Abstract
This experiment examined whether age-related changes in CREB and pCREB contribute to the rapid forgetting seen in aged animals. Young (3-month-old) and aged (24-month-old) Fischer-344 rats received inhibitory avoidance training with a low (0.2 mA, 0.4 sec) or moderate (0.5 mA, 0.5 sec) footshock; memory was measured 7 days later. Other rats were euthanized 30 minutes after training, and CREB and pCREB expression levels were examined in the hippocampus, amygdala, and piriform cortex using immunohistochemistry. CREB levels decreased with age in the hippocampus and amygdala. After training with either shock level, young rats exhibited good memory and increases in pCREB levels in the hippocampus and amygdala. Aged rats exhibited good memory for the moderate but not the low shock but did not show increases in pCREB levels after either shock intensity. These results suggest that decreases in total CREB and in pCREB activation in the hippocampus and amygdala may contribute to rapid forgetting in aged rats. After moderate footshock, the stable memory in old rats together with absence of CREB activation suggests either that CREB was phosphorylated in a spatiotemporal pattern other than analyzed here or that the stronger training conditions engaged alternate mechanisms that promote long-lasting memory.
Keywords: Memory, CREB, hippocampus, amygdala, inhibitory avoidance
1. INTRODUCTION
As seen in humans, rats and mice exhibit age-related impairments in learning and memory on many tasks. Often, the impairments are characterized in terms of rapid forgetting, in which aged rodents perform similarly to young adult rodents on memory tests soon after training, but have poor memory at later times as compared to young rodents (Barnes, 1991; Foster, 1999; Gold, 2005, 2001; Korol, 2002; Winocur, 1988). There are many examples of accelerated forgetting in aged rodents, with specific time courses that differ by task. In particular, memory for inhibitory avoidance training remains stable in young adult rats for weeks after training but decays within hours to days in old rats. Importantly, the age-related difference in forgetting rate is apparent despite similar or lower foot shock perceptual thresholds in old rats (Foster and Kumar, 2007; Frye et al., 2010; Gold et al., 1981; Morris et al., 2010). Rapid forgetting is also evident in a variety of other tasks, including the water maze (Burke et al., 2008; Gage et al., 1984; Mabry et al., 1996; Rapp et al., 1987), reward reduction (Salinas and Gold, 2005), social transmission of food preference (Countryman and Gold, 2007), visual discriminated avoidance (Gold et al., 1981), Barnes circular maze (Barnes and McNaughton, 1985), spatial reversal (Zornetzer et al., 1982), spontaneous alternation (Da Silva Costa-Aze et al., 2011; McNay and Gold, 2001; Stone et al., 1997), odor-reward association (Roman et al., 1996), and eye-blink classical conditioning (e.g. Solomon et al., 1995; Woodruff-Pak et al., 2007). The breadth of examples of rapid forgetting suggests that this is a key characteristic of age-related changes in memory.
Rapid forgetting seen during aging is analogous to similar findings seen after many treatments that interfere with cell and molecular processes associated with the formation of new memories. Rapidly decaying memory is seen after administration of protein synthesis inhibitors, ERK/MAPK inhibitors, and inhibitors of transcription factors, such as cAMP response element-binding protein (CREB), and is also seen in several knockout and transgenic mice with alterations aimed at these and other molecular targets (e.g. Alberini, 2009; Apergis-Schoute et al., 2005; Costa-Mattioli and Sonenberg, 2008; Goelet et al., 1986; Guzowski et al., 2000; Houpt and Berlin, 1999; Izquierdo et al., 2006; Kandel, 2001; McGaugh, 2000; Taubenfeld et al., 2001; Trifilieff et al., 2006). The parallels between the rapid forgetting in these experiments and those seen in aged rodents suggest that similar cellular mechanisms may be involved.
Of particular relevance to the experiments reported here, considerable evidence suggests that CREB is a transcription factor important to the formation of durable memories, perhaps converting rapidly decaying memories and short-term potentiation to more permanent forms (Benito and Barco, 2010; Carlezon et al., 2005; Colombo et al., 2003; Josselyn, 2010; Silva et al., 1998; Taubenfeld et al., 1999; Yin and Tully, 1996). For example, interfering with CREB function via mutations or inhibitors generally disrupts memory assessed at long but not short times after training (Bourtchuladze et al., 1994; Brightwell et al., 2008, 2005; Frankland et al., 2004; Guzowski and McGaugh, 1997; Josselyn et al., 2004; Yin et al., 1994), and these findings are analogous to the rapid forgetting seen in aged rodents. Several studies have examined CREB functions in aged rodents (cf. Lund et al., 2004). One consistent finding is that aged rodents are impaired in training-related activation of phosphorylated CREB (pCREB) in the hippocampus (Countryman and Gold, 2007; Kudo et al., 2005; Monti et al., 2005; Porte et al., 2008; Xu et al., 2010). Several studies of chronically administered treatments have identified substances that can attenuate age-related memory impairments while also enhancing CREB phosphorylation (Assunção et al., 2010; Li et al., 2009; Trofimiuk et al., 2010; Xu et al., 2010). Mouravlev et al. (2006) demonstrated that somatic gene transfer of CREB protein into the hippocampus of young adult rats prevented later formation of age-associated memory impairments. Also, Brightwell et al. (2004) found that aged rats with poor spatial memory have lower hippocampal CREB levels than do those with good spatial memory. However, prior aging studies have apparently not examined whether altering training conditions to promote stable memory formation in aged rodents would reverse deficits in CREB activation.
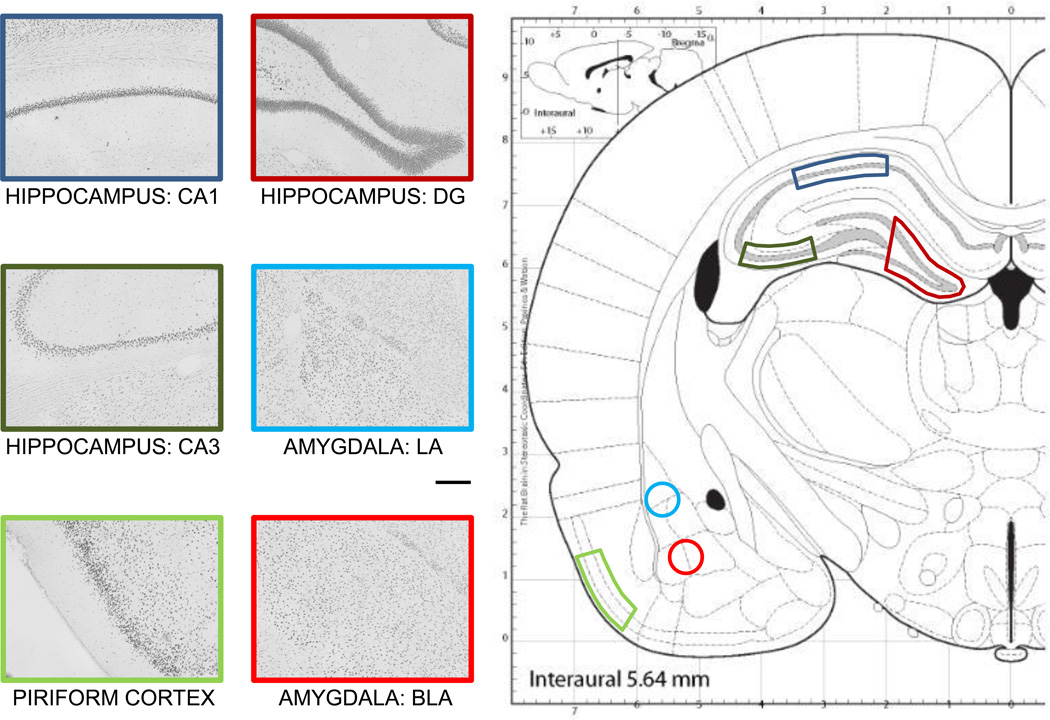
The present report tested the hypothesis that age-related differences in the expression and activation of CREB and pCREB may contribute to the rapid forgetting that is characteristic of aged rodents. Therefore, we used an inhibitory avoidance task in which the rate of forgetting is accelerated in aged rats. In addition, increases in the aversive component of training result in better maintenance of memory, providing a comparison of conditions in which memory is rapidly or slowly forgotten. Thus, in the experiments reported here, rats were trained with either a low intensity foot shock, which led to age-related rapid forgetting, or a moderate intensity foot shock, which led to stable memory formation in both young and old rats. In past studies of aging and memory, CREB and pCREB expression were assessed only in the hippocampus. In the present experiments, CREB and pCREB expression levels were assessed with immunohistochemistry in brain sections collected 30 min after training from the amygdala and piriform cortex, as well as from hippocampal dentate gyrus, area CA3, and area CA1 (see Figure 1).
