Abstract
Objectives
We sought to identify factors associated with death and cardiac transplantation in infants undergoing the Norwood procedure and to determine differences in associations that might favor either the modified Blalock-Taussig shunt (MBTS) or a right ventricle-to-pulmonary artery shunt (RVPAS).
Methods
We used competing risks methodology to analyze death without transplantation, cardiac transplantation, and survival without transplantation. Parametric time-to-event modeling and bootstrapping were used to identify independent predictors.
Results
Data from 549 subjects (follow-up, 2.7±0.9 years) were analyzed. Mortality risk was characterized by early and constant phases; transplant was characterized by only a constant phase. Early phase factors associated with death included lower socioeconomic status (SES; P=.01), obstructed pulmonary venous return (P<.001), smaller ascending aorta (P=.02), and anatomic subtype. Constant phase factors associated with death included genetic syndrome (P<.001) and lower gestational age (GA, P<.001). The RVPAS had better survival in the 51% who were full term with aortic atresia (P<.001). The MBTS was better among the 4% who were preterm with a patent aortic valve (P =.003). Lower pre-Norwood right ventricular fractional area change, pre-Norwood surgery, and anatomy other than hypoplastic left heart syndrome were independently associated with transplantation (all P<.03); but shunt type was not (P=.43).
Conclusions
Independent risk factors for intermediate-term mortality include lower SES, anatomy, genetic syndrome, and lower GA. Term infants with aortic atresia benefited from a RVPAS and preterm infants with a patent aortic valve benefited from a MBTS. Right ventricular function and anatomy, but not shunt type, were associated with transplantation.
INTRODUCTION
The risk of mortality following the Norwood procedure for hypoplastic left heart syndrome (HLHS) and other single right ventricle anomalies with arch obstruction remains significant. Risk factors for death and for transplantation following the Norwood procedure may be thought of as occurring in two categories: factors intrinsic to the patient that are traditionally considered non-modifiable such as anatomic subtype, weight, gestational age, and pulmonary venous obstruction, and modifiable factors that may be subject to practice variation, such as perioperative management, timing of surgery, technique and perfusion strategies. The Pediatric Heart Network (PHN) Single Ventricle Reconstruction (SVR) trial prospectively collected preoperative, operative and postoperative multi-institutional data from over 500 newborns with hypoplastic left heart syndrome and related single right ventricle anomalies. This trial demonstrated that a Norwood procedure with a right ventricle-to-pulmonary artery shunt (RVPAS), compared with a modified Blalock Taussig shunt (MBTS), was associated with better transplantfree survival 12 months after randomization. (1) Using all available follow-up (2.7±0.9 years in event-free subjects), we analyzed the PHN SVR dataset to achieve the following goals: to estimate the cumulative incidence of death and cardiac transplantation in subjects who have had the Norwood procedure, to determine the association of traditionally non-modifiable factors with outcomes using parametric regression modeling, and to determine the interaction of shunt type with these factors. We additionally sought to use this information to develop an algorithm to tailor shunt type to the patient profile.
METHODS
Study Design
The SVR trial has been described in detail in earlier reports. (1, 2) Enrollment occurred between May 2005 and July 2008 at 15 centers in the United States and Canada. Briefly, subjects with single right ventricle anomalies with systemic outflow obstruction were randomly assigned to receive either a MBTS or a RVPAS during the Norwood procedure. Randomization was stratified by the presence vs. absence of aortic atresia and obstructed pulmonary venous return (OPVR), with dynamic balancing within surgeon. The primary trial endpoint was death or cardiac transplantation at one year post-randomization. In addition, an end-of-trial contact was completed in summer 2009 to obtain maximal follow-up on all subjects. A key secondary endpoint was time to death or cardiac transplantation at the time of last follow-up, and is the focus of this report.
The trial protocol was approved by the institutional review board or ethics committee of all institutions, and a parent or legal guardian of each subject provided written informed consent.
Patient Sample
There were 555 neonates randomized in the trial. The analysis cohort included 549 subjects. We excluded 5 subjects who did not undergo a Norwood procedure and one subject who withdrew from the trial in the first week after randomization, and for whom no additional data were collected.
Data Collection
This report is based on an analysis of early and non-modifiable patient factors. Detailed information on birth characteristics, patient demographics, and pre-Norwood procedure medical history was collected on standardized forms. Socioeconomic status (SES) was assigned using a U.S.-census-based score derived from 6 measures related to income, housing, and occupational-related features of the subject’s census block tract (3) at the time of randomization. Cause of death was assigned by a five-member panel that arrived at consensus decision on each event after independent review by each member. (4) The panel consisted of the SVR Study Chair, a pediatric cardiologist, a pediatric cardiac intensivist and two pediatric cardiac surgeons.
Two-dimensional and Doppler echocardiographic data from a standardized echocardiogram obtained prior to the Norwood procedure were used in this analysis. All echocardiograms were interpreted centrally at a core laboratory (Medical College of Wisconsin). Variables used in this report include right ventricular fractional area change, presence vs. absence of antegrade flow in the aorta (patent vs. atretic aortic valve), and no/mild vs. moderate/severe valve regurgitation (aortic, mitral, tricuspid).
Definitions
The type of shunt used in the Norwood procedure (MBTS or RVPAS) was defined as the shunt in place at the end of the Norwood procedure, which differed from the randomly assigned shunt for 48 (9%) of subjects. Preterm was defined as gestational age less than 37 weeks. OPVR was defined as the use of postnatal intervention directed at the atrial septum, including balloon septostomy, open atrial septectomy, or urgent Norwood procedure. (2)
Statistical Methods
The primary endpoint of the SVR trial was a composite of the earliest occurrence of death or transplant. However, we hypothesized that the hazard functions for death and transplant might differ, as well as the factors associated with those endpoints. Therefore, outcomes at the time of last follow-up were examined using a competing risks framework. In addition, we hypothesized that the factors associated with these outcomes might differ according to time domain. Parametric regression modeling methodology allowed us to fit early, constant, and late phase hazard functions and to test their significance.
