SUMMARY
The association between small but still visible lacunar infarcts and cognitive decline has been established by multiple population-based radiological and pathological studies. Microscopic examination of brain sections reveals even smaller but substantially more numerous microinfarcts, the focus of the current review. These lesions often result from small vessel pathologies such as arteriolosclerosis or cerebral amyloid angiopathy. They typically go undetected in clinical-radiological correlation studies that rely on conventional structural MRI, though the largest acute microinfarcts may be detectable by diffusion-weighted imaging. Given their high numbers and widespread distribution, microinfarcts may directly disrupt important cognitive networks and thus account for some of the neurologic dysfunction seen in association with lesions visible on conventional MRI such as lacunar infarcts and white matter hyperintensities. Standardized neuropathological assessment criteria and development of non-invasive means of detection during life would be major steps towards understanding the causes and consequences of the otherwise macroscopically invisible microinfarct.
INTRODUCTION
Many neuropathology and neuroimaging studies have shown that asymptomatic cerebrovascular disease is extremely common.. Further, this so-called “silent” pathology accounts for a surprisingly high portion of dementia.1 Lacunes and white matter lesions (WMLs) in particular have emerged as clear-cut risk factors for dementia in multiple population-based clinical-radiological studies.2, 3
While the association between these visible manifestations of cerebral small vessel disease is strong and consistent, the mechanism remains challenging to explain. Are one or two lacunes, for example, truly sufficient to impair cognition as suggested epidemiologically? An important alternative explanation is that those few readily detectable lesions are instead markers for substantially more numerous and widespread infarcts that are not visible to the naked eye. Tiny microinfarcts are indeed a well described neuropathologic finding. These lesions are characterized by bona fide tissue infarction, but on a scale that renders them unapparent on gross pathologic examination or conventional structural MR imaging. This review synthesizes current knowledge of the detection, appearance, prevalence, distribution, and functional impact of microinfarcts along with recommended areas for future investigation. Although microinfarcts represent just one in a spectrum of small vessel-associated forms of brain injury (a list that also includes white matter T2-hyperintense lesions and cerebral microbleeds), current data suggest that they may comprise the single most widespread form of brain infarction and thus a major component of the causal pathway between cerebral small disease and cognitive dysfunction.
SEARCH STRATEGY AND SELECTION CRITERIA
The PubMed database was searched with the Ovid search engine on December 2 2011 using the terms “microinfarct(s)” and “microscopic infarct(s)” as keywords and MeSH headings, and limited to articles including “brain”, “cerebral” or “central nervous system” as keywords or MeSH headings. The search yielded 535 articles. A single author (EES) screened the abstracts and eliminated 438 articles as not relevant; all study authors reviewed the remaining 97 articles. Another 27 relevant articles were identified by consultation with experts and hand searching of the reference lists of the retrieved articles.
STATISTICAL ANALYSIS
To derive the univariate pooled odds ratio for the relationship between microinfarcts and dementia, we abstracted information on the prevalence of microinfarcts in persons with and without dementia from the studies selected for review (see “Search Strategy and Selection Criteria”). To reduce the risk of bias we only pooled data from community-based studies of all-cause mortality that prospective selected participants during life, without including hospital or clinic-based studies, for example. Univariate odds ratios were graphically displayed as a forest plot and a pooled odds ratio was calculated using the DerSimonian and Laird random effects model.4 Heterogeneity in the odds ratios across studies was quantified by the I-squared measure5 and tested using the chi-square test. Statistical analyses were done using Stata version 9.2 (StataCorp, Texas, USA).
NEUROPATHOLOGY OF MICROINFARCTS
Cerebral microinfarcts are typically defined as sharply delimited microscopic regions of cellular death or tissue necrosis, sometimes with cavitation (that is, a central fluid-filled cavity). The term “microscopic” denotes that these lesions are not visible by gross inspection of the brain but seen by light microscopy (Figure 1).6–9 The term “infarct” is most commonly used for ischemia-related tissue loss, and indeed the pathologic appearance of microinfarcts is consistent with that of known ischemic infarctions. The precise pathophysiologic cause or causes of microinfarcts are not entirely defined, however. To be consistent with prior literature, we will nonetheless continue to refer to these lesions as “microinfarcts.”
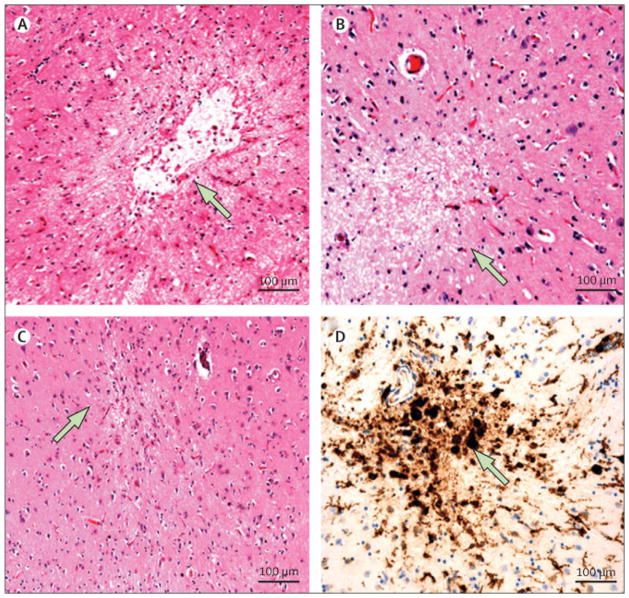
Figure 1. Microinfarct pathology.
A. Cystic (cavitated) microinfarct (diameter: 600 μm) in the basal ganglia; original magnification 10x. B. Incomplete microinfarct without cavitation (diameter: 330 μm) in midfrontal cortex; original magnification 10x. C. Cortical microinfarct with linear scarring (“puckering”) from the middle temporal cortex (diameter 120 μm); original magnification 10x. D. Immunostatin for major histocompatibility complex II human leukocyte antigen DR3 (MHCII HLA-DR3) expression in activated microglia and macrophages in a microinfarct in the basal ganglia; original magnification 10X (diameter: 320 μm).
There are surprisingly few guidelines for the identification of microinfarcts, and many studies provide little information on inclusion or exclusion criteria. The National Institute of Neurologic Disorders and Stroke – Canadian Stroke Network Vascular Cognitive Impairment (NINDS-CSN VCI) Harmonization Standards6 uses the standard approach defining microinfarcts as lesions “not visible to the naked eye but detected on histologic examination” and further suggest that microinfarcts be differentiated from “incomplete ischemic injury” or subinfarctive lesions, which appear as foci of cell loss or tissue rarefaction with reactive changes but without frank tissue loss. However, this differentiation can be difficult in practice. For example, a tissue section at the periphery of a genuine microinfarct may demonstrate only subinfarctive changes, as cavitated infarcts can be surrounded by gliosis some distance from their nidus.10 For this reason, some studies are inclusive of these incompletely infarcted lesions and define microinfarcts to include foci of tissue pallor or astrocytosis,8, 11, 12 whereas others require evidence of frank tissue necrosis or encephalomalacia.7, 13, 14 Some studies further exclude infarcts that are over 2 mm14 or 4 mm12 in diameter; presumably to exclude lesions that should be grossly visible. Another source of variability in microinfarct detection is staining method. Although most studies suggest that microinfarcts are readily identified on routine hematoxylin and eosin (H&E) stain,7, 11 immunohistochemical staining for markers of tissue injury such as reactive astrocytes15 or activated microglia16 may make microinfarcts more recognizable and easier to count.7 However, the sensitivity and specificity of different staining methods for microinfarcts has not been rigorously studied.