Figure 1.
Illustration of the areas targeted in this study, which were the dentate gyrus (DG), CA3, and CA1 of the hippocampus, the lateral (LA) and basolateral (BLA) nuclei of the amygdala, and the piriform cortex. Scale bar = 200 µm. Adapted with permission from Paxinos and Watson (2003).
2. MATERIALS AND METHODS
2.1. Subjects
Young adult (3 to 4 mo.) and old (24 to 25 mo.) male Fischer-344 rats (Taconic Farms, Germantown, NY) were individually housed in translucent cages with a 12-h light/dark cycle (lights on at 07:00 h) and ad libitum access to food and water. Animal pain and discomfort were minimized, and all experiments were conducted in accordance with animal care guidelines established by the National Institute of Health and the University of Illinois, which is fully accredited (AAALAC).
2.2. Inhibitory Avoidance Training
Rats were handled on 5 consecutive days for 5 min each day prior to behavioral training. All training and testing took place between 1200 and 1600 h. The inhibitory avoidance apparatus was a trough-shaped alleyway (91 cm long, 22.9 cm wide at the top, 7.6 cm wide at the bottom, and 15.2 cm deep) divided into lit (31 cm) and dark (60 cm) compartments by a sliding door that could be lowered through the floor. Each rat was placed in the lit chamber facing the door. When the rat turned completely around, the door was lowered to a height approximately 2 cm above the floor. When the rat again turned toward the door, a timer was started to record the latency to enter the dark chamber. Upon entering the dark chamber, the rat received a brief foot shock (either 0.2 mA, 0.4 sec, or 0.5 mA, 0.5 sec) and the door was closed to prevent reentry into the lit chamber. The rat was then returned to the holding cage. For the initial behavioral characterization, retention latencies (max of 600 sec) were tested 7 days after training using the same procedure. To measure CREB and pCREB levels, rats were euthanized 30 min after training and immunostaining procedures were used at a later time. Groups for the CREB and pCREB measures also included a cage control group, in which rats were left in their home cage until euthanasia, and a no shock group, in which rats were placed in the training apparatus and allowed to cross from the lit to dark compartment, but did not receive a foot shock. Ns = 4 for the initial behavioral characterization. For the immunohistochemistry studies, N = 7 for the young cage control group and Ns =4 for all other groups.
2.3. Perfusion and Brain Slicing
Rats were deeply anesthetized with an overdose i.p. injection of sodium pentobarbital (Sigma-Aldrich, St. Louis, MO) and then perfused intracardially with 80 ml of 0.1 M phosphate-buffered saline followed by 80 ml of 4% paraformaldehyde in 0.1 M phosphate buffer. Rats were decapitated and the brains were removed and placed into 4% paraformaldehyde in 0.1 M PB for ~72 hrs. The brains were transferred to 20% glycerol in 0.1 M PBS for ~48 hrs. Frozen sections (40 µM) were collected at −30° C with a Leica 1800 cryostat (Leica Microsystems, Wetzlar, Germany). Slices through the dorsal hippocampus were collected and stored in a cryopreservative solution (250 mM 40 KD polyvinylpyrrolidone, 880 mM sucrose, 30% v/v ethylene glycol, 50 mM sodium phosphate) at −20° C.
2.4. Immunohistochemistry
All steps took place at room temperature. All reactions were performed in duplicate, using alternating slices for CREB and pCREB staining. Slices were washed three times for 10 min each time in 0.05 M PBS initially and between all subsequent steps. Slices were first incubated in blocking solution (1% H2O2, 1% normal goat serum, 0.02% triton x-100, 0.05 M PBS) for 10 min. They were transferred to a pre-incubation solution (2% NGS, 0.4% triton x-100, 0.05 M PBS) for 20 min and then incubated overnight in a solution (1% NGS, 0.4% triton x-100, 0.05 M PBS) containing a rabbit primary antibody for CREB or Ser-133 phosphorylated CREB (Millipore, Billerica, MA) diluted 1:4000. The next day, the slices were placed for 1 hr in a solution (1% NGS, 0.2% triton x-100, 0.05 M PBS) containing a goat anti-rabbit biotinylated secondary antibody (Santa Cruz, Santa Cruz, CA). They were next incubated for 30 min with ABC reagent (Vector, Burlingame, CA) in 0.05 M PBS, followed by incubation with DAB substrate (Vector) for 4 min. Slices were mounted onto slides and allowed to dry overnight. The next morning, slices were dehydrated with a graded ethanol series of washes, then coverslipped using DPX mountant (Sigma-Aldrich).
2.5. Image Acquisition and Analysis
Sections were imaged using a Leica DM 6000B/CTR6000 light microscope and a Leica DFC350 FX video camera, which was interfaced to a PC computer. This system was used in conjunction with Image-Pro software (Media Cybernetics, Inc., Bethesda, MD) for image acquisition and for correction of unevenness in illumination across images. Image J software (NIH, Bethesda, MA) was used to quantify the optical density of CREB and pCREB staining in subregions of the hippocampus, amygdala, and piriform cortex. Figure 1 shows the specific regions that were analyzed. A statistical thresholding method in Image J was used to ensure that only specifically labeled cells were being measured. For each image, the optical density of a nearby region with no or little specific staining was calculated and used for background subtraction.
2.6. Statistical Analyses
All analyses were performed using Statview software. The optical densities of CREB and pCREB immunostaining were analyzed using two-way ANOVAs with post hoc Fisher PLSD tests where appropriate. Behavioral results were analyzed using a non-parametric Kruskal-Wallis one-way analysis of variance, followed by Mann-Whitney tests for individual comparisons.
