
| Flavivirus | PF1S | 8987-9006/forward | NC_002031 | TGYRTBTAYAACATGATGGG | [50] |
| Flavivirus | PF2R | 9239-9258/reverse | NC_002031 | GTGTCCCADCCDGCDGTRTC | [50] |
| Alphavirus | M2W | 164-186/forward | L01443 | YAGAGCDTTTTCGCAYSTRGCHW | [51] |
| Alphavirus | M2W2 | 288-313/forward | L01443 | TGYCCNVTGMDNWSYVCNGARGAYCC | [51] |
| Alphavirus | cM3W | 568-597/reverse | L01443 | ACATDAANKGNGTNGTRTCRAANCCDAYCC | [51] |
| BAV | 9-JKT-S | * 597-618 (A) or 591-611(B)/forward | AF052033 and AF052011 | TGGGATYYHAASAWGATYAAAC | [12] |
| BAV | 9-JKT-R | * 1059-1088 (A) or 1000-1029 (B)/reverse | AF052033 and AF052011 | ACTCAGTKASTACTMYCRRGGGGTGGCTTC | [12] |
| LNV | LNV12s1 | 79-101/forward | AY317110 | GGAAGAATCAATGCCGTAGCCAC | [14] |
| LNV | LNV12r1 | 584-561/reverse | AY317110 | GTGACGATCTTCTCTGAACCAGTG | [14] |
| LNV | LNV12s2 | 105-128/forward | AY317110 | CACTGGCTCCGGCTGTAGTAACAG | [14] |
| LNV | LNV12r2 | 539-516/reverse | AY317110 | CTGTTCGGATCATCTGGAATTTGA | [14] |
| LNV | LNV12S | 13-37/forward | AY317110 | CAACTTGAACTTACTGGTGTGTTTG | This study |
| LNV | LNV12R | 687-665/reverse | AY317110 | GCCTTAGAACTTAAAGTTGTGAG | This study |
| LNV | LNV10S | 21-46/forward | AY317108 | ATGAGTAACGTGACAGAGATTCGTGC | This study |
| LNV | LNV10R | 864-839/reverse | AY317108 | GTTCCCGGACTTTCACAGCTACTTTC | This study |
These include previously published primers for flaviviruses, alphaviruses, Banna virus (BAV), Liao ning virus (LNV) and primers designed in this study for Liao ning virus. *: A and B, refer to genotypes (corresponding to serotypes) A and B of BAV.
Sequence analyses
Sequence analyses of Seg-10 and Seg-12 and comparisons to published data, showed that all 27 isolates belong to LNV-1 (represented by prototype isolate LNV-9712) [14], [15]. The sequences of Seg-10 and Seg-12 from the 27 LNV isolates were deposited in Genbank database under accession numbers HM745506- HM745532and HM745533-HM745554, respectively. The level of nt identity in Seg-12 varied from 99.0% to 100% between the 27 new LNV isolates, while the level of aa identity detected in the deduced VP12 sequences, ranged from 98% to 100%. Comparisons to Seg-12 of the earlier prototype strain of LNV-1 (LNV-9712), showed lower nt identities, ranging from 94 to 94.8%, with VP12 aa identity ranged from 99.1% to 99.93% (indicating that most of the changes were ‘silent’, involving the 3rd base position of the codon). When compared to Seg-12 of the unique LNV-2 isolate (LNV-NE9731), nt identity levels ranged from 87.7% to 87.8%, with 99.8% to 99.9% aa identities in VP12 (again reflecting multiple ‘silent’, third base changes).
LNV Seg-10 encodes the cell-attachment protein VP10, which determines virus serotype [14]. The level of nt identity detected in Seg-10 between the 27 new isolates ranges from 94.3% to 100%, with 99.0% to 100%aa identity in VP10. These levels are consistent with isolates from a single serotype of LNV [14] or BAV [11]. Comparisons of Seg-10 from the 27 new isolates, with the prototype strain of LNV-1 (LNV-NE9712), identified nucleotide substitutions in a total of ninety positions within the sequenced region (nt: 21–864), 76.6% of which were 3rd position changes, 7.8% 2nd position and 15.6% 1st position. Overall, the prototype LNV-1 and the new isolates showed 91.9% to 93.1% nt, and 94.2% to 96.6% aa identity, which is still consistent with membership of the same virus serotype (members of the same LNV serotype show >80% amino acid identity in VP10 [14], while members of the same BAV serotype show >89% amino acid identity in VP9 [11]).
Comparisons of Seg-10 and VP10 from the new LNV isolates with the prototype strain of LNV-2 (LNV-NE9731), showed nt identity levels of only 75.1% to 75.3%, and aa levels of 79% to 80% - consistent with membership of different serotypes [14]. Analysis of base changes at the 1st , 2nd and 3rd codon position of the 27 isolates, showed a total of 203 substitutions relative to LNV-2 (186 of which are ‘segregating’: i.e. showing more than 2 different bases [in different isolates] for the same position). Of these changes 55.4% were at the 3rd position, 19.9% at the 2nd position and 24.7% at the 1st position.
The Tajima D test of neutrality, implemented in MEGA4, was used to assess selection. The expected value for populations that conform to a standard neutral selection model is zero [16]. However the D values for LNV Seg-10 and Seg-12 were −2.13 and −2.55 respectively which therefore reject a ‘null hypothesis’ for neutral selection.
Sequence diversification in LNV infected mice
Previous work [14] has shown that all three BAV, KDV and LNV were able to replicate in mice and could be detected in blood a few days after intra-peritoneal injection. A high level of sequence diversification has previously been reported to occur in LNV Seg-12/VP12, after intra-peritoneal injection of the virus into mice [14]. BAV and KDV infections were not lethal, did not result in severe clinical presentations and produced protective immunity. Re-infection was not accompanied by detectable virus replication. The situation observed after LNV injection was different. Primary infection provoked a 5 days prostration after which all tested animals recovered. However, re-infection with the same serotype or a different serotype of LNV caused a generalised haemorrhage and resulted in death of the mice.