When identified in early stages of their evolution, microinfarcts show the predicted acute ischemic appearance of red neurons (if cortical) and loss of tinctorial staining quality, sometimes with vacuolization from cytotoxic edema. In subacute stages (starting at 3 to 5 days post-infarct), the lesions demonstrate an influx of macrophages that often clearly demarcate the central region of the infarct from surrounding tissue. At approximately 10 days this is followed by a surrounding gemistocytic astrocytosis and fading of the dying neurons. Chronic lesions typically show cavitation with few remaining central macrophages and a surrounding fibrillary gliosis. However, chronic microinfarcts can also appear as a linear “scar” with acellularity and fibrillary gliosis but little or no cavitation, causing invagination of the pial surface and puckering of the surrounding tissue when in the cortex, or if numerous, creating a cortical appearance of “granular atrophy”.17 It is important to note that like other foci of tissue necrosis, microinfarcts can have distant tissue effects such as Wallerian, retrograde, or transynaptic degeneration.7 The combination of local peri-infarct gliosis10 and distant degeneration may thus make the pathologic impact of microinfarcts more widespread than simply the tissue volume occupied by the microinfarcts themselves.
Typical microinfarct size appears to be quite small. In a study of microscopic lesions containing tissue necrosis, mean diameter of the lesions was approximately 0.2 mm.18 In another community-based clinical-pathologic study of aging the average size was much larger, 1mm, with a range of 0.2 to 2.9 mm.19 The small average size highlights the challenges in designing imaging methods for detecting microinfarcts (see later Neuroimaging of Cerebral Microinfarcts).
Microinfarcts are very common in the aging brain. Estimates of the frequency of one or more detectable microinfarcts range from 16% to 46% in unselected elderly persons dying of all causes (Table 1). Microinfarcts were detected in 33% of cognitively normal elderly dying of all causes in a combined analysis of 4 studies.20 The heterogeneity in observed prevalence likely arises in part from differences in cohort characteristics such as age and coexisting medical conditions. An additional source of variation is the sampling strategy. Because microinfarcts are very small lesions within a large brain that is typically only sparsely sampled for microscopic pathology, their prevalence is likely to be affected by both the volume and location of examined tissue. Sampling strategy is not necessarily the overriding determinant of microinfarct prevalence, however; for example, a frequency of 19.3% was reported with 38 regions sampled11 whereas another study found a frequency of 30% with only 9 regions sampled.21
Table 1.
Community-based Autopsy Studies of the Relationship Between Microinfarcts and Dementia
| Study name | Ref | Autopsy rate | Age at Death (years) | N | Population | Prevalence of Microinfarcts | Multivariable-adjusted Odds Ratio (OR) for Dementia (95% Confidence Interval) | ||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Dementia | No dementia | |||||||
| ACT | 8 | 20.1% | Mean 85.01 | 221 | Washington State USA, randomly selected members of Group Health Cooperative | 97/209 (46.4%) | 47/74 (63.5%) | 50/135 (37.8%) | “Negligible” (reference)2 1 or 2 OR 1.13 (0.40–3.04) High 4.80 (1.91–10.26)3 |
| BLSA | 37 | 19.3% | Mean 86.9±8.2 | 179 | Baltimore, USA community | 39/179 (21.8%) | 34/894 (38.2%) | 5/904 (5.6%) | 6.0 (2.1–17)5 |
| Bronx Studies | 27 | NR | Mean 84.4±9.6 | 190 | Bronx, USA community including nursing homes | 30/190 (15.8%) | 20/1314 (15.3%) | 10/594 (16.9%) | 0.6 (0.2–1.9)6 |
| CC75C Study | 82 | NR | Median 90.7 (IQR 88.0–93.4) | 213 | Cambridge, UK, community through general practices | 103/213 (48.4%) | 60/113 (56%) | 43/100 (43%) | 2.2 (1.0–5.1)7 |
| HAAS | 11 | 22.3% | Mean 84.9 | 285 | Honolulu, USA Japanese-American men | 55/285 (19.3%) | 35/118 (29.7%) | 20/167 (12.0%) | Negligible (reference) Intermediate OR 2.36 (0.87–6.41) High 4.59 (2.07–10.19)8 |
| Religious Orders Study | 19 | 94% | Mean 86.5±7.0 | 425 | Older Catholic clergy from multiple U.S. centers | 129/425 (30.4%) | 70/192 (36.5%) | 59/233 (25.3%) | 1.77 (1.07–2.92)9 |
| Rush Study of Memory and Aging | 21 | 81.1% | Mean 87.9±5.6 | 148 | Chicago, USA community mostly from retirement/seniors housing | 35/148 (23.6%) | 14/51 (27.4%) | 21/97 (21.6%) | 0.71 (0.26–1.97)10 |
ACT, Adult Changes in Thought Study; BLSA, Baltimore Longitudinal Study of Aging; Bronx Studies, Bronx Aging Study (n=54), Albert Einstein College of Medicine Teaching Nursing Home Study (n=99), Einstein Aging Study (n=14), and community volunteers (n=23); CC75C, Cambridge City over-75s Cohort Study; HAAS, Honolulu-Asia Aging Study; Rush Study, Rush Memory and Aging Project. Ref, reference; NR, not reported; IQR, interquartile range.
Estimated from data tabulated by age strata.
”Negligible” indicates 2 or fewer microinfarcts in the cerebral cortex and 2 or fewer microinfarcts in the basal ganglia and thalamus; see reference 9 for details.
Including only patients with dementia (n=89) to patients with normal cognition (n=75), excluding patients with cognitive impairment no dementia (n=47). Adjusted for age, sex, education, and apolipoprotein E ε4 genotype.
Percentages of microinfarcts in persons with and without dementia were provided by the authors on request.
Adjusted for age, sex and number of hemispheric macroscopic infarcts.
Adjusted for age, sex, recruitment group, interval from last evaluation to death, macroscopic infarcts, ischemic leukoencephalopathy, Braak NFT stage, and cortical Lewy bodies.
Adjusted for age, time since last clinical assessment while alive, presence of Lewy bodies, presence of white matter pallor and severity grade of neocortical neuritic plaques, entorhinal neuritic plaques, hippocampal diffuse plaques, neocortical neurofibrillary tangles, cerebral amyloid angiopathy and hippocampal atrophy.
Adjusted for age, education, NP, NFT, Lewy Bodies, and hippocampal sclerosis.
Adjusted for age at death, sex, education, macroscopic infarcts, AD pathology (a summary score including measures of neuritic plaques, diffuse plaques and neurofibrillary tangles) and Lewy bodies.
Adjusted for age, sex, education, AD pathology summary measure, and macroscopic infarcts.