3. RESULTS
3.1. Behavioral performance on the inhibitory avoidance task
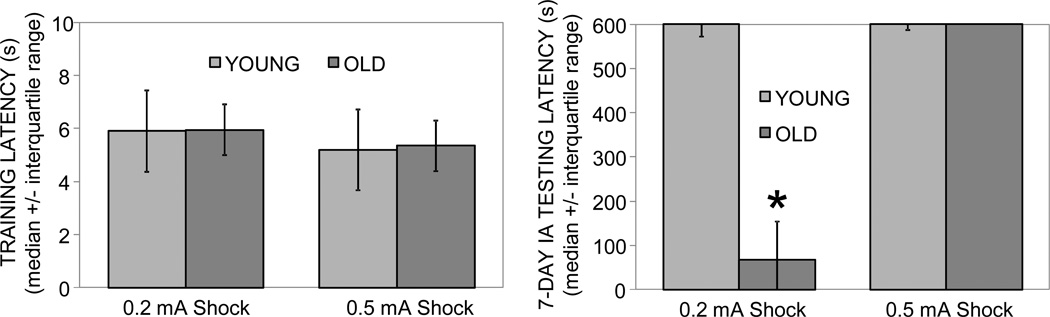
Figure 2 shows training latencies (Left) and 7-day retention latencies (Right) of young and old rats trained on an inhibitory avoidance task. There were no significant age-related differences in training latencies. Young rats had maximum median retention latencies of 600 sec following a 0.2 mA, 0.4 sec or 0.5 mA, 0.5 sec foot shock. The old rats had a maximum median retention latency following the moderate intensity foot shock, but a median retention latency of only 66.9 sec with the lower intensity foot shock. Nonparametric analysis of variance revealed a significant group effect (H = 8.7, p < .05). Post-hoc analyses showed that old rats receiving the low intensity foot shock had significantly lower retention latencies compared to each of the other three groups (ps < .05).
Figure 2.
Training and 7-day retention latencies in young and old rats trained on inhibitory avoidance with a 0.2 or 0.5 mA foot shock. (Left) There were no significant differences in training latencies across groups. (Right) Old rats had significantly lower 7-day retention latencies following the low intensity foot shock than did all other groups (*) ps < .05. Note that old rats exhibited maximal retention latencies after training with the higher foot shock intensity.
3.2. CREB and pCREB immunostaining following training
Differences in immunostaining of pCREB, total CREB, as well as pCREB:CREB ratios, were examined in young and old rats 30 min after inhibitory avoidance training with a low (0.2 mA, 0.4 sec) or moderate (0.5 mA, 0.5 sec) foot shock. These groups were compared to cage controls and to no shock controls, which were trained on inhibitory avoidance without the foot shock. These results are summarized in Table 1.
Table 1.
Summary of Immunohistochemistry Results
| TRAINING EFFECT | AGE EFFECT | ||
|---|---|---|---|
| YOUNG RATS | OLD RATS | ||
| pCREB | |||
| DG | ✓ | ✓ | |
| CA1 | ✓ | ✓ | |
| CA3 | |||
| BLA | ✓ | ✓ | |
| LA | ✓ | ✓ | |
| PIR | |||
| CREB | |||
| DG | |||
| CA1 | |||
| CA3 | ✓ | ||
| BLA | ✓ | ||
| LA | ✓ | ||
| PIR | |||
| pCREB:CREB | |||
| DG | ✓ | ||
| CA1 | ✓ | ||
| CA3 | |||
| BLA | ✓ | ||
| LA | |||
| PIR | |||
✓ denotes significant main effect of age or training with footshock, along with significant effect in at least one post-hoc planned comparison; Brain regions analyzed were dentate gyrus (DG), area CA1, and area CA3 of the hippocampus, basolateral (BLA) and lateral (LA) amygdala, and piriform cortex (PIR).
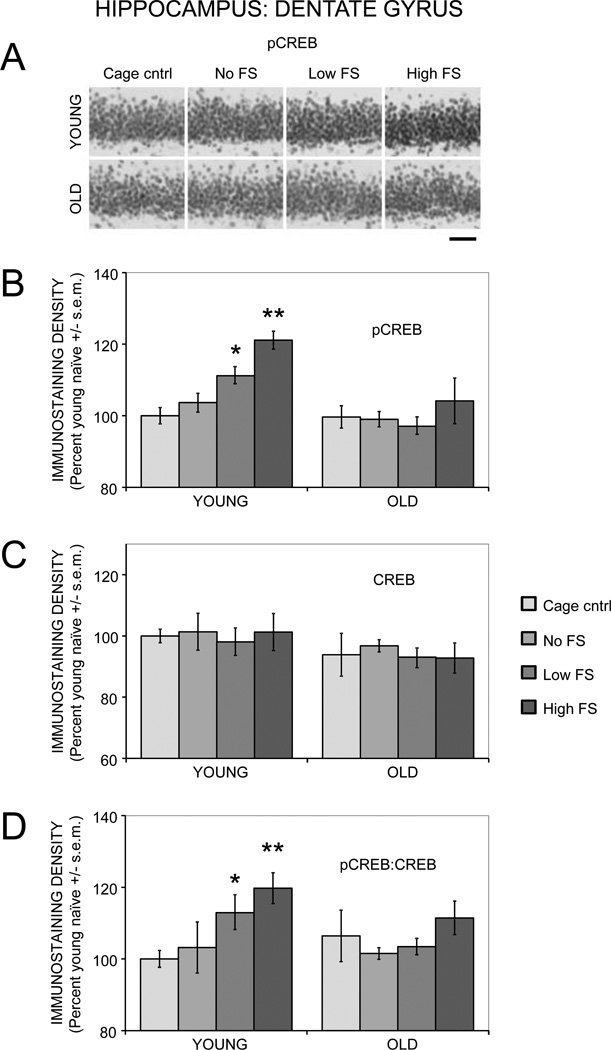
3.2.1. Hippocampus: Dentate Gyrus
Figure 3 (A and B) shows pCREB immunostaining in the dentate gyrus region of the hippocampus. Activation of CREB by training was evident in young but not aged rats. A two-way ANOVA revealed a main effect of training on pCREB staining (F(3,27) = 7.30, p < .01). In young rats, training with a 0.2 mA foot shock significantly enhanced pCREB staining compared to cage controls (p < .01). Training with a 0.5 mA foot shock enhanced pCREB staining compared to cage control, no shock, and 0.2 mA groups (ps < .03). In old rats, there were no significant increases in pCREB staining in response to training at either foot shock intensity. There was a main effect of age on pCREB (F(1,27) = 18.17, p < .001), indicating lower pCREB staining in old compared to young rats. Post-hoc tests were used to identify age-related differences within training groups (e.g. young cage controls vs. old cage controls). There were significant age-related differences between the young and old rats in the 0.2 mA and 0.5 mA shock groups (ps < .05). In addition to the training and age effects, there was a significant age by training interaction (F(3,27) = 3.58, p < .05).
Figure 3.
Age- and training-associated differences in pCREB and CREB immunoreactivity in the dentate gyrus of the hippocampus. (A) Representative photomicrographs of pCREB immunostaining. Scale bar = 50 µm. (B) Young rats had significantly enhanced pCREB levels following training with a low or moderate intensity foot shock, whereas old rats had training-related deficits in pCREB activation. (*) p < .01 vs. young cage controls. (**) ps < .05 vs. young cage control, no shock, and 0.2 mA. (C) Total CREB levels were similar between young and old rats and not altered by training. (D) Young rats had significantly higher ratios of pCREB:CREB after training with a low or moderate intensity foot shock, whereas old rats had training-related deficits in pCREB:CREB ratios. (*) p < .05 vs. young cage controls. (**) ps < .05 vs. young cage controls and young no shock.
There was an overall main effect of age (F(1,27) = 4.36, p < .05) on CREB immunostaining, with lower staining observed in old rats (Figure 3C). There were no main effects of training on CREB staining (F(3,27) = 0.24).
There was a main effect of training (F(3,27) = 4.76, p < .01) but not age (F(1,27) = 1.38) on pCREB:CREB ratios (Figure 3D). In young rats, training with a 0.2 mA shock increased pCREB:CREB ratios above those of cage control rats (p < .03). Training with a 0.5 mA shock increased pCREB:CREB ratios compared to both cage control and no shock groups (ps < .03). In old rats, training did not significantly alter pCREB:CREB ratios, regardless of the foot shock intensity.