We carried out a further study, by injecting subcutaneously LNV-1 (isolate LNV-NE9712) into 4 mice, then re-isolating the virus from mouse blood (collected 3 days post-injection) on BSR and C6/36 cells. Cell culture passage one (P1) of the re-isolated virus was used for molecular studies. Amplicons (506 bp-long) obtained by nested PCR using primers LNV12s2 and LNV12r2 (Table 2) from the infected blood sample, or from the subsequent virus isolate were sequenced. A total of 37 aa changes were detected in VP12 between different clones derived from the blood sample, with only 3 clones identical to the parental sequence. In contrast all of the clones derived from the re-isolated virus P1 in BSR or C6/36 cells were identical to the sequence of the parental virus strain, indicating that passage in cell culture had a significant selective or ‘purifying’ effect.
Twenty cDNA clones were also generated (using primers LNV10S/LNV10R) for Seg-10 of the virus re-isolated in BSR or C6/36 cells. In each case, only one codon change was identified at the same position (nucleotides 64–66: GCA→AAC), changing in alanine (A, position 82) into asparagine (N), compared to all field isolates of LNV-1.
LNV was identified by RT-PCR in the spleen of injected mice, two weeks after injection. Sequencing showed that Seg-10 and Seg-12 of the virus present in the spleen, were identical to those of the virus re-isolated from the blood collected at 3 days post-injection, indicating that the highly divergent sequences were ‘bottlenecked’. All of the blood-derived sequences have been deposited in the Genbank database under accession numbers HM756693, HM756694 and HM745468 to HM745505.
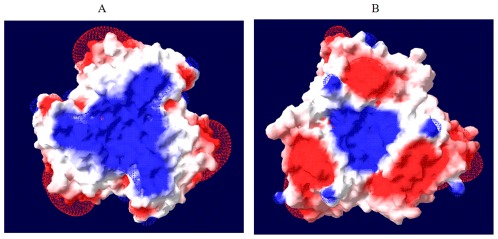
A model for the structure of LNV-1 VP10, was generated using the programme ‘MODELLER’, based on the previously published atomic structure of the homologous BAV VP9 [6] (figure 2). The model indicates that aa position 82 is located within a ‘basic’ depression at the upper/outer surface of the protein trimer. As suggested previously, this basic surface is thought to interact with an ‘acidic’ receptor-molecule on the cell-surface [6]. Most 1st and 2nd base variations that were detected in Seg-10 of the field isolates of LNV-1 are located between nucleotides 369 and 720, which maps to the ‘head’ of the protein monomer (aa 117 to 244), not to the ‘tail’ region where the coiled coils are located.
Figure 2. Theoretical model of the VP10 of LNV based on the atomic structure of the homologous VP9 of BAV.
A: the BAV VP9 atomic structure, B: theoretical model of the VP10 of LNV. The model was constructed using the programme MODELLER. The mutation (A82→N82) found in the VP10 of the virus isolated on cell culture from the mouse blood collected at 3 days post-injection and the virus identified in the spleen 2 weeks post-injection of LNV-9712, mapped to the basic (blue colour) depression on the surface of the VP10 trimer.
Phylogenetic and genealogy analyses
For phylogenetic reconstructions, we determined the shape-parameter alpha to be used for the gamma distance. This shape parameter measures the variability of the rates-of-change between different sites within a set of sequences. When alpha is >1, most sites will have similar rates of change, although when alpha is ≤1 most sites have very low rates, but with evolutionary hot spots with higher rates [17]. The value of the shape parameter calculated for the cloned Seg-12 sequences obtained directly from mouse blood was 0.3, while that calculated for the original mosquito isolates was to 0.5. These values indicate that most sites have very low rates of change, but there are evolutionary hot spots. Calculations of the shape parameter for aa sequences of VP12 showed that the alpha value is 0.3, again suggesting conservation with certain hotspots.
The shape parameter for both the nt and aa sequences of Seg-10 derived from the mosquito isolates was 0.3. The neighbour-joining trees (figures 3 and 4) constructed using sequences of the new field isolates, confirmed their identity as LNV-1. The LNV isolates (from Jiashi and Boshikeranmu) represented a single ‘cluster’.
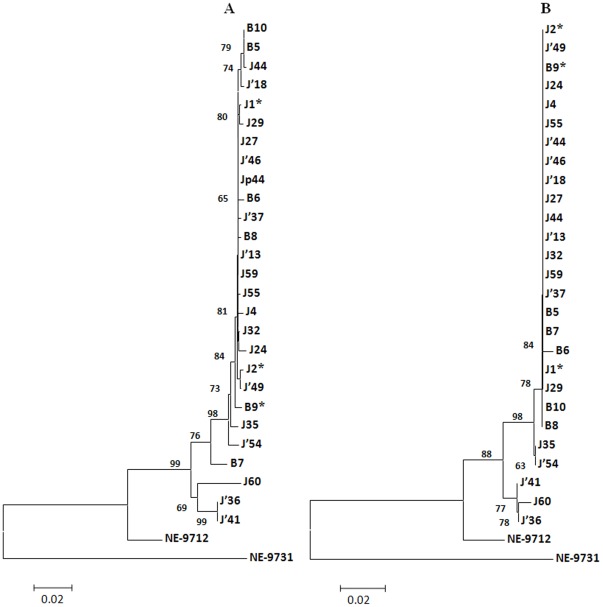
Figure 3. Phylogenetic trees for segment 10 and VP10 of LNV isolates from Xinjiang province.
A: Phylogenetic tree based on the nucleotide sequences. B: Phylogentic tree based on the amino acid sequences. Bootstrap values for 500 replications are indicated at each node. The trees were constructed using the Neighbor joining method and the Kimura 2 parameters method (nucleotides) and Poisson's correction method (amino acids). Similar trees were obtained with the P-distance algorithm. The bar represents the number of substitutions per site. Isolate designations are those reported in table 2. NE-9712 (LNV-NE9712) and NE-9731 (LNV-NE9731) are prototype strains of LNV serotypes 1 and 2, respectively. The letter B in isolates designation refers to Boshikeranmu while J refers to Jiashi county.
Figure 4. Phylogenetic trees for segment 12 and VP12 of LNV isolates from Xinjiang province.