Microinfarct burden is often expressed as the number of microinfarcts per number of blocks from specific regions16 or by semiquantitative estimates across all sampled tissue blocks (e.g. none, one, more than one or more than two).8, 14, 22 The numbers of microinfarcts detected in these studies are likely vast underestimates of the total brain burden, however, as even systematic tissue sampling methods examine only a tiny fraction of the overall brain volume. Calculations based on the sampling protocol used in reference 21 indicate that observation of 1 or 2 microinfarcts in sampled tissue suggests the likely presence of hundreds of microinfarcts across the total brain volume (Schneider and Greenberg, unpublished data). Although direct measurement of total brain microinfarct burden has not been performed (and may indeed be impractical), these calculations suggest that true number is almost certainly orders of magnitude greater than the total burden of grossly visible lacunar infarcts (typically 1 to 15 per brain).23
Determining the distribution of microinfarcts across the brain, like their prevalence and overall burden, is likely influenced by sampling strategy, which varies among studies. A reasonable approach to standardized sampling is the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) protocol for brain sampling with additional sectioning of the anterior (centrum semiovale) and posterior white matter24 as suggested by the NINDS – CSN VCI Harmonization Standards.6 Other important areas for sampling may include the border zones of major arterial territories18, 25, 26 as well as brain regions commonly involved by lacunar infarction, the basal ganglia, thalamus, pons and cerebellum.
Microinfarct location can be classified as cortical or subcortical, a distinction that may be useful for identifying associations with specific diseases.. Some studies of AD, for example, report cortical microinfarcts predominantly in brain arterial border zone areas18, 25, 26 or in motor cortex in the setting of AD with motor impairment.27 Cortical microinfarcts have also been associated with cerebral amyloid angiopathy (CAA),16, 26, 28–32 though the relationship may be complicated by CAA-related perivascular hemosiderin deposition and astroglial scarring that can be difficult to distinguish from incomplete microinfarction. Subcortical microinfarcts, particularly in the putamen, have been described in hypertension33 and hypertensive encephalopathy34, and are probably associated with arteriolosclerosis, whereas periventricular microinfarcts have been described in normal pressure hydrocephalus.35
MICROINFARCTS AND SMALL VESSEL BRAIN DISEASE
In using the term “microinfarcts” for these lesions, the underlying supposition is that these lesions are indeed the results of ischemic injury. Several lines of evidence support this inference. As noted above, microinfarcts appear to share the histopathologic structure and progression of macroscopic infarcts. Another suggestive feature is their association with other markers of cerebrovascular disease such as ischemic macroscopic infarcts, leukoencephalopathy, and intracerebral hemorrhages.7, 21, 36–38 Most notable are the observed associations of microinfarcts with advanced small vessel diseases,14 including both common age-related pathologies such as arteriolosclerosis and CAA16, 26, 28–32 and uncommon hereditary syndromes such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencepalopathy (CADASIL).39, 40 Microinfarcts commonly coexist with degenerative pathologies such as AD8, 9, 21, 37, 41 but the number of microinfarcts does not appear to be related to the severity of AD pathology.19
Relatively few studies have systematically examined the relationship between microinfarcts and vascular or other epidemiologic risk factors. Hypertension was identified as a risk factor in two prospective cohort studies,37, 42 though not in another small study.43 When examined in detail, the association between blood pressure and microinfarcts was seen in those under age 80 but not those 80 and older, and was restricted to higher systolic but not diastolic blood pressure readings.42 There is a reported association between cerebral microinfarcts and history of coronary artery disease.37 Two studies have found associations between microinfarcts and advanced age at death.36 The relationship with gender has been inconsistent.36, 37
NEUROIMAGING OF CEREBRAL MICROINFARCTS
The autopsy-based studies discussed to this point are cross-sectional and therefore limited in their ability to explore the incidence and consequences of microinfarcts. Therefore, there is a need to identify microinfarcts during life in well-characterized clinical populations. The major barrier to this goal is that microinfarcts are, by definition, invisible to the naked eye pathologically and thus likely invisible to conventional structural MRI as well. This quality puts microinfarcts in a distinct category from other features of cerebral small vessel disease that are readily visible on conventional MRI, such as lacunar infarcts, white matter T2 hyperintensities (WMH), enlarged perivascular spaces, microbleeds, and atrophy.44
The paucity of clinical-imaging-pathological correlations45 means that it is difficult to be certain whether some features or consequences of microinfarction might indeed be detectable on structural MRI. Microinfarcts may, for example, contribute to the overall appearance of WMH,46 but cannot easily be distinguished from the other pathologies that contribute to this common MRI finding. It is notable that most imaging-pathological comparisons of patients with small vessel disease to date have focused on periventricular and deep white matter rather than cortical lesions,45, 47 whereas pathological studies of microinfarcts have predominantly sampled the cortex. It is also possible that some of the spherical or ovoid lesions described in recent studies as enlarged perivascular spaces (that is, small CSF containing lesions visible in the white matter and basal ganglia on conventional T2-weighted imaging)48–50 may actually be microinfarcts.
Given a mean diameter of 0.2 to 1 mm,18 the size of most microinfarcts is below the lower limit of spatial resolution (approximately 1 mm3) for MRI at the conventional (1.5 or 3 Tesla) field strengths used in clinical practice. Lesions smaller than 1 mm will only be visible if the lesional signal is strong enough to be detected despite partial volume averaging with normal tissue also contained in the 1 mm3 voxel. Therefore, increased spatial resolution will likely be needed for MRI to be able to detect individual microinfarcts and differentiate them from other tiny lesions. Methods to increase the signal to noise ratio, such as higher field strengths, might improve the visual contrast between microinfarction and normal tissue and thus aid detection. A recent study using 7 Tesla post-mortem MRI, where multiple lengthy acquisitions can be done and averaged to reduce noise, demonstrated infarcts in the sub-millimeter range in a brain affected by CADASIL (Figure 2).51
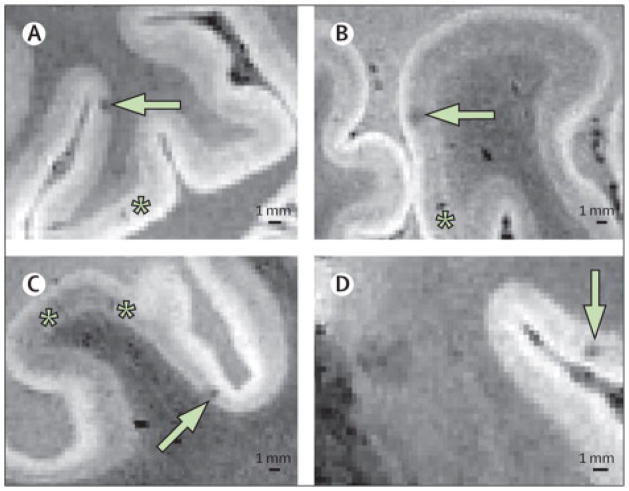
Figure 2. Intra-cortical Small Infarcts Detected on Post-Mortem High-Field Strength MRI.
Examples of pathologically-verified intracortical microinfarcts (white arrows) imaged on post-mortem 7 tesla MRI (voxel size 0.3 mm3) of a 53 year old man who died of CADASIL.51 Asterisks indicate linear hypointensities pathologically proven to be perivascular spaces. The black bar indicates 1 mm. (Figure provided by E. Jouvent).
During the acute phase, macroscopic infarcts can be sensitively52 (but imperfectly53) detected by MRI diffusion-weighted imaging (DWI).54 Acute infarcts are visible as hyperintense regions on DWI images or as hypointense regions on apparent diffusion coefficient (ADC) images, reflecting decreased (restricted) diffusion of water protons in the infracted tissue. In acute infarction, water molecule diffusion decreases within minutes of the onset of ischemia, then increases as barriers to diffusion, such as cellular membranes, are broken down and scavenged.55–57 The rate of evolution is more rapid in gray matter than white matter,58 and may be more rapid for smaller infarcts than larger infarcts and for milder ischemic injuries than more severe. Lesions as small as 1 to 2 mm are detectable on MRI, possibly somewhat enlarged from their pathological dimensions by the “blooming” effects of the strong lesion signal.59 The size of the smallest infarcts detectable on DWI thus approaches the boundary between what is pathologically considered a microinfarct versus a macroscopic lacunar infarct. As the lesion evolves and ADC increases, the size of the lesion may shrink and either cavitate, take on the appearance of WMH or even be indistinguishable from normal tissue.60 There is thus only a relatively short time window (perhaps days) in which a microinfarct might be DWI-detectable.