Summary statistics are reported as mean±standard deviation or median and interquartile range (IQR). Mean or median imputation was used; there was ≤2% missing data in variables on which imputation was performed, with the exception of highest lactate (18%) and fractional area change (8%). Genetic syndrome and non-syndromic abnormalities were analyzed with an explicit category for unknown status. All outcomes were measured as time since randomization. The following independent and mutually exclusive states were defined for analysis: death, cardiac transplantation, and transplant-free survival (i.e., no transition to another state). Nonparametric Kaplan-Meier estimates of freedom from death and transplantation were obtained and used to inform starting values for parametric model estimation of the hazard functions for each state and the number of phases. Competing risks cumulative incidence event rate estimation was employed. Univariate (Online Table 1) and multivariable modeling was conducted using the parametric regression model. (5) Bootstrap resampling was used to estimate the reliability of each factor selected by stepwise regression for the multivariable model. (6–7) We retained main effects in the model if the term had reliability ≥ 50% and a P-value < .05. In addition to the identification of independent associated factors, the interaction of shunt type with each candidate factor was examined unless there were insufficient events to achieve model convergence. The final multivariable model was also solved to predict the time-related probability of each state for selected combinations of associated factors representing plausible patient profiles. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC) and SAS macros from the Cleveland Clinic (5).
RESULTS
The analytic cohort included 549 subjects (268 MBTS, 281 RVPAS). The mean follow-up in subjects who survived and did not undergo cardiac transplant was 2.7±0.9 years, with a maximum follow-up of 4.4 years. There were 178 deaths; 100 in the MBTS group and 78 in the RVPAS group. Over one-third (39%) were of cardiovascular etiology, 25% had unknown etiology, 10% were due to multi-system organ failure, 7% were due to infection, 7% were of pulmonary etiology, 5% were due to surgical complications, and 7% were due to other causes. There were 19 transplants, 7 in the MBTS group (all occurring within one year of randomization) and 12 in the RVPAS group (half occurring within one year of randomization). A genetic syndrome, diagnosed at a median age of 0.5 (interquartile range 0.1 to 9.0) months, was present in 5% (26 subjects), including 8 with CHARGE association; 5 with Turner syndrome; 4 with Kabuki syndrome; one each with DiGeorge, Alagille, Goldenhar, Down or Thode-Leonard syndrome, and 4 others with unspecified syndromes.
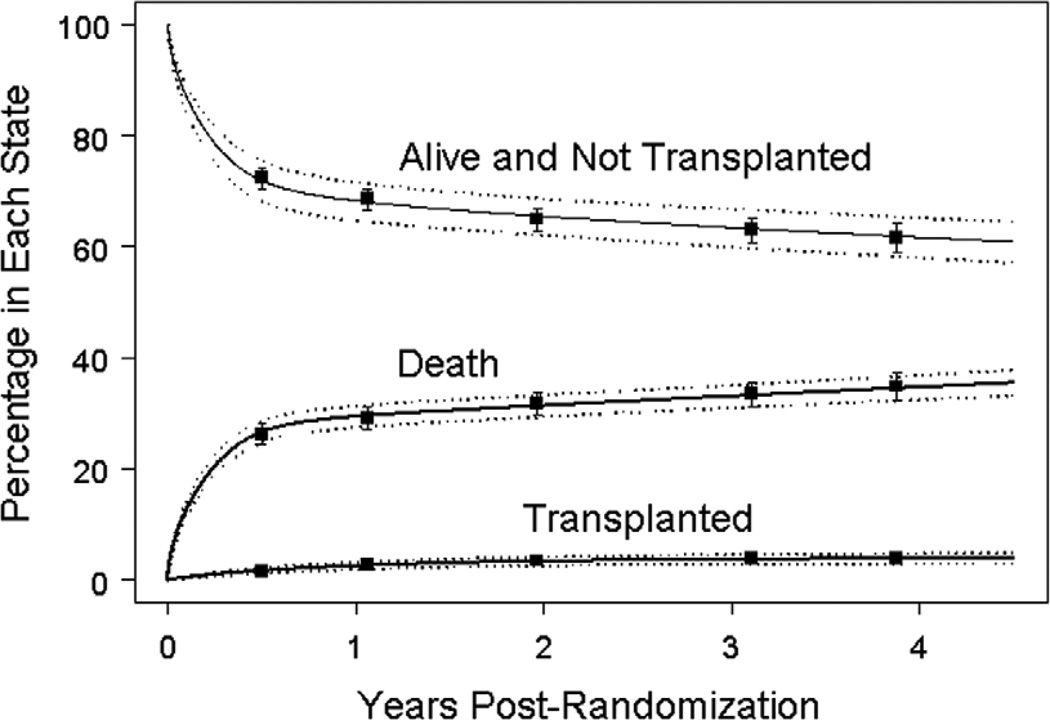
Figure 1 displays competing risk time-related prevalence for the three states (death, transplant, survival without transplant). For the entire cohort, the 1- and 3-year mortality rates were 29% and 33%. The 1- and 3-year transplant prevalence was 2.5% and 3.6%, respectively. The parametric curves provide a very accurate estimate of the event rates derived from nonparametric modeling (shown by the point estimates). This concordance indicates that the parametric regression model provides a suitable fit to the data and is appropriate for identifying factors associated with each state.
Figure 1.
Proportion of subjects in each of three competing mutually exclusive end-states: death, transplant and alive without transplant for all 549 subjects (solid curves). The dashed curves represent the pointwise 68% (1 standard error) confidence bands based on the parametric fit. The squares and associated error bars represent the nonparametric estimates ± one standard error.