A: Phylogenetic tree based on the nucleotide sequences. B: Phylogentic tree based on the amino acid sequences. Bootstrap values for 500 replications are indicated at each node. The trees were constructed using the Neighbor joining method and the Kimura 2 parameters method (nucleotides) and Poisson's correction method (amino acids). Similar trees were obtained with the P-distance algorithm. The bar represents the number of substitutions per site. Isolate designations are those reported in table 2. NE-9712 (LNV-NE9712) and NE-9731 (LNV-NE9731) are prototype strains of LNV serotypes 1 and 2, respectively. The letter B in isolates designation refers to Boshikeranmu while J refers to Jiashi county.
A gene genealogy analysis of Seg-10 showed that 23 of the LNV-1 isolates (out of 27 isolated in Xinjiang) form a ‘network’ with a group of 5 identical isolates that : (J59, J′13, J27, J′46 and J′44et - figure 5A). This analysis is reflected in the tree (Figure 3), which also groups all of these isolates through a common node showing 98% bootstrap confidence. Both the tree and the genealogy analyses indicate that 4 isolates (J60, J′36 and J′41 and B7) are not directly connected to the network and do not form a second network within themselves.
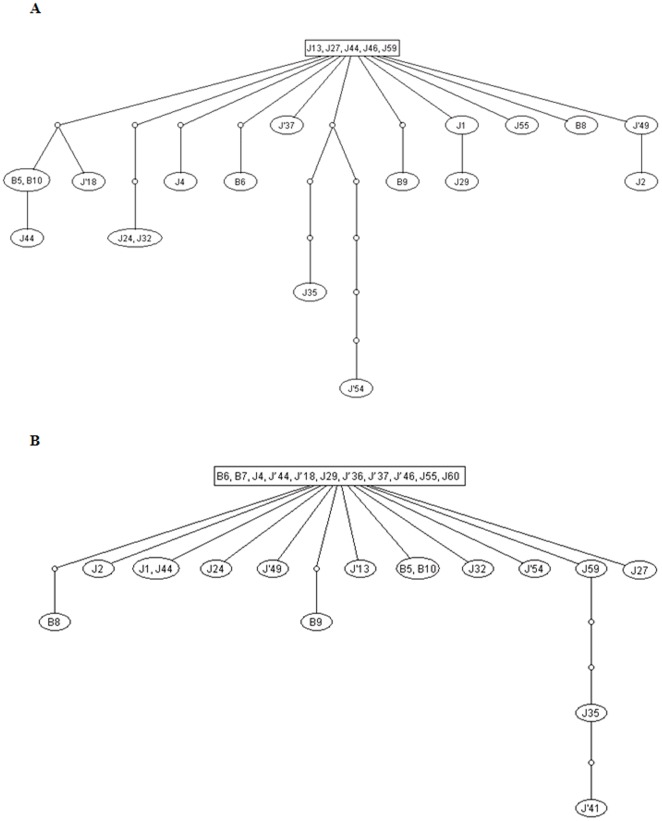
Figure 5. Genealogy of the LNV segments 12 and 10 of the isolates from the Xinjiang province.
Parsimony analysis by TCS of segments 10 (A) and 12 (B) of the LNV isolates from the Xinjiang province. Connecting lines represent a nucleotide substitution. Sequenced haplotypes (oval circles), and putative ancestral virus haplotypes (small circles) are shown. In segment 10, the network show a group of 5 isolates from Jiashi, that is directly linked to other isolates from Jiashi and Boshikeranmu. Only isolates J′36, J′41, J60 and B7 do not figure in this network. In segment 12 a larger group (including 11 isolates) is linked to all remaining isolates.
Genealogy analysis of Seg-12 also shows a network, although in this case it connects all 27 isolates (figure 5B) with a group, showing highest frequency, containing 11 isolates (B6, B7, J4, J′44, J′18, J29, J′36, J′37, J′46, J55 and J60). The deepest node for the cluster of the 27 isolates in the phylogenetic tree is supported by a bootstrap confidence level of 99% (Figure 4).
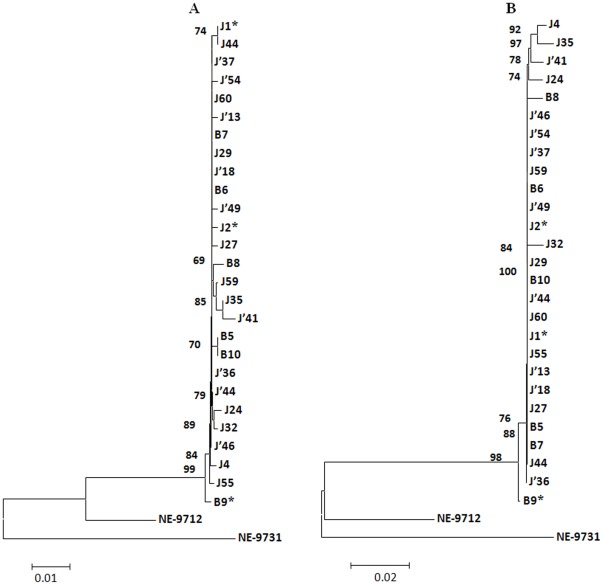
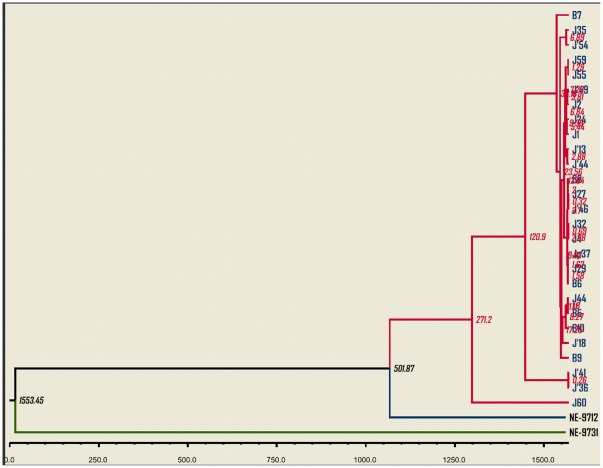
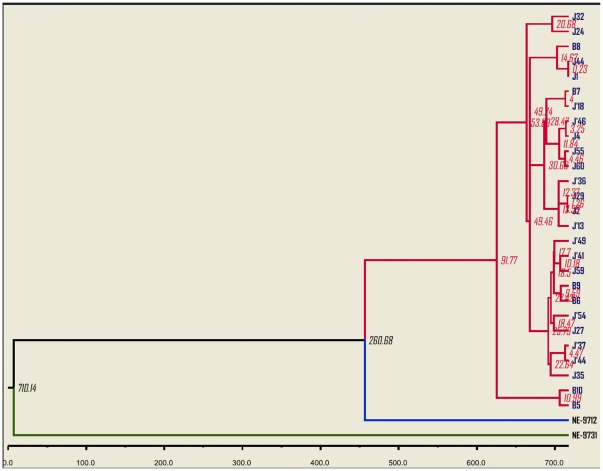
Maximum clade credibility trees (figures 6 and 7) were also generated from nucleotide sequences of Seg-10 and Seg-12, through BEAST analysis. These trees were similar to the neighbor-joining trees and clearly indicate that the prototype isolate of LNV-2 (LNV-NE9731) is distinct from all other isolates described here, and that the prototype isolate of LNV-1 (LNV-NE9712) ‘roots’ the 27 new sequences whether in segment 10 or 12.