Despite these substantial temporal and spatial limitations, a surprisingly high rate of incidental acute small infarction is detectable on DWI in patients with small vessel disease such as CAA and CADASIL.61, 62 In a study of CAA patients, for example, DWI-positive lesions were detected in 12 of 78 (15%) including 5 with 2 lesions (Figure 3).61 Based on these data and a possibly generous estimated 10-day window in which the DWI lesions might remain visible, the authors calculated that the average CAA patient may have 8 incident DWI-detectable small infarcts per year.61 Similarly, incidental small DWI-positive lesions were detected in 16 to 19% of patients with acute lacunar stroke in 2 studies63, 64 and 13 to 31% of patients with acute intracerebral hemorrhage,65–67 with one study finding that DWI-positive lesions were more frequent in the patients with CAA.66
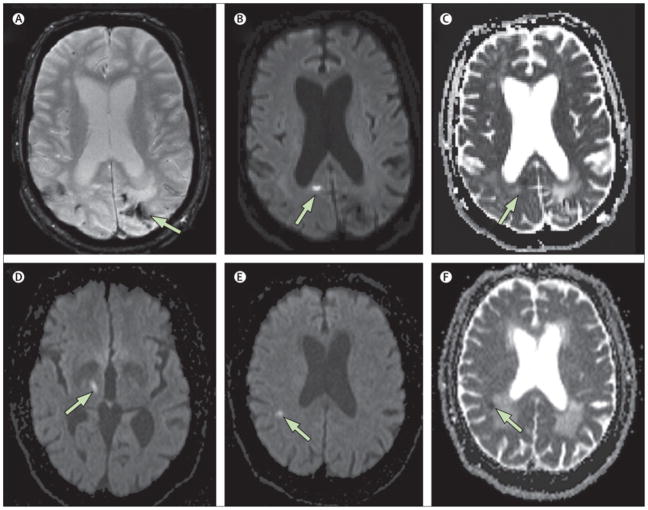
Figure 3. MRI Diffusion-Weighted Imaging of Small Acute Infarction (Sample).
Example of small acute areas of restricted diffusion detected incidentally on MRI. Top panels (A–C): A 70 year man with cerebral amyloid angiopathy who underwent MRI as part of a research study. Hemosiderin staining from prior hemorrhages is seen on the T2*-weighted gradient-recalled echo (GRE) sequence (A). Separate from these prior hemorrhages, an asymptomatic small hyperintensity is seen in the right occipital cortex on the diffusion-weighted image (DWI, panel B) with evidence of restricted diffusion on the apparent diffusion coefficient sequence (ADC, panel C), consist with an acute small cortical infarct. Bottom panels (D–F): A 67-year old man with an acute symptomatic lacunar infarct in the right thalamus (D, DWI sequence) also demonstrates an asymptomatic small 4.5 mm lesion in the right parietal white matter that is hyperintense on DWI (panel E) with restricted diffusion on ADC (panel F). An asymptomatic simultaneous small vessel infarct was suspected because no proximal source of embolism was identified and there was evidence of coexisting chronic cerebral small vessel disease (note confluent white matter lesions exhibiting increased diffusion in panel F).
The apparent association of incidental DWI-positive lesions with advanced small vessel diseases as well as the absence of definite embolic sources in most cases68 support the possibility that some of these lesions represent may acute microinfarction. However, it is not known with certainty whether these DWI-positive neuroimaging lesions correspond pathologically to acute microinfarcts, and direct DWI-pathological correlation therefore remains an important goal for future investigation.
The brief time window for acute infarct detection by DWI imposes substantial limitations to assessing the cumulative impact of ongoing acute small infarction. Detection of chronic microinfarcts would be extremely useful, but faces technical challenges in spatial resolution and in discrimination from other types of very small chronic lesions like focal WMH and enlarged perivascular spaces.48–50 Possible approaches to improved microinfarct detection are discussed below under Areas for Future Investigation.
MICROINFARCTS, COGNITIVE IMPAIRMENT, AND NEUROLOGIC DYSFUNCTION
Data from the autopsy- and DWI-based studies described above suggest that though individually small, microinfarcts may be quite numerous and thus capable of causing clinical symptoms. The clinical syndrome most clearly related to microinfarction is cognitive dysfunction.
Microinfarcts are frequently seen in autopsies of persons with vascular dementia or mixed dementia.7, 28, 43, 69–73 However, determining whether microinfarcts contribute to the risk of dementia is challenging, for two reasons. First, the frequency of microinfarcts is also relatively high in brains of non-demented persons..72–74 Second, other vascular pathologies—such as macroinfarcts, WMLs or CAA—are often seen along with microinfarcts in the same brains. For these reasons, large samples including both demented and non-demented persons with statistical modeling to control for the presence of other pathologies are needed to determine the independent effect of microinfarcts on the risk of cognitive impairment..
A series of hospital-based autopsy studies from a single center suggests that microinfarcts are indeed an independent predictor of cognitive dysfunction when simultaneously accounting for other pathologies, including macroinfarcts. In these studies microinfarcts were independently associated with increased odds of worse cognitive function in selected populations with low75, 76 or high77 levels of neurofibrillary tangle pathology, and with76, 77 or without75 accompanying macroinfarcts (including lacunes). In their most inclusive cohort containing all levels of neurofibrillary tangle pathology and macroscopic infarcts, the cortical microinfarct score (range, 0–3) was independently associated with a 1.21-fold increase in the odds of antemortem dementia per additional point on the score (95% confidence interval 1.12–1.31, P<0.001).77 In patients with low or intermediate levels of neurofibrillary tangle pathology (defined as Braak stage <III), cortical microinfarcts were the single pathology that explained the most variation in Clinical Dementia Rating (CDR) scale score even when simultaneously controlling for macroinfarcts and lacunes. 76, 78, 79 The authors suggested that cortical microinfarcts may make the clinical expression of dementia more likely when there are intermediate levels of AD pathology present, as has also been observed with macroscopic lacunar infarcts.80, 81 Limitations of these studies is that cases were selected from a geriatric and psychiatric hospital and thus may not be representative of the general population.
Most recently, the cognitive impact of microinfarcts has been investigated by several autopsy studies based on prospective cohorts that are more representative of the community than hospital- or clinic-based series. These studies recruited participants from the community, including non-impaired persons, and prospectively followed them with cognitive testing until death. After death, brain autopsies were conducted to identify the neuropathologies associated with cognitive impairment or dementia (Table 1).8, 11, 19, 21, 27, 37, 82 The prevalence of microinfarcts ranged from 18% to 30% in 5 of the 7 studies. The other two studies reported higher prevalences of micoinfarcts (>40%); in one, the mean age at death was relatively high (more than 90 years),82 while in the other, the reasons for a higher prevalence of microinfarcts were unclear but could possibly be related to the sampling strategy.8 Four of the 7 studies found a higher prevalence of microinfarcts in persons with dementia compared to persons without dementia (30 to 64% versus 12 to 38%),8, 11, 37, 82 and 5 of the 7 studies found an independent association between microinfarcts and dementia in multivariable-adjusted analyses.8, 11, 19, 37, 82 Microinfarcts were not only common in clinically-diagnosed vascular dementia (18/25, 75%) or mixed dementia (4/4, 100%) but also common in probable AD dementia (32/76, 44%).82 Pooling the 7 studies, the prevalence of microinfarcts was nearly twice as high in persons who died with dementia (280/488, 57%) than those who died without dementia (208/673, 31%) (Figure 4).