The multivariable model for death included several independent factors and two interactions with shunt type (Table 1A). The hazard for death was characterized by an early phase and a constant phase that when combined, describe the pattern of mortality over time. Clinical factors associated with the constant phase contribute to the likelihood of death equally across the entire time period of analysis. Although early and constant phase factors contribute in varying degrees to the hazard of death across the entire time spectrum, the early phase factors characterized the hazard predominantly in the first year following the Norwood procedure. After this time, the hazard of death up to 4.4 years was predominantly attributable to constant phase factors. Early phase factors associated with death included lower SES score (hazard ratio [HR]=1.28 per 5-point decrease, a decrement roughly comparing subjects from two adjacent SES quartiles, P=.012), a smaller ascending aorta diameter (HR=1.23 per 1 mm decrease, P=.014), the presence of OPVR, and certain subtypes of cardiac anatomy (Table 1A, 1B). Subjects with HLHS-aortic stenosis (AS)/mitral atresia (MA), HLHS-aortic atresia (AA)/mitral stenosis (MS), and those with a diagnosis other than HLHS were at highest risk of death and had similar mortality. Among subjects with HLHS and AA there was no difference in mortality among those with MA compared with those with MS (HR=0.85, P=.46). Mortality was lowest in subjects with HLHS-AS/MS (Table 1A). Two factors were associated with a constant hazard of death (both P<.001): the presence of a genetic syndrome (12 of 26 subjects with a genetic syndrome died) and lower gestational age (HR=1.56 per decreasing week).
Table 1.
| A. Multivariable Model for Mortality (Total N=549, 178 deaths) | ||||
|---|---|---|---|---|
| Hazard Ratio |
95% CI | P-value | Reliability | |
| Early Phase | ||||
| OPVR vs. No OPVR | 4.75 | (2.65, 8.52) | <.001 | 88% |
| No HLHS vs. HLHS with AS/MS | 4.00 | (1.91, 8.37) | <.001 | |
| No HLHS vs. HLHS with AA/MS | 1.75 | (0.98, 3.13) | .06 | |
| No HLHS vs. HLHS with AS/MA | 1.28 | (0.46, 1.56) | .63 | |
| No HLHS vs. HLHS with AA/MA | 2.07 | (1.12, 3.82) | .021 | |
| Lower SES score (per 5-point decrease) | 1.28 | (1.06, 1.56) | .012 | 64% |
| Smaller ascending aorta diameter, per mm | 1.23 | (1.04, 1.45) | .013 | 63% |
| RVPAS vs. MBTS | 56%, 55% | |||
| Term, Aortic valve not patent | 0.36 | (0.22, 0.60) | <.001 | |
| Term, Aortic valve patent | 0.57 | (0.49, 1.47) | .52 | |
| Preterm, Aortic valve not patent | 1.25 | (0.66, 2.38) | .49 | |
| Preterm, Aortic valve patent | 2.97 | (1.43, 6.19) | .004 | |
| Constant Phase | ||||
| Lower Gestational age, per wk | 1.56 | (1.28, 1.90) | <.001 | 50% |
| Genetic syndrome present vs. | 9.34 | (3.07, 28.40) | <.001 | 55% |
| None | ||||
| Genetic syndrome unknown vs. | 4.74 | (2.10, 10.72) | <.001 | 78% |
| None | ||||
| RVPAS vs. MBTS* | 1.10 | (0.43, 2.83) | .85 | 78% |
| B. Additional Pairwise Anatomic Diagnosis Comparisons from Multivariable Model for Death in Table 1A. | ||||
|---|---|---|---|---|
| Comparison | Hazard Ratio | 95% CI | P-value | |
| AS/MS vs. | ||||
| AA/MA | 0.52 | (0.23, 1.15) | .11 | |
| AA/MS | 0.44 | (0.20, 0.95) | .04 | |
| AS/MA | 0.32 | (0.10, 1.00) | .05 | |
| AA/MA vs. | ||||
| AA/MS | 0.84 | (0.54, 1.31) | .46 | |
| AS/MA | 0.62 | (0.23, 1.67) | .35 | |
| AA/MS vs. AS/MA | 0.73 | (0.27, 1.98) | .54 | |
AA = aortic atresia; AS = aortic stenosis; HLHS = hypoplastic left heart syndrome; MA = mitral atresia; MBTS = modified Blalock-Taussig shunt; RVPAS = right ventricle-to-pulmonary-artery shunt; MS = mitral stenosis; OPVR = obstructed pulmonary venous return; SES = socioeconomic score
Shunt type forced into model
AA = aortic atresia; AS = aortic stenosis; MA = mitral atresia; MS = mitral stenosis
In the multivariable model for death, there were two significant two-way interactions. Shunt type had a significant interaction with preterm status (P=.029) and aortic valve patency (P=.018). Four subgroups resulted from these two factors: term infants with an atretic aortic valve (51% of subjects); term infants with a patent aortic valve (38% of subjects); preterm infants with an atretic aortic valve (7% of subjects), and preterm infants with a patent aortic valve (4% of subjects). We found no significant benefit of either shunt type for term infants with a patent aortic valve (P=.57), nor for preterm infants with an atretic aortic valve (P=.49); this represents nearly half the cohort (45%). The RVPAS was associated with lower mortality for term infants with an atretic aortic valve (RVPAS vs. MBTS HR=0.36, P<.001), which represent the other half of the cohort. The MBTS was associated with lower mortality for the small subgroup of preterm infants with a patent aortic valve (RVPAS vs. MBTS HR=2.97, P=.004).