Figure 6. Maximum clade credibility tree based on segment 10 of LNV-1 and LNV-2 prototype isolates and including the 27 LNV isolates obtained from mosquitoes in 2005.
The axis shows the estimated dates for most recent common ancestors as years before 2005. NE-9712 (LNV-NE9712) and NE-9731 (LNV-NE9731) are prototype strains of LNV serotypes 1 and 2, respectively. Values at the nodes are those for the most recent common ancestor of segment 10.
Figure 7. Maximum clade credibility tree based on segment 12 of LNV-1 and LNV-2 prototype isolates and including the 27 LNV isolates obtained from mosquitoes in 2005.
The axis shows the estimated dates for most recent common ancestors as years before 2005. NE-9712 (LNV-NE9712) and NE-9731 (LNV-NE9731) are prototype strains of LNV serotypes 1 and 2, respectively. Values at the nodes are those for the most recent common ancestor of segment 12.
Estimating substitution rates and demographic history
The best fit model for datasets of both Seg-10 and Seg-12 was found to be general time reversible (GTR), with a gamma distribution (Γ 4) model of site variation (GTR+Γ 4). Substitution rate (expressed as substitutions/site/year) in Seg-10 was 2.29×10−4 (95%HPD: 1.29–3.99×10−4). Substitution rate in Seg-12 was 1.57×10−4 (95%HPD: 0.54×10−4–4.13×10−4). Based on these estimates, the mean time for the most recent common-ancestor for Seg-10 of LNV is 1553 years overall (95%HPD: 500.03–2919.37), with 501 (201.52–702.71) years for Seg-10 of LNV-1, and 271 (50.38–399.92) years for Seg-10 of the isolates made in 2005. In contrast, the most recent common-ancestor of segment 12, was 710 years (95%HPD: 357.50–1024.27 years), with 260 (95%HPD: 125.23–399.74 years), years for Seg-12 of LNV-1 and 91 years (95%HPD: 24.69–227.75 years), for Seg-12 of the isolates made in 2005.
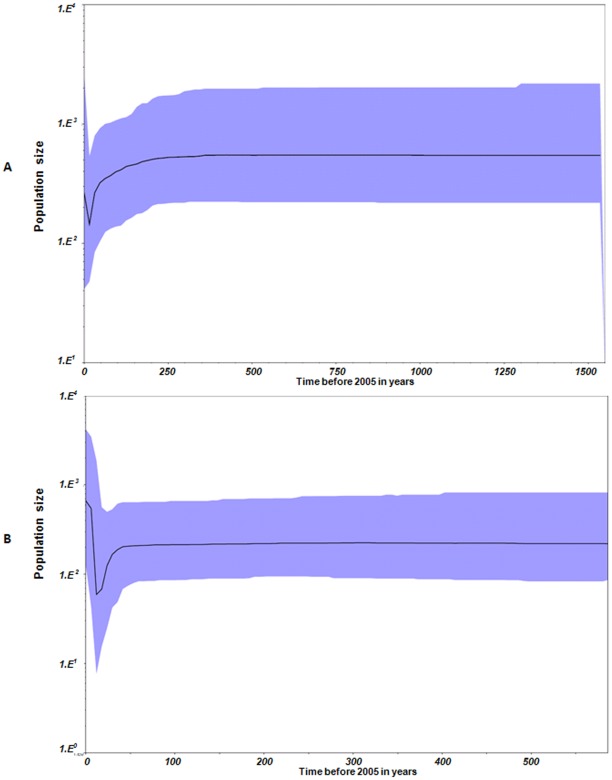
Analyses of their demographic history, using Bayesian skyline plots for both genes, indicate that the virus maintained relatively stable population sizes over the last 1553 years. However, Seg-10 and -12, both experienced a temporary population decline during the late 1980s and early 1990s (figure 8A and 8B).
Figure 8. Bayesian skyline plots for segments 10 (A) and 12 (B) of LNV.
The Bayesian skyline plots for both genes indicated that the virus had maintained relatively stable population sizes over the last 1553 years. However, in both segments 10 and 12, data suggested a temporary population decline during the late 1980s and early 1990s. Seg-12 data also suggest that a beginning of an expansion started in the late 1990s.
Discussion
All known seadornaviruses are able to replicate in mice causing viraemia, although isolates of Banna virus and Kadipiro virus do not cause disease in mice [12], [14]. LNV however causes lethal haemorrhage in mice (when mice are injected at two separate occasions), although death was not attributed to an antibody facilitating-effect as previously reported [14]. Although BAV can productively infect mosquito cells, the only mammalian cells in which it has been shown to replicate are BSR cells [13]. KDV has only been grown successfully in mosquito cell lines.
Isolations of LNV from Culex species described in this study, and from Aedes dorsalis mosquitoes from a previous study [14], show that LNV has a wide vector range. The mosquito Aedes dorsalis exists in North America, in Europe and Asia [18], [19] in a variety of brackish and fresh water habitats. The adult feeds on mammals (including humans and domestic animals) and birds, and could therefore potentially transmit LNV to these host-species. A. dorsalis is a vector for Western equine encephalitis virus (genus Alphavirus) [20], [21] and is considered as a possible North American vector for West Nile virus (genus Flavivirus) [22], [23].