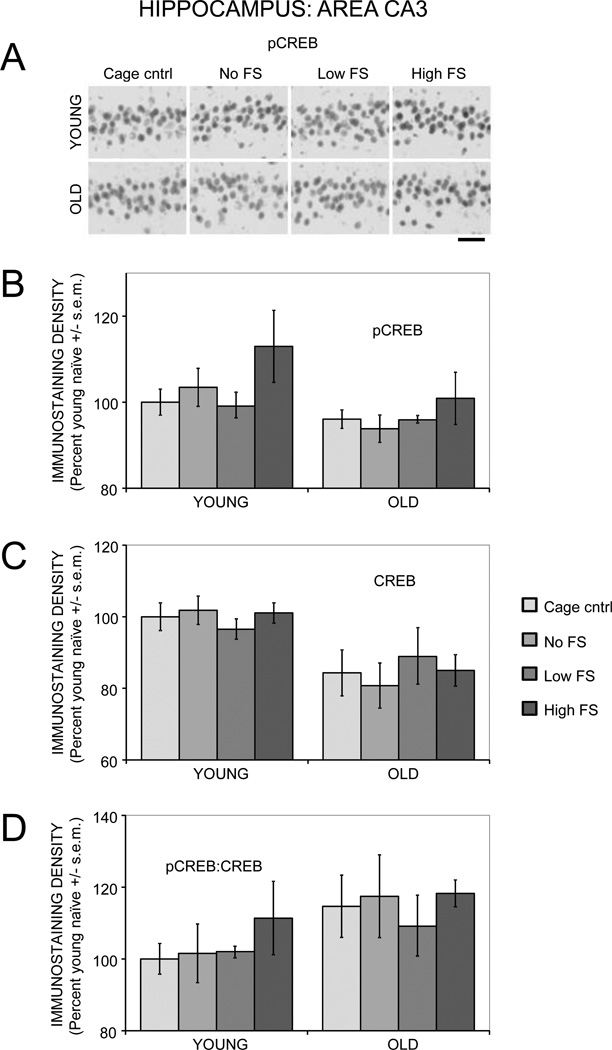
3.2.2. Hippocampus: Area CA3
There was a main effect of age (F(1,27) = 6.45, p < .03) but not training (F(3,27) = 2.40) on pCREB immunostaining in area CA3 (Figure 4A and 4B). However, post-hoc analyses revealed no significant differences between groups. There was also a main effect of age (F(1,27) = 22.74, p < .0001) but not training (F(3,27) = 0.06) on CREB staining (Figure 4C). Post-hoc analyses revealed significantly lower CREB staining in old compared to young rats in the cage control, 0.2 mA shock, and 0.5 mA shock groups (ps < .05), but not in the no shock group. There was a main effect of age (F(1,27) = 4.87, p < .05) on pCREB:CREB ratios (Figure 4D), suggesting higher ratios in old rats. There were no training effects on pCREB:CREB ratios (F(3,27) = 0.78).
Figure 4.
Age- and training-associated differences in pCREB and CREB immunoreactivity in area CA3 of the hippocampus. (A) Representative photomicrographs of pCREB immunostaining. Scale bar = 50 µm. (B) pCREB levels were similar between young and old rats and not significantly altered by training. (C) There were significantly lower CREB levels in old compared to young rats. (D) There were no significant age- or training-related differences in pCREB:CREB ratios.
3.2.3. Hippocampus: Area CA1
In area CA1, the effects on pCREB, CREB, and pCREB:CREB ratios were very similar to those seen in the dentate gyrus, and are therefore not presented in detail here.
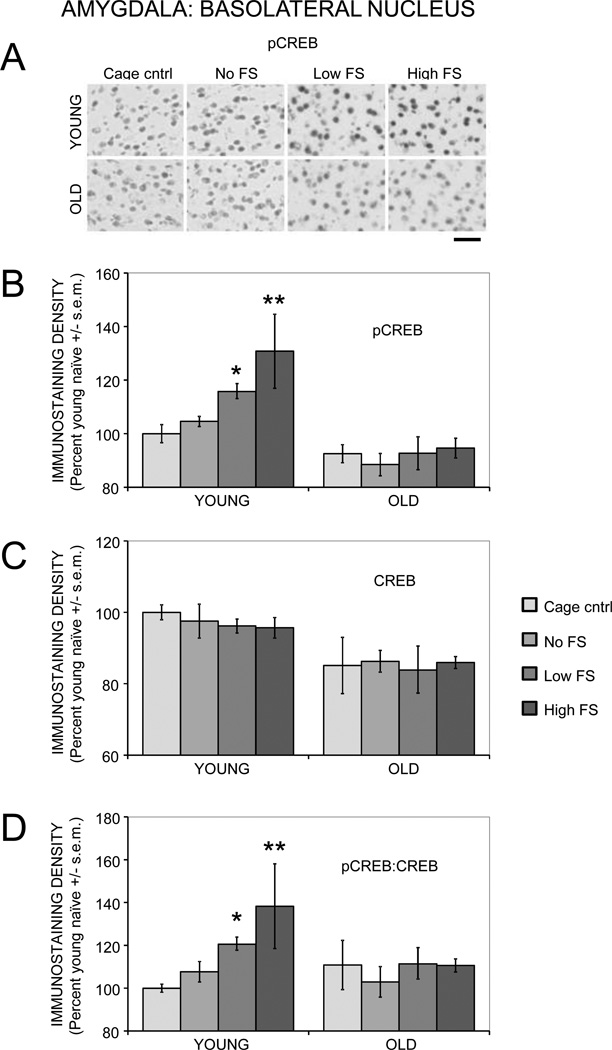
3.2.4. Basolateral Amygdala
Both training (F(3,27) = 5.19, p < .01) and age (F(1,27) = 36.89, p < .0001) significantly affected pCREB immunostaining in the basolateral amygdala (Figure 5A and 5B). In young rats, training with a 0.2 mA foot shock increased pCREB staining compared to cage controls (p < .05). Training with a 0.5 mA foot shock increased pCREB staining compared to both cage and no shock controls (ps < .01). In old rats, training did not significantly alter pCREB staining, regardless of the shock intensity. Compared to cage controls, age-related deficits in pCREB activation were evident in the no shock, 0.2 mA shock, and 0.5 mA shock groups (ps < .05). There was also a significant age by training interaction (F(3,27) = 3.29, p < .05).
Figure 5.
Age- and training-associated differences in pCREB and CREB immunoreactivity in the basolateral amygdala. (A) Representative photomicrographs of pCREB immunostaining. Scale bar = 50 µm. (B) Young rats had significantly enhanced pCREB levels following training with a low or moderate intensity foot shock, whereas old rats had training-related deficits in pCREB activation. (*) p < .05 vs. young cage controls. (**) ps < .01 vs. young cage controls and no shock. (C) Total CREB levels were significantly lower in old compared to young rats. (D) Young rats had significantly higher ratios of pCREB:CREB after training with a low or moderate intensity foot shock, whereas old rats had training-related deficits in pCREB:CREB ratios. (*) p < .05 vs. young cage controls. (**) ps < .05 vs. young cage controls and no shock.
There was a main effect of age (F(1,27) = 21.39, p < .0001) but not training (F(3,27) = 0.20) on CREB immunostaining (Figure 5C). Post-hoc analyses revealed significantly lower CREB staining in old rats in the cage control and 0.5 mA shock groups (ps < .05).
There was a main effect of training (F(3,27) = 3.39, p < .05) but not age (F(1,27) = 2.36) on pCREB:CREB ratios (Figure 5D). In young rats, the 0.2 mA shock group had significantly higher ratios compared to cage control rats (p < .05). The 0.5 mA shock group had significantly higher ratios compared to both the cage control and no shock groups (ps < .05). In old rats, training did not have a significant effect on pCREB:CREB ratios.