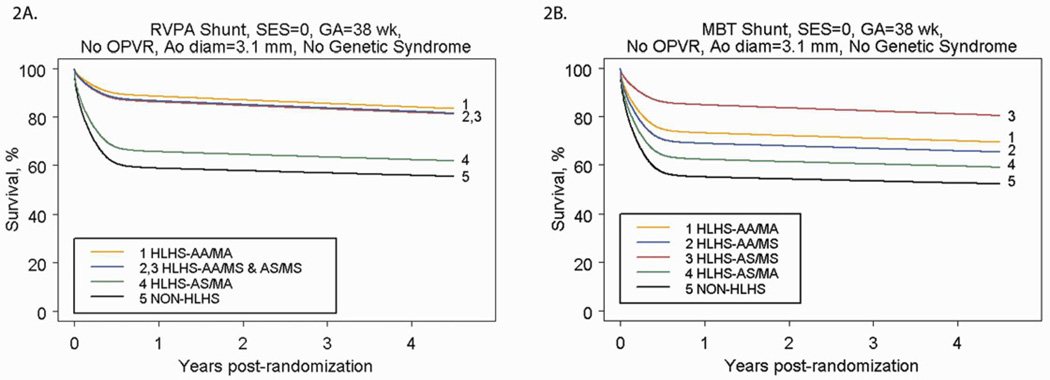
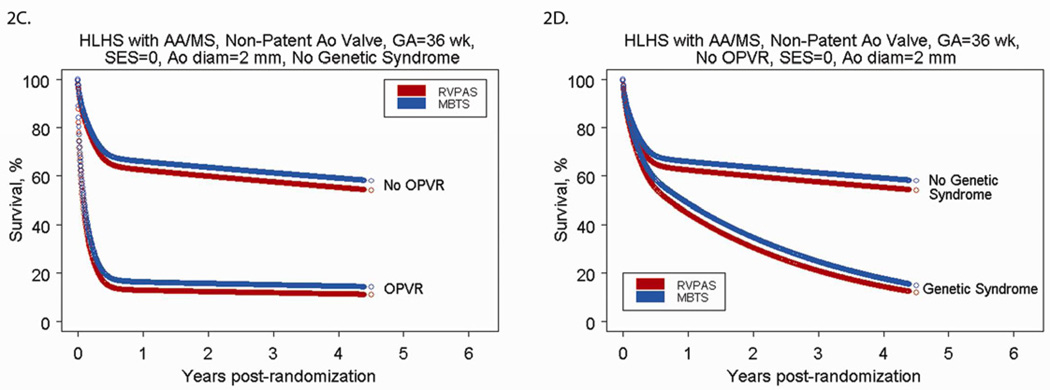
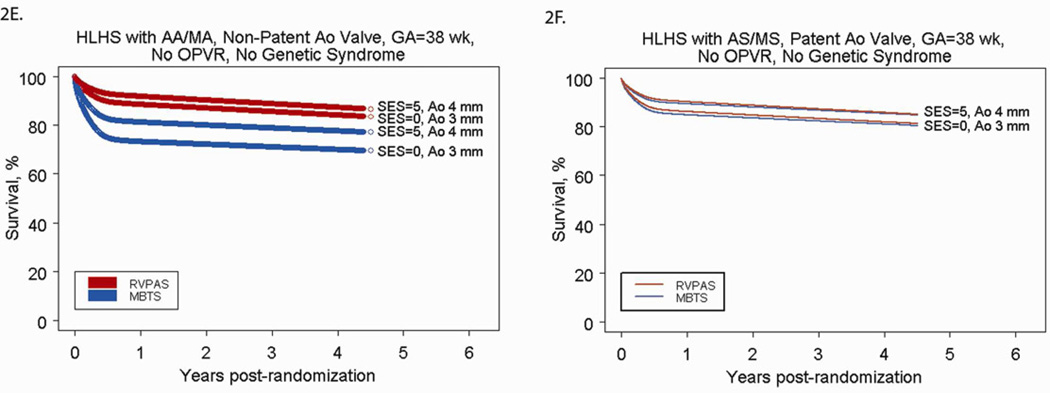
We used the parametric regression model to generate predicted cumulative incidence of death by cardiac anatomy and shunt type for low risk subjects (Figures 2A and 2B). The predicted mortality for any patient profile can be obtained from the model. Figures 2C and 2D demonstrate selected scenarios of poor outcome in, as an example, those with HLHS and AA/MS: subjects with OPVR who are preterm and have a smaller than average ascending aortic diameter have less than a 20% chance of survival to one year, regardless of shunt type. Because OPVR is a risk factor associated with the early phase of the hazard for death, mortality occurs rapidly (by 6–8 months, usually before the Stage II procedure). Subjects with no OPVR, but who have a genetic syndrome, are preterm and have a small aortic diameter also have less than a 20% chance of survival at 4 years, but the mortality occurs over time, because genetic syndrome is a risk factor associated with the underlying constant (rather than early) hazard of death. Conversely, Figures 2E and 2F demonstrate selected scenarios of very good outcome. These profiles are for subjects with HLHS with AS/MS (2E) or AA/MA (2F) with other characteristics fixed at SES at the third quartile, no OPVR, term status, and an above-average ascending aorta diameter. For these subjects, all have survival over 70% at 4 years, regardless of shunt type.
Figure 2.
A) Parametric survival curve by anatomic subtype of subjects undergoing RVPAS. B) Parametric survival curve by anatomic subtype of subjects undergoing MBTS. C) Parametric survival curve for higher-risk subjects, showing the impact of OPVR on survival among subjects undergoing the Norwood procedure with a MBTS vs. a RVPAS. D) Parametric survival curve for higher-risk subjects, showing the impact of a genetic syndrome on survival among subjects undergoing the Norwood procedure with a MBTS versus a RVPAS. E) Parametric survival curve for lower-risk subjects, showing the impact of shunt type, SES, and aortic diameter on survival of subjects with HLHS-AA/MA. F) Parametric survival curve for lower-risk subjects, showing the impact of shunt type, SES, and aortic diameter on survival of subjects with HLHS-AS/MS.