JEV exists in an enzootic cycle between mosquitoes and pigs and/or water birds [24]. Pig farming is not practiced in Xinjiang, where sheep are the major livestock species and a search for JEV in both the human and mosquito populations in Xinjiang province was unsuccessful. However, LNV was isolated from 27 pools of wild caught mosquitoes from Xinjinag (table 1). Twenty four of these isolates from mosquitoes were collected in animal barns although 3 isolates were obtained from human accommodation. All of these isolates were identified as LNV-1 (as indicated by the high level of aa identity in the cell-attachment protein VP10). Besides mosquito vectors, the natural host or reservoir for LNV is not clearly identified. However, like Banna virus, humans, rodents, cattle and sheep [5], [9] may represent hosts of LNV.
Sequence and phylogenetic analyses indicate that different sites in Seg-10/VP10 sequences of LNV-1 have low evolutionary rates, although there are also hot spots with higher rates. These analyses also point towards purifying selection and confirm that all of the isolates belong to a single serotype.
Diversified sequences of segment 12 that were identified in the mouse blood relate to encapsidated genomic dsRNA. Viruses that contain these diverse sequences may not be viable and may not be able to reinitiate a productive infection in susceptible/permissive cells and would therefore be bottlenecked. The stability of the genome of this arbovirus also reflects the need to replicate in both the mosquito vector and a mammalian host.
Phylogenetic trees show that LNV isolates from Boshikeranmu village and Jiashi County (approximately 50 Km apart), do not form separate clusters (at the nt or aa level) either in Seg-10 or Seg-12. Clustering within the nt tree indicates that Seg-10 of some LNV-1 isolates (J′36, J′41, J60) is ancestral to the others. ‘Gene genealogy’ analysis also indicates that Seg-10 from LNV isolates J60, J′36 and J′41 and B7, is not part of a larger Seg-10 ‘network’, or any network among themselves. In contrast all of the Seg-12 sequences were connected in a single network, indicating that the Seg-12 gene pool (in Xinjiang province) is less heterogeneous than that of Seg-10. These analyses strongly indicate that reassortment events, involving exchange of Seg-10 and Seg-12, have occurred during the evolution of these virus strains.
As seen for other arboviruses, changes in vector-host densities will also influence transmission frequency and the incidence of disease. These changes will also affect the population size of the pathogen, as it follows these fluctuations [25], [26], [27]. Genetic data analyses for demographic expansion of the LNV population suggested a lack of demographic fluctuations. However, a decline in LNV population was indicated by data for both Seg-10 and -12, during the late 1980s early 1990s. It is unclear if this could be attributed to changes in the abundance or distribution of competent vectors in Northern China. It is also noticeable from the skyline plot of Seg-12 that there is a beginning of an expansion in the late 1990s.
The original LNV isolates were obtained from Aedes dorsalis mosquitoes in 1997, while all isolates described here were from Culex species in 2005. Banna virus, the prototype of genus Seadornavirus, was originally isolated in Indonesia in 1981, subsequently in Southern China in 1987, then 20 years later in the Northern China. The identification of LNV during 1997 [14] may be the result of a relatively recent introduction into Northern China.
Recent analyses of Aedes aegypti genome [28] showed that genome segment 5 of LNV (which encodes VP5, a 476 aa long non-structural protein) was found integrated in the mosquito genome in the form of two distinct but contiguous DNA inserts (aa2-aa48: 76% identity, 86% similarity and aa314-aa470: 88% identity, 93% similarity to LNV-NE9712). This finding was confirmed in an Aedes aegypti cell line (A20) that was cultured in 1969 from first instar larvae of the London strain of Ae. aegypti (colonized in 1957 [29]). The London strain also originates from West Africa. Wild caught Ae. aegypti mosquitoes from Pakistan were also found positive for the inserted Seg-5 sequence (data not shown). This indicates that insertion occurred before the introduction of Ae aegypti into [30], [31], [32] Asia which coincides with the development of shipping industry in 18th and 19th century, and that LNV could have originated from Africa where the Ae aegypti originates from.
The haemorrhage caused by the second injection of LNV into mice was not attributed to an antibody facilitating effect [14]. LNV was identified in mouse blood at 3 days post-injection. A significant level of diversification occurred during the early stages of LNV infection in mice. However, only the parental sequence was detected in Seg-12 of the virus after re-isolation in BSR or C6/36 cells, showing a strong ‘purifying’ effect. Analyses of Seg-10 from the re-isolated virus, showed an alanine to asparagine change in the deduced aa sequence (of each clone). The change maps to the surface of VP10 that is thought to interact with cell surface receptors. This aa position is also an asparagine in the published sequence of LNV-2 [14]. Both amino acids are non-charged and the significance of this change is unclear, although this might relate to the nature of the cell receptor.
Phylogenetic analyses of 40 clones of Seg-12 from mouse blood (4 mice) identified separate clusters of cDNA clones. The nucleotide sequences of mice 1, 2, 3 or 4 formed each a cluster containing sequences from a single animal. The identification of LNV in the spleen (two weeks post-injection) with identical sequences to the virus re-isolated from mouse blood, indicates that the highly divergent sequences identified directly in the blood, had been ‘bottlenecked’.
Phylogenetic analyses, genealogy, animal and cell culture work on the field samples show that LNV Seg-12 varies less than Seg-10. This mirrors findings concerning the variability of genome segments encoding cell-attachment outer-capsid proteins, of other dsRNA arboviruses, particularly the orbiviruses [33], [34], [35]. Evolutionary rates of 4 genome segments of another dsRNA arbovirus (bluetongue virus, BTV: a Culicoides-borne orbivirus), were previously found to be within a similar order of magnitude and ranged from 0.5×10−4 to 7×10−4 substitutions/nt/year [36].
Banna virus is implicated as a cause of disease in humans [5], [7], [10]. LNV is capable of replicating in a wide range of cell types, is lethal in mice and can replicate in multiple mosquito species. The recent emergence of other arboviral diseases, linked to climate change suggests that LNV could potentially emerge as an important agent of disease in animals including livestock and/or humans.