3.2.5. Lateral Amygdala
In the lateral amygdala, the effects on pCREB, CREB, and pCREB:CREB ratios were similar those seen in the basolateral amygdala, with the major exception being lack of a training effect on pCREB:CREB ratios in the lateral amygdala (see Table 1). Therefore, these results are not presented in detail here.
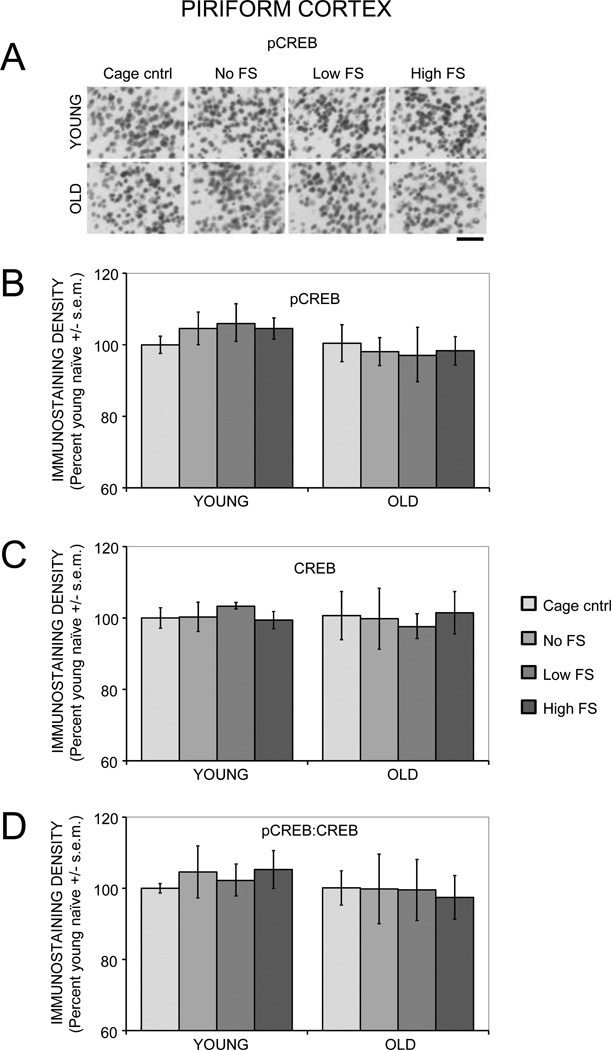
3.2.6. Piriform cortex
There were no main effects of age (Fs(1,27) < 3.67) or training (Fs(3,27) < 0.07) on pCREB, CREB, or pCREB:CREB ratios in the piriform cortex (Figure 6).
Figure 6.
Age- and training-associated differences in pCREB and CREB immunoreactivity in the piriform cortex. (A) Representative photomicrographs of pCREB immunostaining. Scale bar = 50 µm. There were no age- or training-related differences in pCREB (B), CREB (C), or ratio of pCREB:CREB (D).
4. DISCUSSION
4.1. Age-Related Differences in Memory
Training with a low intensity foot shock produced stable 7-day memory in young rats, but led to poor 7-day memory in old rats. These results support prior work demonstrating rapid age-related forgetting following inhibitory avoidance training (Gold et al., 1981; Morris et al., 2010). In particular, Gold et al. (1981) trained rats with a low intensity foot shock and training-testing intervals ranging from 2 hours to six weeks. Young rats had stable memory for at least 3 weeks after training. Old rats had comparable or even better memory than young rats 2 hours after training. However, the old rats exhibited slight deficits after 1 day, and were significantly impaired after 7 days. Thus, old rats exhibited rapid forgetting for inhibitory avoidance, with memory intact for hours after training, but with memory impairments emerging during the days after training. Importantly, this operational definition of forgetting may reflect memories with variable time courses depending on the salience of the initiating events.
In contrast to the results with the low foot shock, training with a moderate intensity foot shock resulted in stable 7-day memory in both young and old rats. These results are similar to findings by Gold et al. (1981), and indicate that forgetting rates in old rats can be slowed by increasing the saliency of the training foot shock, perhaps with concomitant increases in neural modulators of memory associated with the higher training-related arousal levels. Together, the behavioral results presented here provide a good model with which to examine changes in CREB expression and activation that might contribute to rapid forgetting in aged rats.
4.2. Age-Related Differences in CREB and pCREB Expression at Baseline
At baseline, CREB levels were lower in aged vs. young adult rats in area CA3 and in the basolateral and lateral amygdala. Comparable changes were not evident in the dentate gyrus, area CA1, or piriform cortex, although the latter brain region had high CREB expression levels as seen previously (Ferrer et al., 1996; Herdegen et al., 1993). The age-related reductions in hippocampal CREB observed here are consistent with past findings revealing decreased expression of hippocampal CREB in old Fischer-344 rats (Countryman and Gold, 2007). Other studies have shown age-related reductions in CREB levels in whole hippocampal homogenates from Long Evans (Brightwell et al., 2004) and Wistar rats (Trofimiuk et al., 2010). The age-related decreases in CREB expression in the amygdala have not, to our knowledge, been noted before. The decreased expression of CREB in the hippocampus and amygdala with age, as well as the decreased training-related activation of CREB described below, may contribute to age-related memory impairments. The memory impairments are analogous to those seen in CREB knockout mice and in rats and mice treated with CREB antisense oligonucleotides, RNA interference, or dominant negative mutations, any of which impairs the formation of long-lasting memory and reduces the durability of long-term potentiation (Bourtchuladze et al., 1994; Canal et al., 2008; Florian et al., 2006; Guzowski and McGaugh, 1997; Josselyn, 2010; Peters et al., 2009; Pittenger et al., 2002; Won and Silva, 2008).
Importantly, age-related changes in CREB, as well as changes in expression of pCREB described below, do not likely reflect differences in cell numbers or cell morphology between young and old rats. Studies utilizing stereological methods for quantification have found that neuron numbers are preserved in the hippocampus of old rats (Gallagher et al., 2003; Rapp and Gallagher, 1996; Rasmussen et al., 1996). Likewise, another study reported no significant volume or cell loss in the hippocampus or amygdala of aged mice (von Bohlen und Halbach and Unsicker, 2002).
The age-related decreases in CREB levels in several brain regions suggest that some estimates of age-related changes in behaviorally elicited increases in pCREB levels may reflect the loss of CREB per se. Several recent studies of changes during aging in pCREB levels in the hippocampus have measured only pCREB or have used total CREB levels to normalize for changes in pCREB (e.g. Assunção et al., 2010; Hattiangady et al., 2005; Li et al., 2009; Trofimiuk et al., 2010; Xing et al., 2010; Xu et al., 2010; Zhao et al., 2009). The present results suggest that quantifying total CREB levels is important to interpret correctly age- or activity-dependent changes in pCREB in old rats. Further, using CREB levels for normalization in aging studies may make it difficult to compare directly results across age groups, since total CREB levels may decline with age in a brain area-specific manner.
4.3. Age-Related Differences in pCREB Expression in Response to Training
CREB phosphorylation in the piriform cortex has been shown to be responsive to several stimuli (Alvarez-López et al., 2004; Dere et al., 2008; Estrada and Isokawa, 2009; Kim et al., 2006; Pandey et al., 2001a, b; Wang et al., 2007). However, this is the first study, to our knowledge, to examine CREB activation in the piriform cortex after inhibitory avoidance training, revealing no evidence for pCREB increases after training in either young or aged rats. The results contrast with those showing c-Fos expression and ERK activation in piriform cortex induced by active avoidance training in young adult rats (Radwanska et al., 2010).