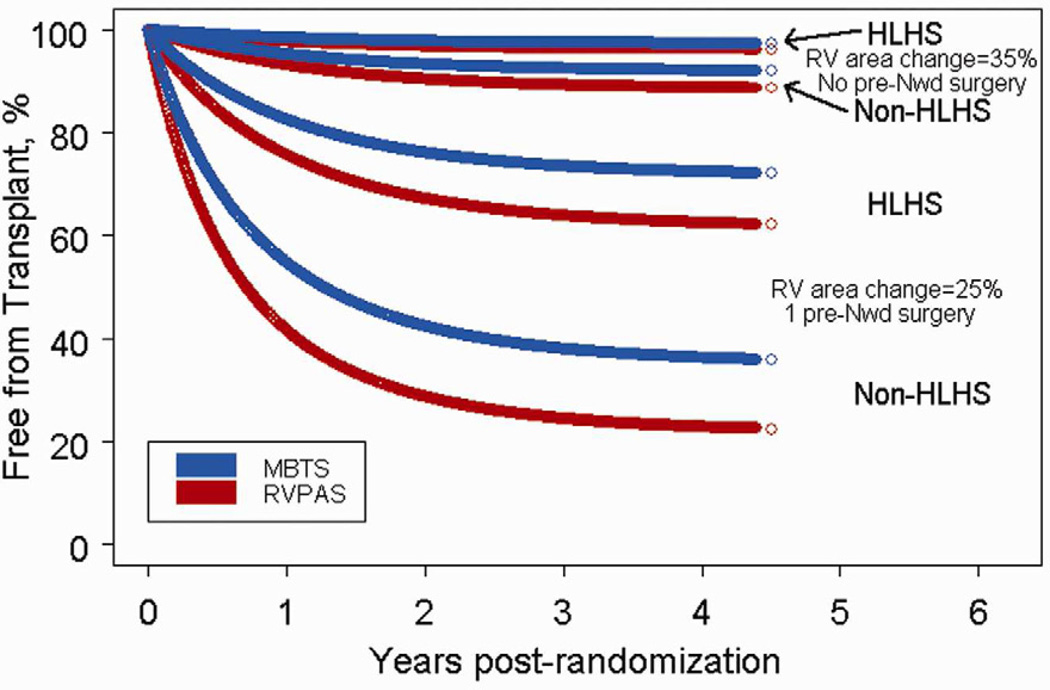
Eight variables were associated with an increased risk of transplant at the P<.10 level (Online Table 2). We found no significant interactions of shunt type and other variables. The multivariable model for transplant (Table 2) had three independent factors: lower RV fractional area change from the pre-Norwood echocardiogram, a diagnosis other than HLHS, and a larger number of pre-Norwood surgeries (see Online Table 2 for procedure listing). Shunt type was not associated with transplant (RVPAS vs. MBTS HR=1.46, 95% CI 0.57 to 3.70, P=.43). The presence of moderate/severe tricuspid regurgitation was also clinically significant (HR=2.61), but was not statistically significant (P=.07) due to its correlation with cardiac anatomy and number of prior surgeries. Figure 3 displays from the cumulative incidence of transplant for 8 patient profiles. For example, 11% of infants with 35% fractional area change on pre-Norwood echocardiogram and no pre-Norwood surgery are predicted to undergo transplant by 3 years regardless of anatomy, whereas 27–36% of infants with HLHS, 25% fractional area change and 1 pre-Norwood surgery will undergo transplant by that time. One high risk scenario is an infant with a diagnosis other than HLHS, 25% fractional area change and 1 pre-Norwood surgery (62–76% three-year probability of transplant).
Table 2.
Multivariable Model for Cardiac Transplantation
| Variable | Hazard Ratio | 95% CI | P-value | Reliability |
|---|---|---|---|---|
| RVPAS vs. MBTS* | 1.46 | (0.57, 3.70) | .43 | - |
| Lower pre-Norwood RV fractional area change, % | 1.49† | (1.12, 1.97) | .006 | 60% |
| HLHS vs. other diagnosis | 0.32 | (0.11, 0.89) | .029 | 51% |
| Number of pre-Norwood surgeries | 5.64 | (1.69, 18.77) | .005 | 54% |
Shunt type was forced into the model.
per 5 unit decrease
HLHS = Hypoplastic left heart syndrome; MBTS = Modified Blalock-Taussig shunt; RV = right ventricular; RVPAS = Right ventricle to pulmonary artery shunt;;
Figure 3.
Predicted probability of freedom from transplant for subjects with differing cardiac anatomy, RV function, and numbers of pre-Norwood surgeries.
DISCUSSION
The goal of this analysis of the PHN SVR trial dataset was to identify patient and preoperative factors associated with the intermediate-term occurrence of death and cardiac transplant in infants with single right ventricle anomalies undergoing staged palliation. The candidate factors chosen for this analysis include those with little or no potential for modification prior to surgery. As these characteristics are unique to each patient at the time of diagnosis, they are the factors the teams caring for these neonates must use to formulate decisions prior to surgical intervention. Consideration of primarily non-modifiable factors was chosen with the additional goal of identifying those patient-specific factors that might allow better assignment to either a MBTS or a RVPAS for the Norwood procedure.
Using parametric regression, we were able to characterize the hazard of death as having a constant phase and an early phase. Many of these risk factors, specifically OPVR, shunt type, smaller ascending aorta, non-HLHS anatomy, genetic syndromes and lower gestational age have been identified in previous retrospective and prospective studies. (1, 8–12)
Although a genetic syndrome was present in only 5% of the cohort, it had a strong negative association with survival. Only 14 of 26 subjects with an identified genetic syndrome were alive at last follow-up. In a recent analysis of the Society of Thoracic Surgeons and Congenital Heart Surgeons’ Society databases, the risk of death after the Norwood procedure among patients with chromosomal defects, the majority of which were Turner Syndrome, was increased and found to be greatest within the first year of the Norwood procedure. (13) Similarly, we found that 10 of the 12 deaths occurring in subjects with a genetic syndrome occurred in the first year. The specific mechanism of increased risk of mortality is unknown but it is likely due to abnormalities of organ system function specific to each genetic syndrome. (12,13) The poor survival of this group is important to consider during prenatal and preoperative counseling.