An ELISA, based on the serotype-specific protein VP10 of the two known serotypes of LNV, has been developed and is being used for epidemiological surveys of LNV in China, particularly in Xinjiang. Real time RT-PCR systems based on Seg-10 and Seg-12 of LNV have also been developed for the same purpose. These studies include humans, domesticated and wild animals (particularly rodents, sheep, birds) and will help clarify if LNV is the causative agent of disease outbreaks in China, and help to identify potential risks that may be involved. We will also attempt to identify the epidemiological impact of LNV in neighboring Asian countries.
Materials and Methods
Ethics statement
No specific permits were required for the described field studies. The work was done under the general supervision of the Chinese centers for disease control and prevention. No specific permission was required for trapping of mosquitoes. The trapping locations are not protected in any way. The field studies did not involve endangered or protected species.
The ethics review committee of the Institute has reviewed and approved the proposed experimental infection of LNV in mice.
Mosquito collection, treatment and virus isolations
Mosquitoes were collected during July–August of 2005, using light traps, in human accommodations of patients with febrile manifestations, and in animal barns where sheep represent the main livestock, in Xinjiang province of China. Collections were made in Jiashi County and in Boshikeranmu village, situated in the Kashi prefecture of Xinjiang province in the north-west of China. The village and county are both located ∼50 Km from Kashi city. The collected mosquitoes were frozen for 30 min for −20°C, then sorted on an ice plate.
Blood-fed and male mosquitoes were excluded. Eighty to two hundred mosquitoes were sorted into each collecting tube and stored in liquid nitrogen.
For virus isolation, 2 ml of minimal essential medium (MEM, HyClone), supplemented with 2% foetal bovine serum, 100 U penicillin/ml and 100 U streptomycin/ml, was added to each mosquito pool, followed by homogenisation in pre-cooled sterile plastic ‘grinding’ tubes. The ground samples were centrifuged at 12,000 g for 20 min at 4°C, and the clarified supernatant was assayed for virus by inoculation onto Aedes albopictus C6/36 [37] mosquito cells (obtained from the American type culture collection) and BSR [38] cells (a gift from Dr. Noel Tordo, Institut Pasteur, France ). The development of cytopathic effects was followed up daily. Viruses were propagated in Aedes albopictus C6/36 cells at 28°C in MEM for further agarose gel electropherotyping, PCR amplifications and sequencing. Virus isolates were checked for their relatedness to Japanese encephalitis virus, Banna virus and Liao ning virus, by RT-PCR, using the primers shown in table 2.
Inoculation of mice with LNV
The prototype strain of LNV serotype 1 (isolate LNV-NE9712: obtained from Aedes dorsalis mosquitoes, in the Liaoning province in northwest China in 1997) of serotype 1 was injected subcutaneously into mice. Viremic blood was collected at 3 days post-injection, before antibodies had been produced (absence of anti-LNV IgMs or IgGs: data not shown). LNV was re-isolated from mouse blood on BSR or C6/36 cells.
Preparation of the viral RNA, reverse transcription and PCR amplification
Viral RNA was extracted from virus-infected C6/36 cells and converted into cDNA using hexaprimers as previously described [12].
RNA was extracted from mouse blood using Trizol® as previously described [12], [39].
Viral RNA was also extracted from cell cultures (BSR and C6/36) infected by the virus from mouse blood using Trizol. RNA extracts were converted to cDNA as described above.
Specific primers were designed from sequences of genome segment 12 (Seg-12) of LNV (Table 2). These were used to amplify and sequence Seg-12 from field isolates (LNV12S/LNV12R), viremic blood and cell culture re-isolated virus (first round and nested primer pairs LNV12s1/LNV12r1 and LNV12s2/LNV12r2, respectively). Primers designed from Seg-10 were only used to amplify cDNAs from virus re-isolated in cell culture. PCR was performed using the triple-master PCR (Eppendorf). Amplified products were analysed by agarose gel electrophoresis and purified by QIAquick-PCR purification (QIAGEN) and sequenced directly. Each nucleotide position was sequenced three times.
Amplicons derived from the blood and cell culture re-isolated virus were ligated into the pGEM-T vector (Promega) and transformed into bacteria. Forty plasmid clones from mouse blood (10 from each mouse) were sequenced using M13 primers. Another 80 clones from cell cultures were also sequenced (20 clones each for Seg-12 and Seg-10 from BSR cells and another 20 clones each for Seg-12 and Seg-10 from C6/36 cells).
Sequence analysis and phylogenetic comparisons
Nucleotide (nt) and amino acid (aa) sequence alignments were generated using ClustalX version 1.8 [40]. Phylogenetic analysis was performed using MEGA4 [41], and the Neighbour-joining method was used for phylogenetic reconstructions of trees. P-distance or Kimura-2 parameters algorithms were used for the trees constructed using nucleotide sequences, while P-distance or Poisson correction algorithms were used for aa trees.
For gamma distance calculations (Kimura-2 parameter and Poisson correction models), the shape parameter alpha was determined using the PAML package [42].
Gene genealogy analysis was carried out using the programme TCS [43]. The programme collapses identical sequences into haplotypes and calculates the frequencies of the haplotypes in the sample. These frequencies are used to estimate haplotype outgroup probabilities, which correlate with haplotype age.
The best fit model of nucleotide substitution to be used in Bayesian coalescent analyses was determined using jModelTest (v 0.1.1) [44].