A striking result was that there were substantial age-related differences in activation of CREB in response to training in the hippocampus and amygdala. In young adult rats, pCREB expression in the dentate gyrus, area CA1, and the lateral and basolateral amygdala increased 30 min after training with a low foot shock as compared to expression levels in young cage controls. Training with a moderate intensity foot shock further enhanced pCREB levels in the same brain regions. In parallel groups of young rats, both shock intensities produced high avoidance latencies in memory tests 7 days after training. These results are consistent with the view that, in young adult rats, CREB activation may be an important component or marker of long-term memory processes occurring after training. The results obtained in hippocampal regions agree with a number of other studies showing increased hippocampal CREB phosphorylation following inhibitory avoidance training in young adult rats, which can be abolished by treatments that interfere with memory processes (Cammarota et al., 2000; Taubenfeld et al., 2001, 1999; Viola et al., 2000). The amygdala results also agree with several studies demonstrating enhanced CREB phosphorylation in the basolateral and/or lateral amygdala following fearful or stressful stimuli (Bilang-Bleuel et al., 2002; Hubbard et al., 2007; Ilin and Richter-Levin, 2009; Kogan and Richter-Levin, 2008; Lin et al., 2003; Saha and Datta, 2005; Shen et al., 2004; Stanciu et al., 2001).
In marked contrast to the results obtained with young adult rats, aged rats did not show significant increases in pCREB expression in any brain region after training with either the low or moderate shock intensity. In old rats, pCREB levels in the dentate gyrus, area CA1, and lateral and basolateral amygdala, i.e. those areas responsive to training in young rats, were similar to those of aged cage controls and were generally significantly lower than those in corresponding groups and samples of young rats. Viewed across all brain areas, age-related decreases in total CREB cannot entirely account for these impairments in CREB phosphorylation, since basal CREB levels in area CA1 and the dentate gyrus were similar between young and old rats. Rather, the findings suggest that age-related impairments upstream to CREB activation may be an important contributor to age-related impairments in memory. This interpretation is consistent with recent evidence that overexpression of CREB in the hippocampus improves memory in young but not middle-aged rats (East et al., 2011), perhaps due to an inability of middle aged rats to engage upstream activators of CREB phosphorylation when needed.
The present results are the first to identify substantial age-related impairments in CREB activation in the amygdala and hippocampus after inhibitory avoidance training. The failure to activate CREB in the amygdala may have significant consequences to modulation of memory in other neural systems, such as the hippocampus, and may contribute to the decreased pCREB after training in the hippocampus. Consistent with this possibility, intra-amygdala injections of β-adrenergic agonists and antagonists, drugs that enhance and impair memory respectively, increase and decrease expression of activity-regulated cytoskeletal protein (Arc) in the hippocampus in conjunction with modulation of memory (McIntyre et al., 2005). These findings suggest that age-related deficiencies in amygdala modulation of hippocampal functions may be important in mediating the rapid forgetting and rapid decay of neural plasticity seen in aged rodents. The present results also agree with a number of studies showing age-related impairments in CREB activation in a variety of hippocampal-dependent tasks (Countryman and Gold, 2007; Kudo et al., 2005; Monti et al., 2005; Porte et al., 2008; Xu et al., 2010). Recent studies also indicate that long-term administration of certain compounds, including procyandins (Xu et al., 2010), green tea (Assunção et al., 2010; Li et al., 2009), and St. John’s wort (Trofimiuk et al., 2010), can reverse age-related deficits in CREB activation in concert with memory enhancement. Thus, these findings are consistent with the view that a failure to activate CREB after training contributes to age-related memory impairments.
The present experiments included a condition, moderate intensity foot shock used during training, which tested further the importance of CREB activation for long-lasting memory. On the basis of past findings (Gold et al., 1981), we expected the moderate shock condition to result in longer maintenance of memory for inhibitory avoidance training, a result confirmed here. We further expected that the moderate training shock would also result in greater CREB activation that would parallel the better memory scores. This result was clearly not evident: Raising the foot shock intensity reversed age-related impairments in 7-day memory but did not increase CREB phosphorylation in the hippocampus or amygdala of old rats, as it did in young rats. Thus, CREB activation was dissociated from memory formation in old rats trained with the moderate foot shock intensity.
There are several possible explanations for the apparent dissociation between CREB activation and memory formation. In the present experiment, measurements of CREB activation were limited to a single time point, 30 min after training. Although some studies suggest there is rapid and long-lasting CREB activation following training (e.g. Taubenfeld et al., 2001, 1999), others have observed specific and even delayed time windows of CREB phosphorylation after training (Bernabeu et al., 1997; O’Connell et al., 2000). Therefore, an earlier or later time point might reveal training-related increases in pCREB levels that would be associated with the stable memory observed here after training with the moderate shock intensity. It is also possible that the quantitative histochemistry methods used here were not sufficiently sensitive to reveal increases after the moderate shock intensity. Given the greater increase in pCREB expression levels seen in young rats after the moderate shock intensity, this seems unlikely, though the possibility cannot be fully discounted. Although pCREB activation in young rats appeared more or less uniform across large numbers of cells, such widespread activation may not be necessary for memory formation. For instance, Han et al. (2009, 2007) found that altering CREB function in small numbers of sparsely distributed cells in the lateral amygdala can affect formation of auditory fear memory (Han et al., 2009, 2007; Zhou et al., 2009). Sparse distribution of critical neurons expressing CREB activation would likely be missed using the immunohistochemistry methods employed here. Utilization of a cell counting strategy or of more quantitative techniques, such as ELISAs or western blots, may be helpful in providing corroborative molecular evidence of the optical density measurements presented here.
Another possibility is that the dissociation between CREB and memory may indicate that CREB activation is not required for memory formation in old rats. This view is consistent with a number of studies showing stable formation of memory and long-term potentiation in CREB knockout and mutant mice (Balschun et al., 2003; Gass et al., 1998; Rammes et al., 2000). Although several studies do indicate deficits in long-term memory in CREB mutant mice (Bourtchuladze et al., 1994; Sekeres et al., 2010), these deficits can often be reversed by altering training procedures or by administering memory-enhancing agents (Frankland et al., 2004; Kogan et al., 1997; Canal et al., 2008). Further, the idea that CREB activation is not necessary for the formation of stable memories after the moderate shock is consistent with the possibility that the moderate shock level recruits additional molecular components of memory formation compared to those seen with the lower shock. In young rats, the behavioral measure of memory is maximal at both shock intensities, thereby obscuring the utility of additional molecular mechanisms. However, in aged rats, the stable memory after training with the moderate shock, coupled to the absence of CREB activation, may reflect the importance of these additional molecular mechanisms. In this regard, the study of stable memory in aged rats may be a condition favorable to investigation of these additional factors.
Highlights.
Old rats exhibit memory impairments following inhibitory avoidance training.
Memory impairments are evident following a low but not moderate foot shock.
Old rats have deficits in total CREB and in pCREB activation following training.
pCREB deficits in old rats are present regardless of foot shock intensity.