The presence of OPVR also had a very high impact on mortality. This condition is associated with profound hypoxia and the need for either emergent Norwood procedure or pre-Norwood intervention. Although creation of an unrestrictive atrial septal communication can usually be accomplished at the time of surgery, these patients frequently have persistent abnormalities of the pulmonary vasculature. The wall of the pulmonary veins can be thickened by the presence of multiple elastic lamina, normally not seen in pulmonary veins. (14,15) Despite anatomic decompression of the left atrium, these patients have ongoing hypoxia with a pulmonary vascular bed unsuitable for subsequent single ventricle palliation. While we have confirmed the profound risk associated with OPVR, effective treatment remains elusive. Attempts at prenatal intervention are promising but as yet have not resulted in significantly improved outcomes. (15)
There were important differences in survival with respect to anatomic subtype. Consistent with single-center reports, subjects with a non-HLHS diagnosis, such as unbalanced atrioventricular canal defect, double outlet right ventricle with mitral atresia or heterotaxy syndrome had poorer survival. (10) The superior survival among subjects with AS/MS was observed even after adjustment for ascending aortic diameter, but may be due in part to the presence of antegrade flow with more reliable coronary circulation and possibly a larger aortic root and arch, simplifying the Norwood procedure. Furthermore, the presence of even a small left ventricle that can contribute to cardiac output may be helpful. Two large single-center retrospective studies have suggested that patients with AA/MS may be at higher risk for death following stage 1 palliation, possibly due to the higher prevalence of coronary artery fistulas. (16, 17) In this analysis, there was no significant difference in mortality between subjects with AA and MA and those with AA and MS. Our data do not provide justification for alternative strategies in this group of patients.
We found an important interaction between shunt type and aortic valve patency, as well as between shunt type and prematurity in association with early phase mortality. Specifically, term infants with an atretic aortic valve had better survival if they underwent a RVPAS compared with a MBTS. The benefit of the RVPAS at 12 months, as previously reported for the SVR trial, appears to be confined, based on mean follow-up of 2.7±0.9 years, to this group of 272 subjects (51% of the total cohort). (1) The risk of mortality for a term patient with an atretic aortic valve undergoing a RVPAS was one-third that of a similar patient undergoing a MBTS. In contrast, among 21 preterm infants (4% of the cohort) with a patent aortic valve, survival was better with a MBTS. For the 45% of the subjects outside of these two groups, specifically term patients with a patent aortic valve and preterm patients with aortic atresia, we were unable to demonstrate a significant association between shunt type and mortality. Among the postulated benefits of the RVPAS is improved coronary artery perfusion. (18–20) The RVPAS results in a higher diastolic pressure, and thus a higher coronary artery driving pressure, which may provide an important benefit to the patient with aortic atresia. In contrast to patients with a patent aortic valve, patients with AA are more likely to have coronary artery anomalies including fistulas. (21,22) Furthermore, patients with an atretic aortic valve are more likely to have a smaller ascending aorta, making surgical construction of an unobstructed connection between the ascending aorta and main pulmonary artery more technically challenging. Indeed, obstruction in this connection, which can impair coronary artery blood flow in the presence of AA, has been cited as a cause of death among patients undergoing the Norwood procedure. (23) The more favorable hemodynamics of the RVPAS may ameliorate mild obstruction that could otherwise result in critical coronary artery insufficiency, myocardial ischemia and death among similar patients with a MBTS. Therefore, the benefit of the RVPAS over the MBTS in term newborns with aortic atresia may be explained by better coronary artery blood flow. Why a MBTS would be favorable among preterm subjects with a patent aortic valve is less clear. Perhaps the ventriculotomy in a small heart is less well tolerated and overwhelms other risk factors. It must be emphasized that only 11% of the subjects in this trial were preterm.
This analysis studied factors that are known about the newborn with HLHS prior to surgical intervention, and are the basis for decision-making by the teams caring for the patient with HLHS. They are generally considered non-modifiable, but in fact two of the factors, gestational age and SES, might be potentially modifiable. This study and single-center studies of outcome have identified the importance of gestational age as a risk factor for mortality, as well as for neurodevelopmental outcome. (9–12) Although some early deliveries are inevitable, the increased risk of mortality should be taken into consideration if earlier elective delivery is considered. In the case of SES, the impact on survival is thought to be due to limited access to health care resources. (24) Therefore, provision of services to compensate for the challenges associated with low SES could positively impact outcome.
Given the high mortality of subjects following the Norwood procedure, it seems at first surprising that there was such a low utilization of transplant. Although the decision-making process concerning the use of transplant in this group is beyond the scope of this study, it is noteworthy that many of the factors associated with mortality, such as OPVR and a genetic syndrome, are often considered contraindications for transplant. (25) Furthermore, multi-organ dysfunction that occurs as a consequence of inadequate cardiac output following the Norwood procedure may also preclude the use of transplantation as salvage therapy. The relative lack of donor availability also limits use of this therapy. These data suggest that transplant may be an alternative for only a small fraction of patients at risk for mortality following the Norwood procedure.