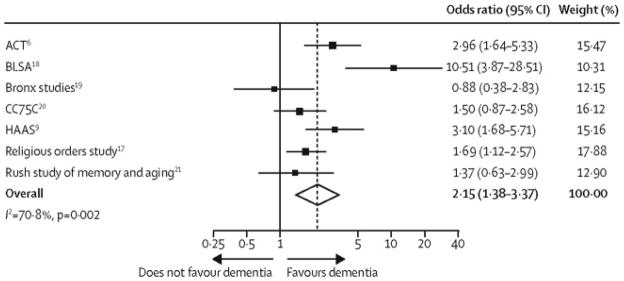
Figure 4. Pooled Odds of Dementia in Persons with Microinfarcts (Unadjusted Analysis).
The unadjusted odds of dementia were more than doubled in the presence of microinfarcts (odds ratio 2.31, 95% confidence interval 1.40 to 3.82). Significant heterogeneity in the odds across studies (p=0.002) may be related to different methods for sampling for microinfarcts. See the legend to Table 1 for an explanation of the study acronyms.
In most cases microinfarcts co-exist with other pathologies, and are infrequently the sole neuropathology in cognitively impaired persons. In two studies, only 13/118 (11%)11 and 3/179 (2%)22 of demented persons were found to have microinfarcts as the sole potential cause of dementia. About half of patients with microinfarcts also had grossly visible macroinfarcts, particularly lacunar infarcts; conversely, about half of patients with macroinfarcts had accompanying microinfarcts.19, 22
To disentangle the relative contribution of these correlated pathologies—microinfarction and macroinfarction—in the same set of brains, multivariable statistical modeling can be employed. When controlling for the presence of larger infarcts including lacunes, microinfarcts were independently associated with the risk of dementia in a series of single center hospital-based studies,75–77, 79 and in three prospective community-based studies.11, 19, 37 In several of these studies larger infarcts were also associated with the risk of dementia,19, 37, 76, 77 while in others only microinfarcts, but not larger infarcts, were associated with dementia in the fully adjusted models. 11, 75, 79 In another study microinfarcts, but not larger infarcts, were associated with dementia in univariate analysis.8 Conversely, only a single study found the opposite—that larger (macroscopic) infarcts, but not microscopic infarcts, were associated with dementia in univariate and multivariable-adjusted analyses.21 Finally, a single study found that neither microinfarcts nor larger infarcts were associated with dementia when controlling for each other.27 The preponderance of the evidence therefore suggests that while both microinfarcts and lacunar infarcts may increase the risk of dementia, it is the smaller but more numerous microinfarcts that may be the relatively stronger contributor. More, larger studies with careful pathological assessment are needed to clarify the clinical relevance of microinfarcts compared to the entire spectrum of abnormalities due to small vessel disease, including white matter lesions, microbleeds and ischemic lesions.
There are few data on the prevalence of microinfarcts in persons with mild cognitive impairment (MCI) rather than dementia. One study found an intermediate prevalence of microinfarcts in persons with CDR greater than 0 but no dementia (26/47, 44%) compared to persons who were cognitively normal (25/88, 28%) or demented (47/74, 64%),8 while another study found no differences between cognitively normal and MCI (normal: 41/170, 24%; MCI 28/134, 20.9%; AD dementia 57/179, 31.8%).22
It is reasonable to surmise that the clinical impact of microinfarcts is determined by location as well as number, but this aspect has not been extensively studied. Two studies suggest that cortical microinfarcts may be more closely associated with dementia than subcortical microinfarcts,19, 37 while another found that both cortical and basal ganglia or thalamic infarcts independently contributed to likelihood of dementia.11
The relationship between microinfarcts and neuropsychological test performance has been investigated in a prospective community-based autopsy study.19 Microinfarcts were associated with episodic memory, semantic memory and visual perceptual speed, but not working memory or visuospatial function, in models controlling for age, sex, education, macroinfarcts, AD pathology and Lewy bodies.19 Cortical microinfarcts but not subcortical microinfarcts were associated with poor cognitive performance.19 Microinfarcts were associated with age-related cognitive decline but not the more rapid terminal cognitive decline preceding death.83
There is limited information on the relationship between microinfarcts and clinical findings other than cognitive dysfunction. Gait impairment has been linked to microinfarcts in a small number of cases,69, 84 and a large community-based autopsy study showed that multiple microinfarcts were associated with Parkinsonism after controlling for macroinfarcts and other neuropathologies.85 Microinfarcts are more prevalent in patients with Parkinson’s disease or Lewy body disease compared to healthy controls.86 Reports on associations with depression are inconsistent. Two studies from the same group failed to find an association between microinfarcts and depression87 or post-stroke depression,88 while another smaller study of patients with vascular dementia or Alzheimer’s dementia did find an association with depression.70
AREAS FOR FUTURE INVESTIGATION
There are many important directions for future studies that could move the field in the direction of new treatment approaches for preventing microinfarct-related neurologic dysfunction. Some salient possibilities are listed below.
Develop standardized neuropathologic methods
A standardized approach to the detection, diagnosis, and classification of microinfarcts would allow valid comparisons across populations and research groups, and potentially provide clues to microinfarct pathophysiology. Therefore, development of new consensus standards should be a priority. The standardized method would need to include specific guidelines on which brain regions should be sampled, how tissue should be stained, and how identified lesions should be classified. General guidelines provided by the NINDS – CSN VCI Harmonization Standards6 provide a reasonable starting point for standard blocking protocols (to include the traditional CERAD regions24 plus sections of the anterior and posterior white matter43, 71, 86); in addition we suggest that basal ganglia, thalamus, pons, cerebellum, occipital cortex, periventricular, arterial borderzone25–27 and motor regions27 should be sampled as well. Microinfarct load in both cortical and subcortical brain regions should be recorded. With regard to staining methods, H&E appears to be a good technique for identifying microinfarcts, but with unclear sensitivity and specificity relative to immunohistochemical methods. Given that non-infarct pathologies may result in the clustering of reactive astrocytes or microglia, one may hypothesize that immunohistochemical markers for inflammation or injury may increase sensitivity but decrease specificity for microinfarct detection, suggesting they should be used as an adjunctive but not primary measure at present. Finally, the major outstanding question for microinfarct classification is how to categorize milder injuries without clear cavitation, including the distinct foci of tissue pallor or gliosis that can occur with small vessel disease. Though sometimes referred to as incomplete ischemic injury or subinfarctive injury, there are no detailed standardized definitions or gradations of these injuries, and their relationship with complete microinfarcts is not well established. In the absence of a better understanding of the relationship between “complete” and “incomplete” lesions, it seems reasonable to record and analyze the two lesions individually. Data collection should also include the presumed age of the microinfarct, since established or cavitated microinfarcts might be better correlates of clinical status than recent microinfarcts representing perimortem rather than ante-mortem events. However, by analogy with lacunar lesions, we should not assume that microinfarcts all cavitate or that those that cavitate do so at the same rate.60 Finally, total counts of microinfarcts should be collected and reported along with the sampling strategy employed, to determine the total burden of microinfarct pathology.
