Abstract
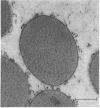
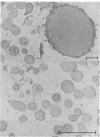
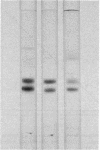
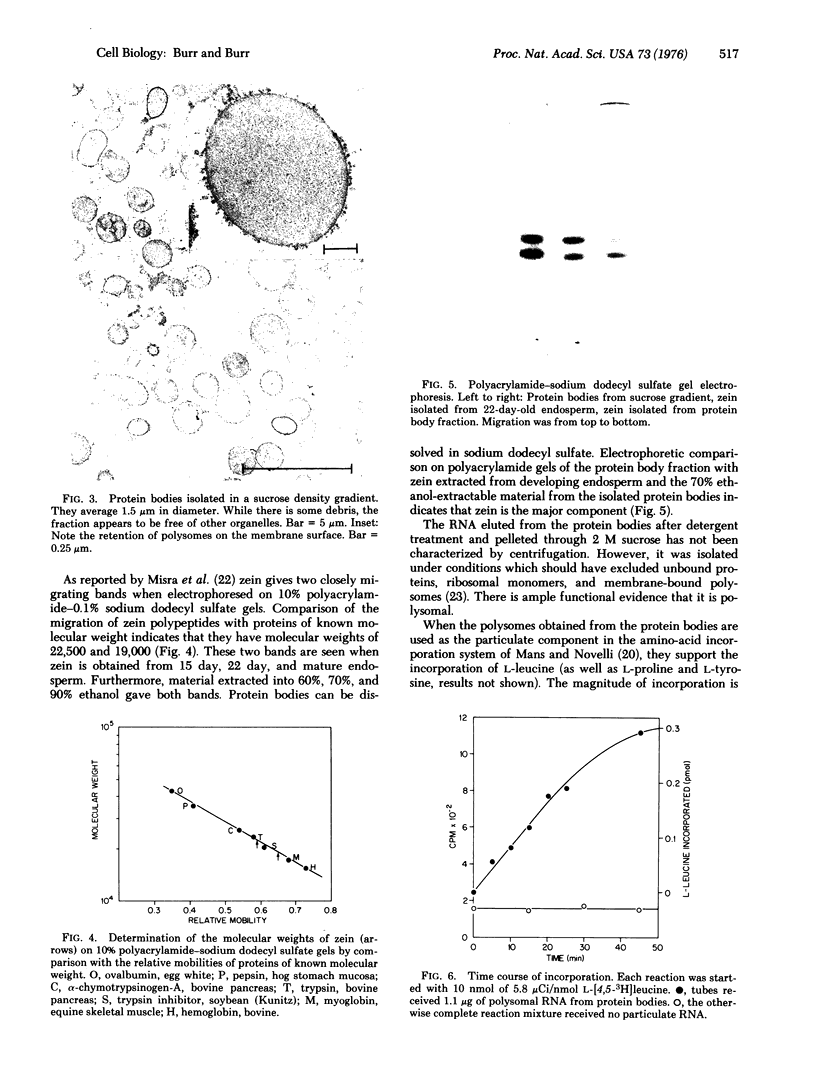
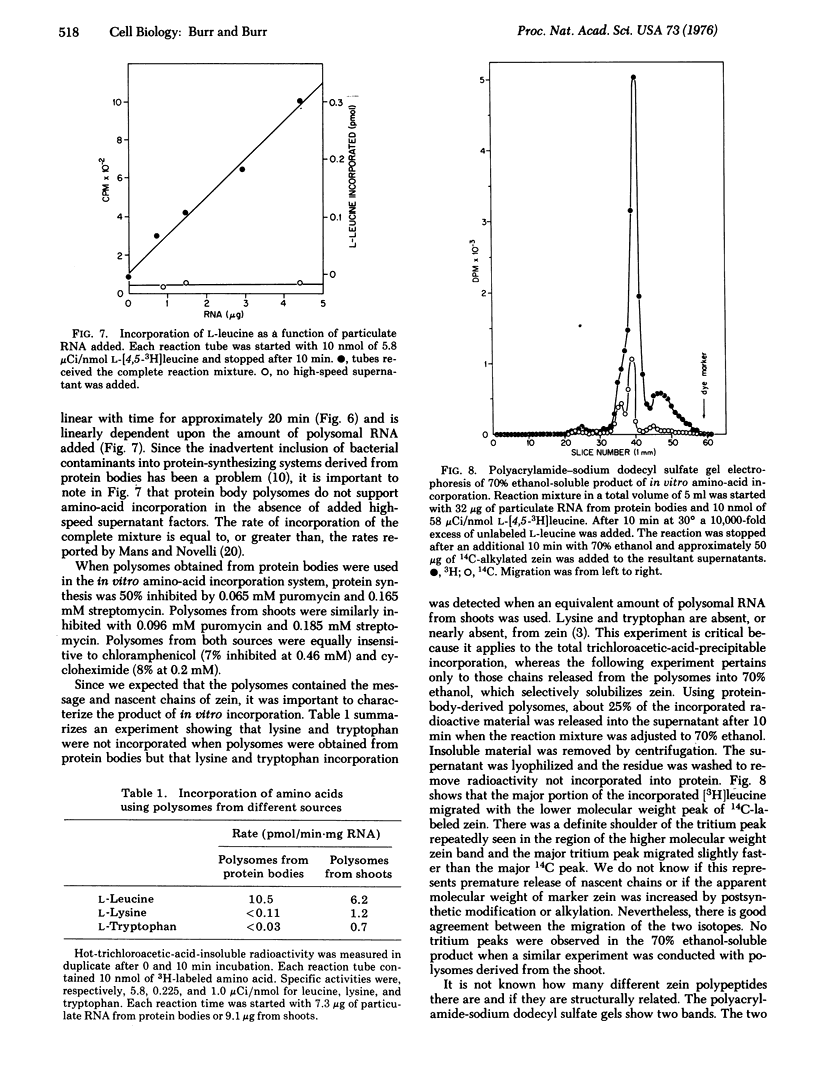
The protein bodies in maize endosperm are the sites of zein deposition. They are single membrane-bound vesicles with polysomes associated with the exterior surface of the membrane. These protein bodies were isolated by sucrose density gradients and characterized by electron microscopy and polyacrylamide gel electrophoresis. Polyribosomes dissociated from the surface of the membrane by detergent treatment were placed into an amino-acid incorporating system. Based on alcohol solubility, amino-acid composition, and molecular weight distribution, the product synthesized appeared to be largely, or entirely, zein. This suggests the existence of components which are specific for the synthesis of zein at the protein body membrane surface.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalby A., Davies I. I. Ribonuclease activity in the developing seeds of normal and opaque-2 maize. Science. 1967 Mar 24;155(3769):1573–1575. doi: 10.1126/science.155.3769.1573. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Graebe J. E., Novelli G. D. Amino acid incorporation in a cell-free system from submerged tissue cultures of Zea mays L. Exp Cell Res. 1966 Mar;41(3):521–534. doi: 10.1016/s0014-4827(66)80103-9. [DOI] [PubMed] [Google Scholar]
- Gray J. C. The inhibition of ribonuclease activity and the isolation of polysomes from leaves of the french bean, Phaseolus vulgaris. Arch Biochem Biophys. 1974 Jul;163(1):343–348. doi: 10.1016/0003-9861(74)90485-8. [DOI] [PubMed] [Google Scholar]