This analysis has several limitations. We had limited power to detect interactions of shunt type and patient subgroup when subgroup sizes were small, and to detect differences in mortality for selected subgroups regardless of shunt. For example, the difference in mortality between HLHS patients with AA/MA vs. those with AS/MS was two-fold, clinically significant, but was of marginal statistical significance. There was also low power to identify statistically significant risk factors for transplant due to the small number of such events. Nevertheless, the risk models developed for both mortality and transplant included multiple independent factors and have clinical relevance. Finally, the presence vs. absence of a genetic syndrome was unknown for one-third of subjects, and the increased hazard ratio for death relative to subjects without a genetic syndrome reflects in part mortality that occurred before a genetic evaluation could be performed.
Conclusions
These data suggest that term patients with aortic atresia have better outcomes with a RVPAS and that preterm infants with patent aortic valve may have better outcomes with a MBTS. For slightly less than half of the cohort, non-modifiable factors did not aid in shunt selection. Lower gestational age and SES as well as the presence of a genetic syndrome are also independent predictors of mortality. Transplant was used in only 3% of subjects following the Norwood procedure. Right ventricular function and anatomy, but not shunt type, were associated with transplantation. Patient-specific factors associated with mortality following the Norwood procedure are frequently contraindications to transplantation. Effective alternative therapies for the highest risk Norwood candidates remain elusive.
Supplementary Material
Acknowledgements
The authors wish to thank Dr. Eugene Blackstone and Jeevanantham Rajeswaran for their guidance on this analysis.
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute
APPENDIX
National Heart, Lung, and Blood Institute: Gail Pearson, Victoria Pemberton, Rae-Ellen Kavey*, Mario Stylianou, Marsha Mathis*.
Network Chair: University of Texas Southwestern Medical Center, Lynn Mahony
Data Coordinating Center: New England Research Institutes, Lynn Sleeper (PI), Sharon Tennstedt (PI), Steven Colan, Lisa Virzi*, Patty Connell*, Victoria Muratov, Lisa Wruck*, Minmin Lu, Dianne Gallagher, Anne Devine*, Julie Schonbeck, Thomas Travison*, David F. Teitel
Core Clinical Site Investigators: Children’s Hospital Boston, Jane W. Newburger (PI), Peter Laussen, Pedro del Nido, Roger Breitbart, Jami Levine, Ellen McGrath, Carolyn Dunbar-Masterson, John E. Mayer, Jr., Frank Pigula, Emile A. Bacha, Francis Fynn-Thompson; Children’s Hospital of New York, Wyman Lai (PI), Beth Printz*, Daphne Hsu*, William Hellenbrand, Ismee Williams, Ashwin Prakash*, Seema Mital*, Ralph Mosca*, Darlene Servedio*, Rozelle Corda, Rosalind Korsin, Mary Nash*; Children’s Hospital of Philadelphia, Victoria L. Vetter (PI), Sarah Tabbutt*, J. William Gaynor (Study Co-Chair), Chitra Ravishankar, Thomas Spray, Meryl Cohen, Marisa Nolan, Stephanie Piacentino, Sandra DiLullo*, Nicole Mirarchi; Cincinnati Children’s Medical Center, D. Woodrow Benson (PI), Catherine Dent Krawczeski, Lois Bogenschutz, Teresa Barnard, Michelle Hamstra, Rachel Griffiths, Kathryn Hogan, Steven Schwartz*, David Nelson, Pirooz Eghtesady*; North Carolina Consortium: Duke University, East Carolina University, Wake Forest University, Page A. W. Anderson (PI) – deceased, Jennifer Li (PI), Wesley Covitz, Kari Crawford*, Michael Hines, James Jaggers*, Theodore Koutlas, Charlie Sang, Jr., Lori Jo Sutton, Mingfen Xu; Medical University of South Carolina, J. Philip Saul (PI), Andrew Atz, Girish Shirali, Scott Bradley, Eric Graham, Teresa Atz, Patricia Infinger; Primary Children’s Medical Center and the University of Utah, Salt Lake City, Utah, L. LuAnn Minich (PI), John A. Hawkins-deceased, Michael Puchalski, Richard V. Williams, Peter C. Kouretas, Linda M. Lambert, Marian E. Shearrow, Jun A. Porter*; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Joel Kirsh, Chris Caldarone, Elizabeth Radojewski, Svetlana Khaikin, Susan McIntyre, Nancy Slater; University of Michigan, Caren S. Goldberg (PI), Richard G. Ohye (Study Chair), Cheryl Nowak*; Children’s Hospital of Wisconsin and Medical College of Wisconsin, Nancy S. Ghanayem (PI), James S. Tweddell, Kathleen A. Mussatto, Michele A. Frommelt, Peter C. Frommelt, Lisa Young-Borkowski.
Auxiliary Sites: Children’s Hospital Los Angeles, Alan Lewis (PI), Vaughn Starnes, Nancy Pike; The Congenital Heart Institute of Florida (CHIF), Jeffrey P. Jacobs (PI), James A. Quintessenza, Paul J. Chai, David S. Cooper*, J. Blaine John, James C. Huhta, Tina Merola, Tracey Griffith; Emory University, William Mahle (PI), Kirk Kanter, Joel Bond*, Jeryl Huckaby; Nemours Cardiac Center, Christian Pizarro (PI), Carol Prospero; Julie Simons, Gina Baffa, Wolfgang A. Radtke; University of Texas Southwestern Medical Center, Ilana Zeltzer (PI), Tia Tortoriello*, Deborah McElroy, Deborah Town.
Angiography Core Laboratory: Duke University, John Rhodes, J. Curt Fudge
Echocardiography Core Laboratories: Children’s Hospital of Wisconsin, Peter Frommelt; Children’s Hospital Boston, Gerald Marx.
Genetics Core Laboratory: Children’s Hospital of Philadelphia, Catherine Stolle.
Protocol Review Committee: Michael Artman (Chair); Erle Austin; Timothy Feltes, Julie Johnson, Thomas Klitzner, Jeffrey Krischer, G. Paul Matherne.