Acknowledgments
Supported by an NIH NRSA F30AG034803 (KAM), by NIH grants AG07648 and DA024129, NSF grants IOS-08-43175 and IOS 10-52464, and by an award from the Alzheimer’s Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-López C, Cernuda-Cernuda R, Paniagua MA, Alvarez-Viejo M, Fernández-López A, García-Fernández JM. The transcription factor CREB is phosphorylated in neurons of the piriform cortex of blind mice in response to illumination of the retina. Neurosci. Lett. 2004;357:223–226. doi: 10.1016/j.neulet.2003.12.099. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyère V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J. Neurosci. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção M, Santos-Marques MJ, Carvalho F, Andrade JP. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free Radic. Biol. Med. 2010;48:831–838. doi: 10.1016/j.freeradbiomed.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schütz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory changes with age: neurobiological correlates. In: Martinez JL, Kesner RP, editors. Learning and Memory: A Biological View. New York: Academic Press; 1991. pp. 259–296. [Google Scholar]
- Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav. Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Rech J, De Carli S, Holsboer F, Reul JM. Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures in the rat. Eur J. Neurosci. 2002;15:1048–1060. doi: 10.1046/j.1460-9568.2002.01934.x. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Gallagher M, Colombo PJ. Hippocampal CREB1 but not CREB2 is decreased in aged rats with spatial memory impairments. Neurobiol. Learn. Mem. 2004;81:19–26. doi: 10.1016/j.nlm.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Smith CA, Countryman RA, Neve RL, Colombo PJ. Hippocampal overexpression of mutant creb blocks long-term, but not short-term memory for a socially transmitted food preference. Learn. Mem. 2005;12:12–17. doi: 10.1101/lm.85005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightwell JJ, Smith CA, Neve RL, Colombo PJ. Transfection of mutant CREB in the striatum, but not the hippocampus, impairs long-term memory for response learning. Neurobiol. Learn. Mem. 2008;89:27–35. doi: 10.1016/j.nlm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Yang Z, Navratilova Z, Barnes CA. Glutamate receptor-mediated restoration of experience-dependent place field expansion plasticity in aged rats. Behav. Neurosci. 2008;122:535–548. doi: 10.1037/0735-7044.122.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, Medina JH. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: abolition by NMDA receptor blockade. Brain Res. Mol. Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- Canal CE, Chang Q, Gold PE. Intra-amygdala injections of CREB antisense impair inhibitory avoidance memory: role of norepinephrine and acetylcholine. Learn. Mem. 2008;15:677–686. doi: 10.1101/lm.904308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Brightwell JJ, Countryman RA. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J. Neurosci. 2003;23:3547–3554. doi: 10.1523/JNEUROSCI.23-08-03547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sonenberg N. Translational control of gene expression: a molecular switch for memory storage. Prog. Brain Res. 2008;169:81–95. doi: 10.1016/S0079-6123(07)00005-2. [DOI] [PubMed] [Google Scholar]
- Countryman RA, Gold PE. Rapid forgetting of social transmission of food preferences in aged rats: relationship to hippocampal CREB activation. Learn. Mem. 2007;14:350–358. doi: 10.1101/lm.524907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva Costa-Aze V, Dauphin F, Boulouard M. Serotonin 5-HT6 receptor blockade reverses the age-related deficits of recognition memory and working memory in mice. Behav. Brain Res. 2011;222:134–140. doi: 10.1016/j.bbr.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Dere E, Zheng-Fischhöfer Q, Viggiano D, Gironi Carnevale UA, Ruocco LA, Zlomuzica A, Schnichels M, Willecke K, Huston JP, Sadile AG. Connexin31.1 deficiency in the mouse impairs object memory and modulates open-field exploration, acetylcholine esterase levels in the striatum, and cAMP response element-binding protein levels in the striatum and piriform cortex. Neuroscience. 2008;153:396–405. doi: 10.1016/j.neuroscience.2008.01.077. [DOI] [PubMed] [Google Scholar]
- East BS, Kathirvelu B, Kochli DE, Colombo PJ. Wasted on the young: overexpression of CREB in the hippocampus improves spatial memory in young, but not middle-aged, rats. Soc. Neurosci. 2011 Abst. 81.17. [Google Scholar]
- Estrada NM, Isokawa M. Metabolic Demand Stimulates CREB Signaling in the Limbic Cortex: Implication for the Induction of Hippocampal Synaptic Plasticity by Intrinsic Stimulus for Survival. Front. Syst. Neurosci. 2009;3:5. doi: 10.3389/neuro.06.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Rivera R, Carmona M, Ballabriga J, Olivé M, Planas AM. CREB-1 and CREB-2 immunoreactivity in the rat brain. Brain Res. 1996;712:159–164. doi: 10.1016/0006-8993(95)01527-2. [DOI] [PubMed] [Google Scholar]
- Florian C, Mons N, Roullet P. CREB antisense oligodeoxynucleotide administration into the dorsal hippocampal CA3 region impairs long- but not short-term spatial memory in mice. Learn. Mem. 2006;13:465–472. doi: 10.1101/lm.249306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res. Brain Res. Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol. Learn. Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA, Anagnostaras SG, Kogan JH, Takahashi E, Silva AJ. Consolidation of CS and US representations in associative fear conditioning. Hippocampus. 2004;14:557–569. doi: 10.1002/hipo.10208. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Lephart ED, Walf AA. 3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front. Aging Neurosci. 2010;2:15. doi: 10.3389/fnagi.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Björklund A. Spatial learning and motor deficits in aged rats. Neurobiol. Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp. Gerontol. 2003;38:71–77. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp HP, Schütz G. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn. Mem. 1998;5:274–288. [PMC free article] [PubMed] [Google Scholar]
- Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory--a molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Gold PE. Drug enhancement of memory in aged rodents and humans. In: Carroll ME, Overmier JB, editors. Animal Research and Human Health: Advancing Human Welfare through Behavioral Science. Washington, DC: American Psychological Association; 2001. pp. 293–304. [Google Scholar]
- Gold PE. Glucose and age-related changes in memory. Neurobiol. Aging. 2005;26S:S60–S64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gold PE, McGaugh JL, Hankins LL, Rose RP, Vasquez BJ. Age-dependent changes in retention in rats. Exp. Aging Res. 1981;8:53–58. [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, Neve RL, Guzowski JF, Silva AJ, Josselyn SA. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- Han JH, Kushner SA, Yiu AP, Hsiang HL, Buch T, Waisman A, Bontempi B, Neve RL, Frankland PW, Josselyn SA. Selective erasure of a fear memory. Science. 2009;323:1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Sandkühler J, Gass P, Kiessling M, Bravo R, Zimmermann M. JUN, FOS, KROX, and CREB transcription factor proteins in the rat cortex: basal expression and induction by spreading depression and epileptic seizures. J. Comp. Neurol. 1993;333:271–288. doi: 10.1002/cne.903330212. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Berlin R. Rapid, labile, and protein synthesis-independent short-term memory in conditioned taste aversion. Learn. Mem. 1999;6:37–46. [PMC free article] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilin Y, Richter-Levin G. ERK2 and CREB activation in the amygdala when an event is remembered as "Fearful" and not when it is remembered as "Instructive". J. Neurosci. Res. 2009;87:1823–1831. doi: 10.1002/jnr.21994. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Josselyn SA. Continuing the search for the engram: examining the mechanism of fear memories. J. Psychiatry Neurosci. 2010;35:221–228. doi: 10.1503/jpn.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Kida S, Silva AJ. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol. Learn. Mem. 2004;82:159–163. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci. Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Kim HH, Puche AC, Margolis FL. Odorant deprivation reversibly modulates transsynaptic changes in the NR2B-mediated CREB pathway in mouse piriform cortex. J. Neurosci. 2006;26:9548–9559. doi: 10.1523/JNEUROSCI.1727-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Current Biology. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Kogan I, Richter-Levin G. Activation pattern of the limbic system following spatial learning under stress. Eur. J. Neurosci. 2008;27:715–722. doi: 10.1111/j.1460-9568.2008.06034.x. [DOI] [PubMed] [Google Scholar]