Bayesian coalescent analysis based on Markov Chain Monte Carlo (MCMC) sampling [45] was implemented in BEAST (Bayesian evolutionary analysis by sampling trees) [46]. Unrooted models of phylogeny and strict molecular clock models are two extremes of a continuum [47]. Substitution rates for segments 10 and 12 of LNV were therefore calculated in BEAST using a relaxed uncorrelated lognormal clock model. As applied in BEAST, this model estimates phylogenies and divergence times in the face of uncertainty in evolutionary rates and calibration times. The most general Bayesian skyline coalescent prior [48], which allows for both constant and complex changes in population size through time, was used. Differences in sampling time between 1997 and 2005 (dates of isolations of LNV strains) are used to scale branch length estimates to obtain the expected rate of genetic change per unit time. As a measure of estimate uncertainty, the program returns the 95% highest posterior density (HPD) interval. Based on the evolutionary rate, the age of the root (or of any other node) can also be estimated under the molecular clock model. In addition, the program can produce an estimate of the demographic history of the sampled population as a Bayesian skyline plot [48]. Analyses were carried out using a chain length of 10,000,000 states with the first 10% removed as burn-in. Output log files of 4 independent BEAST runs were combined together using LogCombiner (v1.5.4). This increased the effective sample sizes, and checked whether the various runs are converging on the same distribution in the MCMC run. The program Tracer (v1.5) was used to inspect posterior distributions and estimate evolutionary parameters. Maximum credibility trees were identified using TreeAnnotator (v1.5.4) and displayed using FigTree (v1.3.1).
Models for the outer capsid protein of LNV were generated using the Programme MODELLER [49] based on the previously determined atomic structure of the homologous VP9 protein of BAV.
Footnotes
Competing Interests: Dr. Houssam Attoui is a PLoS ONE Editorial Board member (joined on 07/15/2011). The authors confirm that this does not alter their adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This study was supported by funding from Biotechnology and Biological Sciences Research Council (BBSRC) and the Department for Environment, Food and Rural Affairs (DEFRA) in the UK. The study was also supported by the Ministry of Science and Technology, China (contracts: 2003BA712A08-01; 2008ZX10004-001) and by the Development Grant of State Key Laboratory for Infectious Disease Prevention and Control (contract: 2008SKLID105). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Attoui H, Mertens PPC, Becnel J, Belaganahalli M, Bergoin M, et al. The Double Stranded RNA Viruses. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy Ninth Report of the International Committee on Taxonomy of Viruses. London: Academic press; 2010. pp. 497–637. [Google Scholar]
- 2.Attoui H, Mohd Jaafar F, de Lamballerie X, Mertens PPC. Seadornavirus, Reoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy Eighth Report of the International Committee on Taxonomy of Viruses. London: Elsevier/Academic Press; 2005. pp. 504–510. [Google Scholar]
- 3.Brown SE, Gorman BM, Tesh RB, Knudson DL. Coltiviruses isolated from mosquitoes collected in Indonesia. Virology. 1993;196:363–367. doi: 10.1006/viro.1993.1490. [DOI] [PubMed] [Google Scholar]
- 4.Nabeshima T, Thi Nga P, Guillermo P, Parquet Mdel C, Yu F, et al. Isolation and molecular characterization of Banna virus from mosquitoes, Vietnam. Emerg Infect Dis. 2008;14:1276–1279. doi: 10.3201/eid1408.080100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Tao S. Arbovirus survey in China in recent ten years. Chin Med J (Engl) 1996;109:13–15. [PubMed] [Google Scholar]
- 6.Mohd Jaafar F, Attoui H, Bahar MW, Siebold C, Sutton G, et al. The structure and function of the outer coat protein VP9 of Banna virus. Structure. 2005;13:17–28. doi: 10.1016/j.str.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Li QP. First isolation of 8 strains of new orbivirus (Banna) from patients with innominate fever in Xinjiang. endemic diseases bulletin. 1992;7:77–82. [Google Scholar]
- 8.Xu P, Wang Y, Zuo J, Lin J. New orbiviruses isolated from patients with unknown fever and encephalitis in Yunnan province. Chinese Journal of Virology. 1990;6:27–33. [Google Scholar]
- 9.Tao SJ, Chen BQ. Studies of coltivirus in China. Chin Med J (Engl) 2005;118:581–586. [PubMed] [Google Scholar]
- 10.Liting S, Chen B, Chou Z. Isolation and identification of new members of coltivirus from mosquitoes collected in China. Chinese J Exp Clin Virol. 1995;9:7–10. [Google Scholar]
- 11.Attoui H, Charrel RN, Billoir F, Cantaloube JF, de Micco P, et al. Comparative sequence analysis of American, European and Asian isolates of viruses in the genus Coltivirus. J Gen Virol. 1998;79(Pt 10):2481–2489. doi: 10.1099/0022-1317-79-10-2481. [DOI] [PubMed] [Google Scholar]
- 12.Billoir F, Attoui H, Simon S, Gallian P, de Micco P, et al. Molecular diagnosis of group B coltiviruses infections. J Virol Methods. 1999;81:39–45. doi: 10.1016/s0166-0934(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhai Y, Attoui H, Mohd Jaafar F, Wang HQ, Cao YX, et al. Isolation and full-length sequence analysis of Armigeres subalbatus totivirus, the first totivirus isolate from mosquitoes representing a proposed novel genus (Artivirus) of the family Totiviridae. J Gen Virol. 2010;91:2836–2845. doi: 10.1099/vir.0.024794-0. [DOI] [PubMed] [Google Scholar]
- 14.Attoui H, Mohd Jaafar F, Belhouchet M, Tao S, Chen B, et al. Liao ning virus, a new Chinese seadornavirus that replicates in transformed and embryonic mammalian cells. J Gen Virol. 2006;87:199–208. doi: 10.1099/vir.0.81294-0. [DOI] [PubMed] [Google Scholar]
- 15.Attoui H, Mohd Jaafar F, de Micco P, de Lamballerie X. Coltiviruses and seadornaviruses in North America, Europe, and Asia. Emerg Infect Dis. 2005;11:1673–1679. doi: 10.3201/eid1111.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barreiro LB, Quintana-Murci L. From evolutionary genetics to human immunology: how selection shapes host defence genes. Nat Rev Genet. 2010;11:17–30. doi: 10.1038/nrg2698. [DOI] [PubMed] [Google Scholar]