Collect data on pathologies and risk factors associated with microinfarcts
Despite the emerging impression that these lesions reflect infarction related to advanced small vessel disease, the precise pathogenesis of microinfarcts is not known. It will thus be important to establish which pathologies, risk factors and medical conditions are associated with microinfarcts in different populations. Potential mechanisms for microinfarction include occlusive vascular disease, embolism, hypoperfusion, and disruptions to the blood brain barrier. Focal injury related to oxidative stress, inflammation or other mechanisms are also possible. Indeed, the pathogenesis of microinfarcts may be the result of more than one mechanism. Unraveling these potential mechanisms could be best accomplished by clinical-imaging correlation studies using an accurate, noninvasive method for microinfarct detection during life (see below). In the absence of such an imaging modality, detailed prospective clinical-pathological correlation studies that collect extensive information on associated clinical risk factors and vascular pathologies remain the most compelling approach.
Determine the full anatomic extent and physiologic impact of microinfarcts
It will be important to establish the mechanisms by which these very small foci of tissue injury result in impaired neurologic function. As noted above, the finding of even a few microinfarcts in a small volume of sampled brain tissue implies a large global burden of microinfarcts and volume of tissue loss; working this out with precision will require more detailed information on variations in microinfarct density across various brain regions. An intriguing potential approach to this question is post mortem MRI at high field strength and spatial resolution, which offers the theoretical possibility of imaging microinfarcts throughout the entire brain.89 There may also be related tissue injuries extending beyond the immediate microinfarct focus. These distant injuries could include regional oxidative stress, inflammation or other tissue changes. Alternatively, microinfarcts may be a surrogate marker of other pathophysiologic disruptions such as disruptions in the blood brain barrier or generalized hypoperfusion. Future studies are needed to estimate microinfarct load, related tissue injuries, and the potential roles of inflammation, oxidative stress or blood brain barrier disruptions in promoting brain dysfunction.
Identify noninvasive methods for detection of microinfarcts in life
Establishing accurate means of identifying microinfarcts during life would represent perhaps the greatest step towards elucidating the causes and effects of these lesions. Enhanced detection on MRI could be enabled by higher field strengths51, 90 and higher spatial resolution, although trade-offs in increased scan acquisition time may make current research approaches challenging for even the most cooperative patients.
Given the technical challenges in identifying individual microinfarcts, a potentially fruitful alternative approach is to infer their presence by identifying patterns of associated lesions. MRI-defined cerebral small vessel disease is associated with increased vascular permeability on MRI,91 and retinal microvascular abnormalities,92, 93 but it is not currently known whether these abnormalities are also specifically associated with microinfarction. Other consequences of microinfarction could include regional or global brain atrophy, or subtle alterations in white matter water proton diffusivity or fractional anisotropy detectable using diffusion-tensor imaging.94 Some small MRI lesions currently considered to be enlarged perivascular spaces might actually represent visible microinfarcts.
Incorporate incident microinfarcts as a neuroimaging marker in clinical studies and trials
Current MRI DWI sequences, though limited in spatial and temporal resolution, offer a convenient and readily available method for detecting at least some incident acute microinfarcts in individuals undergoing MRI in observational or interventional studies. New microinfarcts, if at the larger end of the spectrum (greater than 1 mm), may remain visible as focal hyperintensities on T2-weighted images or lesions with signal characteristics similar to enlarged perivascular spaces. Incident microinfarcts, and possibly incident enlarged perivascular space-like lesions, could thus reasonably be incorporated as ancillary markers in studies aimed at preventing progression of small vessel brain disease. This possibility is particularly attractive for vascular conditions such as hypertensive brain disease or advanced CAA,61 in which the incidence of these lesions may be high enough to allow a treatment-related reduction to be detectable. An important goal for future investigations in this area will be to collect more data on the appearance, location, and associated risk factors for these small, clinically silent DWI lesions, a promising noninvasive approach towards visualizing the “invisible” microinfarct.
CONCLUSIONS
Despite the large number of unanswered questions about cerebral microinfarcts, it is possible to make some preliminary conclusions. Microinfarcts are quite numerous in the aged brain, particularly in individuals with cognitive impairment or underlying small vessel diseases. Nonetheless, they are difficult to detect directly and specifically during life based on current neuroimaging capabilities. However, MRI DWI detects a surprisingly high incidence of acute small infarctions in patients with symptomatic small vessel diseases, approaching the upper size limit of pathologically-defined microinfarcts. Mounting evidence from clinical-autopsy correlation studies suggest that the numerous foci of tissue loss related to cerebral microinfarcts may play a direct causal role in cognitive impairment, particularly when present in combination with other age-related brain pathologies. Future work should focus on achieving consensus pathological definitions, improving our understanding of the pathogenesis and consequences to brain structure and function, and developing non-invasive means of detection during life.
Acknowledgments
There was no external funding for this review. Dr. Smith receives salary support from Alberta Innovates – Health Solutions (funded by the Alberta Heritage Fund for Medical Research) and the Canadian Institutes of Health Research. Dr. Wardlaw receives partial funding support from the Scottish Funding Council. Dr. Greenberg receives salary support from the National Institutes of Health, including for the study of microinfarcts in cerebral amyloid angiopathy (2R01 AG26484-01). The corresponding author Dr. Greenberg had full access to all the data and had the final responsibility for the decision to submit for publication. We gratefully acknowledge Dr. Chunhui Yang for contributions to Figure 1; Dr. Eric Jouvent and Dr. Hugues Chabriat for contributing Figure 2; Drs. Dorothea Strozyk, Joe Verghese and Richard O’Brien for contributing unpublished data used in Table 1 and Figure 4; and Dr. Chelsea Kidwell for providing details of her studies presented at the 2010 International Stroke conference and published in abstract form.
Footnotes
CONFLICT OF INTEREST STATEMENT
Dr. Smith reports no conflicts of interest. Dr. Schneider reports serving as a consultant to AVID Radiopharmaceuticals, and serving on advisory boards to Eli Lilly and Company and GE Healthcare. Dr. Wardlaw reports no conflicts of interest. Dr. Greenberg reports no conflicts of interest.