- Korol DL. Enhancing cognitive function across the life span. Ann. N.Y. Acad. Sci. 2002;959:167–179. doi: 10.1111/j.1749-6632.2002.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Kudo K, Wati H, Qiao C, Arita J, Kanba S. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res. 2005;1054:30–37. doi: 10.1016/j.brainres.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Cai MY, Li Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J. Neurosci. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund PK, Hoyt EC, Bizon J, Smith DR, Haberman R, Helm K, Gallagher M. Transcriptional mechanisms of hippocampal aging. Exp. Gerontol. 2004;39:1613–1622. doi: 10.1016/j.exger.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Mabry TR, McCarty R, Gold PE, Foster TC. Age and stress history effects on spatial performance in a swim task in Fischer-344 rats. Neurobiol. Learn. Mem. 1996;66:1–10. doi: 10.1006/nlme.1996.0038. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc. Natl. Acad. Sci. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus. 2005;15:1041–1049. doi: 10.1002/hipo.20099. [DOI] [PubMed] [Google Scholar]
- Morris KA, Chang Q, Mohler EG, Gold PE. Age-related memory impairments due to reduced blood glucose responses to epinephrine. Neurobiol. Aging. 2010;31:2136–2145. doi: 10.1016/j.neurobiolaging.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouravlev A, Dunning J, Young D, During MJ. Somatic gene transfer of cAMP response element-binding protein attenuates memory impairment in aging rats. Proc. Natl. Acad. Sci. 2006;103:4705–4710. doi: 10.1073/pnas.0506137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell C, Gallagher HC, O'Malley A, Bourke M, Regan CM. CREB phosphorylation coincides with transient synapse formation in the rat hippocampal dentate gyrus following avoidance learning. Neural Plast. 2000;7:279–289. doi: 10.1155/NP.2000.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the creb gene transcription factor in rat cortex. J. Pharmacol. Exp. Ther. 2001a;296:857–868. [PubMed] [Google Scholar]
- Pandey SC, Roy A, Xu T, Mittal N. Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. J. Neurochem. 2001b;77:943–952. doi: 10.1046/j.1471-4159.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier Academic Press; 2003. [Google Scholar]
- Peters M, Bletsch M, Catapano R, Zhang X, Tully T, Bourtchouladze R. RNA interference in hippocampus demonstrates opposing roles for CREB and PP1alpha in contextual and temporal long-term memory. Genes Brain Behav. 2009;8:320–329. doi: 10.1111/j.1601-183X.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Porte Y, Buhot MC, Mons N. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol. Aging. 2008;29:1533–1546. doi: 10.1016/j.neurobiolaging.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Radwanska K, Nikolaev E, Kaczmarek L. Central noradrenergic lesion induced by DSP-4 impairs the acquisition of avoidance reactions and prevents molecular changes in the amygdala. Neurobiol. Learn. Mem. 2010;94:303–311. doi: 10.1016/j.nlm.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Rammes G, Steckler T, Kresse A, Schütz G, Zieglgänsberger W, Lutz B. Synaptic plasticity in the basolateral amygdala in transgenic mice expressing dominant-negative cAMP response element-binding protein (CREB) in forebrain. Eur. J. Neurosci. 2000;12:2534–2546. doi: 10.1046/j.1460-9568.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav. Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sørensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Roman FS, Alescio-Lautier B, Soumireu-Mourat B. Age-related learning and memory deficits in odor-reward association in rats. Neurobiol. Aging. 1996;17:31–40. doi: 10.1016/0197-4580(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Saha S, Datta S. Two-way active avoidance training-specific increases in phosphorylated cAMP response element-binding protein in the dorsal hippocampus, amygdala, and hypothalamus. Eur. J. Neurosci. 2005;21:3403–3414. doi: 10.1111/j.1460-9568.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- Salinas JA, Gold PE. Glucose regulation of memory for reward reduction in young and aged rats. Neurobiol. Aging. 2005;26:45–52. doi: 10.1016/j.neurobiolaging.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Sekeres MJ, Neve RL, Frankland PW, Josselyn SA. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn. Mem. 2010;17:280–283. doi: 10.1101/lm.1785510. [DOI] [PubMed] [Google Scholar]
- Shen CP, Tsimberg Y, Salvadore C, Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Barth CL, Wood MS, Velazquez E, Groccia-Ellison M, Yang BY. Age-related deficits in retention of the classically conditioned nictitating membrane response in rabbits. Behav. Neurosci. 1995;109:18–23. doi: 10.1037//0735-7044.109.1.18. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res. Mol. Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Stone WS, Rudd RJ, Parsons MW, Gold PE. Memory scores in middle-aged rats predict later deficits in memory, paradoxical sleep, and blood glucose regulation in old age. Exp. Aging Res. 1997;23:287–300. doi: 10.1080/03610739708254285. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Bear MF, Alberini CM. A molecular correlate of memory and amnesia in the hippocampus. Nat. Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein [beta] and [delta] Co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J. Neurosci. 2001;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn. Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimiuk E, Holownia A, Braszko JJ. Activation of CREB by St. John's wort may diminish deletorious effects of aging on spatial memory. Arch. Pharm. Res. 2010;33:469–477. doi: 10.1007/s12272-010-0318-y. [DOI] [PubMed] [Google Scholar]
- Viola H, Furman M, Izquierdo LA, Alonso M, Barros DM, de Souza MM, Izquierdo I, Medina JH. Phosphorylated cAMP response element-binding protein as a molecular marker of memory processing in rat hippocampus: effect of novelty. J. Neurosci. 2000;20:RC112. doi: 10.1523/JNEUROSCI.20-23-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bohlen und Halbach O, Unsicker K. Morphological alterations in the amygdala and hippocampus of mice during ageing. Eur. J. Neurosci. 2002;16:2434–2340. doi: 10.1046/j.1460-9568.2002.02405.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav. Brain Res. 2007;178:262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Winocur G. A neuropsychological analysis of memory loss with age. Neurobiol Aging. 1988;9:487–494. doi: 10.1016/s0197-4580(88)80102-7. [DOI] [PubMed] [Google Scholar]
- Won J, Silva AJ. Molecular and cellular mechanisms of memory allocation in neuronetworks. Neurobiol. Learn. Mem. 2008;89:285–292. doi: 10.1016/j.nlm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Seta SE, Roker LA, Lehr MA. Effects of paradigm and inter-stimulus interval on age differences in eyeblink classical conditioning in rabbits. Learn. Mem. 2007;14:287–294. doi: 10.1101/lm.504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Meng X, Wei S, Li S. Influence of dopamine D3 receptor knockout on age-related decline of spatial memory. Neurosci. Lett. 2010;481:149–153. doi: 10.1016/j.neulet.2010.06.071. [DOI] [PubMed] [Google Scholar]
- Xu J, Rong S, Xie B, Sun Z, Deng Q, Wu H, Bao W, Wang D, Yao P, Huang F, Liu L. Memory Impairment in Cognitively Impaired Aged Rats Associated With Decreased Hippocampal CREB Phosphorylation: Reversal by Procyanidins Extracted From the Lotus Seedpod. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:933–940. doi: 10.1093/gerona/glq094. [DOI] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr. Opin. Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li Q, Pei X, Zhang Z, Yang R, Wang J, Li Y. Long-term ginsenoside administration prevents memory impairment in aged C57BL/6J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav. Brain Res. 2009;201:311–317. doi: 10.1016/j.bbr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 2009;12:1438–1443. doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornetzer SF, Thompson R, Rogers J. Rapid forgetting in aged rats. Behav. Neural Biol. 1982;36:49–60. doi: 10.1016/s0163-1047(82)90234-5. [DOI] [PubMed] [Google Scholar]