- 17.Attoui H, Sailleau C, Mohd Jaafar F, Belhouchet M, Biagini P, et al. Complete nucleotide sequence of Middelburg virus, isolated from the spleen of a horse with severe clinical disease in Zimbabwe. J Gen Virol. 2007;88:3078–3088. doi: 10.1099/vir.0.83076-0. [DOI] [PubMed] [Google Scholar]
- 18.Romanowski M. Aedes dorsalis in New dorsalis: larval habitate and identification. Proc N J Mosquito Control Assoc; 1989. pp. 58–62. [Google Scholar]
- 19.Ciolpan O, Nicolescu G, Pop G. The mosquitoes (Diptera: Culicidae) in the area of the middle course of the river Somesul Mare (Romania): faunistical and ecological data. Roum Arch Microbiol Immunol. 1998;57:77–91. [PubMed] [Google Scholar]
- 20.Fulhorst CF, Hardy JL, Eldridge BF, Presser SB, Reeves WC. Natural vertical transmission of western equine encephalomyelitis virus in mosquitoes. Science. 1994;263:676–678. doi: 10.1126/science.8303276. [DOI] [PubMed] [Google Scholar]
- 21.Kramer LD, Reisen WK, Chiles RE. Vector competence of Aedes dorsalis (Diptera: Culicidae) from Morro Bay, California, for western equine encephalomyelitis virus. J Med Entomol. 1998;35:1020–1024. doi: 10.1093/jmedent/35.6.1020. [DOI] [PubMed] [Google Scholar]
- 22.Medlock JM, Snow KR, Leach S. Potential transmission of West Nile virus in the British Isles: an ecological review of candidate mosquito bridge vectors. Med Vet Entomol. 2005;19:2–21. doi: 10.1111/j.0269-283X.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 23.Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monath TP, Heinz FX. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JP, et al., editors. Fields Virology. 3 ed. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 25.Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 26.Fischer JR, Stallknecht DE, Luttrell P, Dhondt AA, Converse KA. Mycoplasmal conjunctivitis in wild songbirds: the spread of a new contagious disease in a mobile host population. Emerg Infect Dis. 1997;3:69–72. doi: 10.3201/eid0301.970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yates TL, Mills JN, Parmenter CA, Ksiazek TG, Parmenter RR, et al. The ecology and evolutionary history of an emergent disease: Hantavirus pulmonary syndrome. Bioscience. 2002;52:989–998. [Google Scholar]
- 28.Katzourakis A, Gifford RJ. Endogenous viral elements in animal genomes. PLoS Genet. 2010;6:e1001191. doi: 10.1371/journal.pgen.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varma MG, Pudney M. The growth and serial passage of cell lines from Aedes aegypti (L.) larvae in different media. J Med Entomol. 1969;6:432–439. doi: 10.1093/jmedent/6.4.432. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald WW. Aedes aegypti in Malaya. II. Larval and adult biology. Ann Trop Med Parasitol. 1956;50:399–414. [PubMed] [Google Scholar]
- 31.Macdonald WW. Aedes aegypti in Malaya. I. Distribution and dispersal. Ann Trop Med Parasitol. 1956;50:385–398. [PubMed] [Google Scholar]
- 32.Macdonald WW. A mosquito survey at Kuala Lumpur airport with special reference to Aedes aegypti. Med J Malaya. 1956;10:232–245. [PubMed] [Google Scholar]
- 33.Attoui H, Billoir F, Biagini P, de Micco P, de Lamballerie X. Complete sequence determination and genetic analysis of Banna virus and Kadipiro virus: proposal for assignment to a new genus (Seadornavirus) within the family Reoviridae. J Gen Virol. 2000;81:1507–1515. doi: 10.1099/0022-1317-81-6-1507. [DOI] [PubMed] [Google Scholar]
- 34.Attoui H, Mohd Jaafar F, Biagini P, Cantaloube JF, de Micco P, et al. Genus Coltivirus (family Reoviridae): genomic and morphologic characterization of Old World and New World viruses. Arch Virol. 2002;147:533–561. doi: 10.1007/s007050200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maan S, Maan NS, Samuel AR, Rao S, Attoui H, et al. Analysis and phylogenetic comparisons of full-length VP2 genes of the 24 bluetongue virus serotypes. J Gen Virol. 2007;88:621–630. doi: 10.1099/vir.0.82456-0. [DOI] [PubMed] [Google Scholar]
- 36.Carpi G, Holmes EC, Kitchen A. The evolutionary dynamics of bluetongue virus. J Mol Evol. 2010;70:583–592. doi: 10.1007/s00239-010-9354-y. [DOI] [PubMed] [Google Scholar]
- 37.Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- 38.Sato M, Maeda N, Yoshida H, Urade M, Saito S. Plaque formation of herpes virus hominis type 2 and rubella virus in variants isolated from the colonies of BHK21/WI-2 cells formed in soft agar. Arch Virol. 1977;53:269–273. doi: 10.1007/BF01314672. [DOI] [PubMed] [Google Scholar]
- 39.Attoui H, Billoir F, Cantaloube JF, Biagini P, de Micco P, et al. Strategies for the sequence determination of viral dsRNA genomes. J Virol Methods. 2000;89:147–158. doi: 10.1016/s0166-0934(00)00212-3. [DOI] [PubMed] [Google Scholar]
- 40.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 43.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 44.Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 45.Drummond AJ, Nicholls GK, Rodrigo AG, Solomon W. Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics. 2002;161:1307–1320. doi: 10.1093/genetics/161.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drummond A, Pybus OG, Rambaut A. Inference of viral evolutionary rates from molecular sequences. Adv Parasitol. 2003;54:331–358. doi: 10.1016/s0065-308x(03)54008-8. [DOI] [PubMed] [Google Scholar]
- 47.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drummond AJ, Rambaut A, Shapiro B, Pybus OG. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol Biol Evol. 2005;22:1185–1192. doi: 10.1093/molbev/msi103. [DOI] [PubMed] [Google Scholar]
- 49.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 50.Crochu S, Cook S, Attoui H, Charrel RN, De Chesse R, et al. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J Gen Virol. 2004;85:1971–1980. doi: 10.1099/vir.0.79850-0. [DOI] [PubMed] [Google Scholar]
- 51.Pfeffer M, Proebster B, Kinney RM, Kaaden OR. Genus-specific detection of alphaviruses by a semi-nested reverse transcription-polymerase chain reaction. Am J Trop Med Hyg. 1997;57:709–718. doi: 10.4269/ajtmh.1997.57.709. [DOI] [PubMed] [Google Scholar]