AUTHOR CONTRIBUTIONS
Dr. Smith performed the literature search, reviewed relevant articles, carried out meta-analysis, wrote the first draft of sections of the manuscript, and made revisions to the manuscript content. Dr. Schneider reviewed relevant articles from the literature search, identified additional relevant articles, wrote the first draft of sections of the manuscript, and made revisions to the manuscript content. Dr. Wardlaw reviewed relevant articles from the literature search, identified additional relevant articles, wrote the first draft of sections of the manuscript, and made revisions to the manuscript content. Dr. Greenberg conceived the project, reviewed relevant articles from the literature search, identified additional relevant articles, and made revisions to the manuscript content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet neurol. 2007;6:611–9. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 3.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Bmj. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 5.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 7.Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–45. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 8.Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 9.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–25. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 10.Marshall VG, Bradley WG, Jr, Marshall CE, Bhoopat T, Rhodes RH. Deep white matter infarction: correlation of MR imaging and histopathologic findings. Radiology. 1988;167:517–22. doi: 10.1148/radiology.167.2.3357964. [DOI] [PubMed] [Google Scholar]
- 11.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Schneider JA. High blood pressure and microinfarcts: a link between vascular risk factors, dementia, and clinical Alzheimer’s disease. J Am Geriatr Soc. 2009;57:2146–7. doi: 10.1111/j.1532-5415.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 14.Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, et al. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–65. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- 15.Mancardi GL, Romagnoli P, Tassinari T, Gandolfo C, Primavera A, Loeb C. Lacunae and cribriform cavities of the brain. Correlations with pseudobulbar palsy and parkinsonism. Eur Neurol. 1988;28:11–7. doi: 10.1159/000116220. [DOI] [PubMed] [Google Scholar]
- 16.Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, et al. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 2010;20:459–67. doi: 10.1111/j.1750-3639.2009.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pentschew A. Die granulare atrophie der grosshirnrinde. Arch Psychiatry. 1933;101:80–136. [Google Scholar]
- 18.Okamoto Y, Ihara M, Fujita Y, Ito H, Takahashi R, Tomimoto H. Cortical microinfarcts in Alzheimer’s disease and subcortical vascular dementia. Neuroreport. 2009;20:990–6. doi: 10.1097/WNR.0b013e32832d2e6a. [DOI] [PubMed] [Google Scholar]
- 19.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnen JA, Santa Cruz K, Hemmy LS, Woltjer R, Leverenz JB, Montine KS, et al. Ecology of the aging human brain. Arch Neurol. 2011;68:1049–56. doi: 10.1001/archneurol.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 22.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology. 1965;15:774–84. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- 24.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 25.Miklossy J. Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer’s disease. Neurol Res. 2003;25:605–10. doi: 10.1179/016164103101202048. [DOI] [PubMed] [Google Scholar]
- 26.Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–92. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 27.Strozyk D, Dickson DW, Lipton RB, Katz M, Derby CA, Lee S, et al. Contribution of vascular pathology to the clinical expression of dementia. Neurobiol Aging. 2008;31:1710–20. doi: 10.1016/j.neurobiolaging.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haglund M, Passant U, Sjobeck M, Ghebremedhin E, Englund E. Cerebral amyloid angiopathy and cortical microinfarcts as putative substrates of vascular dementia. Int J Geriatr Psychiatry. 2006;21:681–7. doi: 10.1002/gps.1550. [DOI] [PubMed] [Google Scholar]
- 29.Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54:22–31. [PubMed] [Google Scholar]
- 30.Olichney JM, Hansen LA, Lee JH, Hofstetter CR, Katzman R, Thal LJ. Relationship between severe amyloid angiopathy, apolipoprotein E genotype, and vascular lesions in Alzheimer’s disease. Ann N Y Acad Sci. 2000;903:138–43. doi: 10.1111/j.1749-6632.2000.tb06360.x. [DOI] [PubMed] [Google Scholar]
- 31.Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986;45:79–90. [PubMed] [Google Scholar]
- 32.De Reuck J, Deramecourt V, Cordonnier C, Leys D, Maurage CA, Pasquier F. The impact of cerebral amyloid angiopathy on the occurrence of cerebrovascular lesions in demented patients with Alzheimer features: a neuropathological study. Eur J Neurol. 2011;18:1468–331. doi: 10.1111/j.1468-1331.2010.03329.x. [DOI] [PubMed] [Google Scholar]
- 33.Arboix A, Ferrer I, Marti-Vilalta JL. Clinico-anatomopathologic analysis of 25 patients with lacunar infarction. Rev Clin Esp. 1996;196:370–4. [PubMed] [Google Scholar]
- 34.Schiff D, Lopes MB. Neuropathological correlates of reversible posterior leukoencephalopathy. Neurocrit Care. 2005;2:303–5. doi: 10.1385/NCC:2:3:303. [DOI] [PubMed] [Google Scholar]
- 35.Akai K, Uchigasaki S, Tanaka U, Komatsu A. Normal pressure hydrocephalus. Neuropathological study. Acta Pathol Jpn. 1987;37:97–110. [PubMed] [Google Scholar]
- 36.Longstreth WT, Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23:291–4. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi R, Joachim C, Geroldi C, Combrinck M, Esiri MM, Smith AD, et al. Association between subcortical vascular disease on CT and neuropathological findings. Int J Geriatr Psychiatry. 2004;19:690–5. doi: 10.1002/gps.1144. [DOI] [PubMed] [Google Scholar]
- 39.Munoz DG. Small vessel disease: neuropathology. Int Psychogeriatr. 2003;15 (Suppl 1):67–9. doi: 10.1017/S1041610203008986. [DOI] [PubMed] [Google Scholar]
- 40.Viswanathan A, Gray F, Bousser MG, Baudrimont M, Chabriat H. Cortical neuronal apoptosis in CADASIL. Stroke. 2006;37:2690–5. doi: 10.1161/01.STR.0000245091.28429.6a. [DOI] [PubMed] [Google Scholar]
- 41.Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–75. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 42.Wang LY, Larson EB, Sonnen JA, Shofer JB, McCormick W, Bowen JD, et al. Blood pressure and brain injury in older adults: findings from a community-based autopsy study. J Am Geriatr Soc. 2009;57:1975–81. doi: 10.1111/j.1532-5415.2009.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Ser T, Hachinski V, Merskey H, Munoz DG. Alzheimer’s disease with and without cerebral infarcts. J Neurol Sci. 2005;231:3–11. doi: 10.1016/j.jns.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 45.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–35. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 46.Udaka F, Sawada H, Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci. 2002;977:411–5. doi: 10.1111/j.1749-6632.2002.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 47.Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dementia & Geriatric Cognitive Disorders. 1998;9:2–5. doi: 10.1159/000051182. [DOI] [PubMed] [Google Scholar]
- 48.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41:450–4. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 49.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483–90. doi: 10.1161/STROKEAHA.110.591586. [DOI] [PubMed] [Google Scholar]
- 50.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512–20. [PMC free article] [PubMed] [Google Scholar]
- 51.Jouvent E, Poupon C, Gray F, Paquet C, Mangin JF, Le Bihan D, et al. Intracortical infarcts in small vessel disease: a combined 7-T postmortem MRI and neuropathological case study in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2011;42:e27–30. doi: 10.1161/STROKEAHA.110.594218. [DOI] [PubMed] [Google Scholar]
- 52.Gass A, Ay H, Szabo K, Koroshetz WJ. Diffusion-weighted MRI for the “small stuff”: the details of acute cerebral ischaemia. Lancet Neurol. 2004;3:39–45. doi: 10.1016/s1474-4422(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 53.Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross-sectional study. J Neurol Neurosurg Psychiatry. 2010 doi: 10.1136/jnnp.2009.190298. [DOI] [PubMed] [Google Scholar]