- Kabat D. Potentiation of hemoglobin messenger ribonucleic acid. A step in protein synthesis initiation involving interaction of messenger with 18 S ribosomal ribonucleic acid. J Biol Chem. 1975 Aug 10;250(15):6085–6092. [PubMed] [Google Scholar]
- Morton R. K., Raison J. K. The separate incorporation of amino acids into storage and soluble proteins catalysed by two independent systems isolated from developing wheat endosperm. Biochem J. 1964 Jun;91(3):528–539. doi: 10.1042/bj0910528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Nelson O. E. Mutant genes that change the composition of maize endosperm proteins. Fed Proc. 1966 Nov-Dec;25(6):1676–1678. [PubMed] [Google Scholar]
- Ory R. L., Henningsen K. W. Enzymes associated with protein bodies isolated from ungerminated barley seeds. Plant Physiol. 1969 Nov;44(11):1488–1498. doi: 10.1104/pp.44.11.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES E. D., SINGER S. J. A preliminary study of the properties of proteins in some nonaqueous solvents. Arch Biochem Biophys. 1956 Jul;63(1):144–159. doi: 10.1016/0003-9861(56)90018-2. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. Bacteria, antibiotics and amino Acid incorporation into maize endosperm protein bodies. Plant Physiol. 1966 Feb;41(2):325–327. doi: 10.1104/pp.41.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. J., Khoo U., Seckinger H. L. Subcellular structure of endosperm protein in high-lysine and normal corn. Science. 1967 Aug 4;157(3788):556–557. doi: 10.1126/science.157.3788.556. [DOI] [PubMed] [Google Scholar]