- 54.Romero JM, Schaefer PW, Grant PE, Becerra L, Gonzalez RG. Diffusion MR imaging of acute ischemic stroke. Neuroimaging Clin N Am. 2002;12:35–53. doi: 10.1016/s1052-5149(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 55.Lansberg MG, Thijs VN, O’Brien MW, Ali JO, de Crespigny AJ, Tong DC, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol. 2001;22:637–44. [PMC free article] [PubMed] [Google Scholar]
- 56.Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, et al. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35:514–9. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- 57.Schwamm LH, Koroshetz WJ, Sorensen AG, Wang B, Copen WA, Budzik R, et al. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29:2268–76. doi: 10.1161/01.str.29.11.2268. [DOI] [PubMed] [Google Scholar]
- 58.Munoz Maniega S, Bastin ME, Armitage PA, Farrall AJ, Carpenter TK, Hand PJ, et al. Temporal evolution of water diffusion parameters is different in grey and white matter in human ischaemic stroke. J Neurol Neurosurg Psychiatry. 2004;75:1714–8. doi: 10.1136/jnnp.2003.033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–9. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 60.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Counting cavitating lacunes underestimates the burden of lacunar infarction. Stroke. 2010;41:267–72. doi: 10.1161/STROKEAHA.109.566307. [DOI] [PubMed] [Google Scholar]
- 61.Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–5. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gobron C, Viswanathan A, Bousser MG, Chabriat H. Multiple simultaneous cerebral infarctions in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Cerebrovasc Dis. 2006;22:445–6. doi: 10.1159/000095287. [DOI] [PubMed] [Google Scholar]
- 63.Ay H, Oliveira-Filho J, Buonanno FS, Ezzeddine M, Schaefer PW, Rordorf G, et al. Diffusion-weighted imaging identifies a subset of lacunar infarction associated with embolic source. Stroke. 1999;30:2644–50. doi: 10.1161/01.str.30.12.2644. [DOI] [PubMed] [Google Scholar]
- 64.Wessels T, Rottger C, Jauss M, Kaps M, Traupe H, Stolz E. Identification of embolic stroke patterns by diffusion-weighted MRI in clinically defined lacunar stroke syndromes. Stroke. 2005;36:757–61. doi: 10.1161/01.STR.0000158908.48022.d7. [DOI] [PubMed] [Google Scholar]
- 65.Prabhakaran S, Gupta R, Ouyang B, John S, Temes RE, Mohammad Y, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage: a diffusion-weighted imaging study. Stroke. 2010;41:89–94. doi: 10.1161/STROKEAHA.109.566257. [DOI] [PubMed] [Google Scholar]
- 66.Gregoire SM, Gadapa N, Dolan E, Baron JC, Peeters A, Vandermeeren Y, et al. Silent ischemic lesions in patients with acute symptomatic spontaneous intracerebral hemorrhage: Relationship to lobar microbleeds and clinically probable cerebral amyloid angiopathy. Stroke. 2011;42:e122. [Google Scholar]
- 67.Menon RS, Burgess RE, Wing JJ, Gibbons MC, Shara NM, Fernandez S, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Annals of Neurology. 2011 doi: 10.1002/ana.22668. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chowdhury D, Wardlaw JM, Dennis MS. Are multiple acute small subcortical infarctions caused by embolic mechanisms?[see comment] J Neurol Neurosurg Psychiatry. 2004;75:1416–20. doi: 10.1136/jnnp.2004.038653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan JG, Katzman R, Horoupian DS, Fuld PA, Mayeux R, Hays AP. Progressive dementia, visual deficits, amyotrophy, and microinfarcts. Neurology. 1985;35:789–96. doi: 10.1212/wnl.35.6.789. [DOI] [PubMed] [Google Scholar]
- 70.Ballard C, McKeith I, O’Brien J, Kalaria R, Jaros E, Ince P, et al. Neuropathological substrates of dementia and depression in vascular dementia, with a particular focus on cases with small infarct volumes. Dement Geriatr Cogn Disord. 2000;11:59–65. doi: 10.1159/000017215. [DOI] [PubMed] [Google Scholar]
- 71.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–53. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.del Ser T, Bermejo F, Portera A, Arredondo JM, Bouras C, Constantinidis J. Vascular dementia. A clinicopathological study. J Neurol Sci. 1990;96:1–17. doi: 10.1016/0022-510x(90)90052-o. [DOI] [PubMed] [Google Scholar]
- 73.Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol. 1987;74:209–25. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 74.De Reuck J, Deramecourt V, Cordonnier C, Leys D, Pasquier F, Maurage CA. Prevalence of small cerebral bleeds in patients with a neurodegenerative dementia: a neuropathological study. J Neurol Sci. 2011;300:63–6. doi: 10.1016/j.jns.2010.09.031. Epub 2010 Oct 20. [DOI] [PubMed] [Google Scholar]
- 75.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–4. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- 76.Gold G, Kovari E, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–8. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 77.Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain. 2007;130:2830–6. doi: 10.1093/brain/awm228. [DOI] [PubMed] [Google Scholar]
- 78.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 79.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, et al. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology. 2007;68:927–31. doi: 10.1212/01.wnl.0000257094.10655.9a. [DOI] [PubMed] [Google Scholar]
- 80.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–7. [PubMed] [Google Scholar]
- 81.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354:919–20. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 82.Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18:645–58. doi: 10.3233/JAD-2009-1182. [DOI] [PubMed] [Google Scholar]
- 83.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–8. doi: 10.1212/WNL.0b013e3181f39adc. Epub 2010 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baloh RW, Vinters HV. White matter lesions and disequilibrium in older people. II. Clinicopathologic correlation. Arch Neurol. 1995;52:975–81. doi: 10.1001/archneur.1995.00540340067014. [DOI] [PubMed] [Google Scholar]
- 85.Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke. 2011;42:3183–9. doi: 10.1161/STROKEAHA.111.623462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghebremedhin E, Rosenberger A, Rub U, Vuksic M, Berhe T, Bickeboller H, et al. Inverse relationship between cerebrovascular lesions and severity of lewy body pathology in patients with lewy body diseases. J Neuropathol Exp Neurol. 2010;69:442–8. doi: 10.1097/NEN.0b013e3181d88e63. [DOI] [PubMed] [Google Scholar]
- 87.Santos M, Gold G, Kovari E, Herrmann FR, Hof PR, Bouras C, et al. Neuropathological analysis of lacunes and microvascular lesions in late-onset depression. Neuropathol Appl Neurobiol. 2010 doi: 10.1111/j.1365-2990.2010.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Santos M, Gold G, Kovari E, Herrmann FR, Bozikas VP, Bouras C, et al. Differential impact of lacunes and microvascular lesions on poststroke depression. Stroke. 2009;40:3557–62. doi: 10.1161/STROKEAHA.109.548545. [DOI] [PubMed] [Google Scholar]
- 89.Wardlaw JM. Post-mortem MR brain imaging comparison with macro- and histopathology: useful, important and underused. Cerebrovasc Dis. 2011;31:518–9. doi: 10.1159/000326846. [DOI] [PubMed] [Google Scholar]
- 90.Song HS, Kang CK, Kim JS, Park CA, Kim YB, Lee DH, et al. Assessment of pial branches using 7-tesla MRI in cerebral arterial disease. Cerebrovasc Dis. 2010;29:410. doi: 10.1159/000288056. [DOI] [PubMed] [Google Scholar]
- 91.Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, et al. Changes in background blood-brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke. 2008;39:1327–32. doi: 10.1161/STROKEAHA.107.500124. [DOI] [PubMed] [Google Scholar]
- 92.Doubal FN, MacGillivray TJ, Hokke PE, Dhillon B, Dennis MS, Wardlaw JM. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology. 2009;72:1773–8. doi: 10.1212/WNL.0b013e3181a60a71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindley RI, Wang JJ, Wong MC, Mitchell P, Liew G, Hand P, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet neurol. 2009;8:628–34. doi: 10.1016/S1474-4422(09)70131-0. [DOI] [PubMed] [Google Scholar]
- 94.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–48. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]