Data and Safety Monitoring Board: John Kugler (Chair); Rae-Ellen Kavey, Executive Secretary; David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Holly Taylor, Catherine L. Webb.
*no longer at the institution listed
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010 May 27;362(21):1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008 Oct;136(4):968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001 Jul 12;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 4.Ohye RG, Schonbeck JV, Eghtesady P, Laussen PC, Pizarro C, Shrader P, et al. Cause, timing and location of death in the Single Ventricle Reconstruction Trial. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2012.04.028. 20** ;***(*):***-*** [concurrent issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackstone EH, Naftel DC, Turner ME. The decomposition of time-varying hazard Into phases, each Incorporating a separate stream of concomitant Information. Amer Stat Assoc. 1986;81:615–624. [Google Scholar]
- 6.Breiman L. Bagging predictors. Machine Learning. 1996;24(2):123–140. [Google Scholar]
- 7.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, Florida: CRC Press LLC; 1998. [Google Scholar]
- 8.Ashburn DA, McCrindle BW, Tchervenkov CI, Jacobs ML, Lofland GK, Bove EL, et al. Outcomes after the Norwood operation in neonates with critical aortic stenosis or aortic valve atresia. J Thorac Cardiovasc Surg. 2003 May;125(5):1070–1082. doi: 10.1067/mtc.2003.183. [DOI] [PubMed] [Google Scholar]
- 9.Ghanayem NS, Hoffman GM, Mussatto KA, Frommelt MA, Cava JR, Mitchell ME, et al. Perioperative monitoring in high-risk infants after stage 1 palliation of univentricular congenital heart disease. J Thorac Cardiovasc Surg. 2010 Oct;140(4):857–863. doi: 10.1016/j.jtcvs.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006 Feb;131(2):412–417. doi: 10.1016/j.jtcvs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Artrip JH, Campbell DN, Ivy DD, Almodovar MC, Chan KC, Mitchell MB, et al. Birth weight and complexity are significant factors for the management of hypoplastic left heart syndrome. Ann Thorac Surg. 2006 Oct;82(4):1252–1257. doi: 10.1016/j.athoracsur.2006.04.062. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 12.Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002 Jul;22(1):82–89. doi: 10.1016/s1010-7940(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 13.Patel A, Hickey E, Mavroudis C, Jacobs JP, Jacobs ML, Backer CL, et al. Impact of noncardiac congenital and genetic abnormalities on outcomes in hypoplastic left heart syndrome. Ann Thorac Surg. 2010 Jun;89(6):1805–1813. doi: 10.1016/j.athoracsur.2010.02.004. discussion 13-4. [DOI] [PubMed] [Google Scholar]
- 14.Rychik J, Rome JJ, Collins MH, DeCampli WM, Spray TL. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol. 1999 Aug;34(2):554–560. doi: 10.1016/s0735-1097(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 15.Vida VL, Bacha EA, Larrazabal A, Gauvreau K, Thiagaragan R, Fynn-Thompson F, et al. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: surgical experience from a single center. Ann Thorac Surg. 2007 Aug;84(2):581–585. doi: 10.1016/j.athoracsur.2007.04.017. discussion 6. [DOI] [PubMed] [Google Scholar]
- 16.Vida VL, Bacha EA, Larrazabal A, Gauvreau K, Dorfman AL, Marx G, et al. Surgical outcome for patients with the mitral stenosis-aortic atresia variant of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2008 Feb;135(2):339–346. doi: 10.1016/j.jtcvs.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Glatz JA, Fedderly RT, Ghanayem NS, Tweddell JS. Impact of mitral stenosis and aortic atresia on survival in hypoplastic left heart syndrome. Ann Thorac Surg. 2008 Jun;85(6):2057–2062. doi: 10.1016/j.athoracsur.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Bradley SM, Simsic JM, McQuinn TC, Habib DM, Shirali GS, Atz AM. Hemodynamic status after the Norwood procedure: a comparison of right ventricle-to-pulmonary artery connection versus modified Blalock-Taussig shunt. Ann Thorac Surg. 2004 Sep;78(3):933–941. doi: 10.1016/j.athoracsur.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Pizarro C, Norwood WI. Right ventricle to pulmonary artery conduit has a favorable impact on postoperative physiology after Stage I Norwood: preliminary results. Eur J Cardiothorac Surg. 2003 Jun;23(6):991–995. doi: 10.1016/s1010-7940(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 20.Cua CL, Thiagarajan RR, Gauvreau K, Lai L, Costello JM, Wessel DL, Del Nido PJ, Mayer JE, Jr, Newburger JW, Laussen PC. Early postoperative outcomes in a series of infants with hypoplastic left heart syndrome undergoing stage 1 palliation operation with either modified Blalock-Taussig shunt or right ventricle to pulmonary artery conduit. Pediatr Crit Care Med. 2006 May;7(3):238–244. doi: 10.1097/01.PCC.0000201003.38320.63. [DOI] [PubMed] [Google Scholar]
- 21.Smith A, Pozzi M, Anderson RH. The morphology of hypoplasia of the left heart. In: Anderson RH, Pozzi M, Hutchinson S, editors. Hypoplastic left heart syndrome. London: Springer-Verlag; 2005. pp. 1–18. [Google Scholar]
- 22.Baffa JM, Chen SL, Guttenberg ME, Norwood WI, Weinberg PM. Coronary artery abnormalities and right ventricular histology in hypoplastic left heart syndrome. J Am Coll Cardiol. 1992 Aug;20(2):350–358. doi: 10.1016/0735-1097(92)90101-r. [DOI] [PubMed] [Google Scholar]
- 23.Bartram U, Grunenfelder J, Van Praagh R. Causes of death after the modified Norwood procedure: a study of 122 postmortem cases. Ann Thorac Surg. 1997 Dec;64(6):1795–1802. doi: 10.1016/s0003-4975(97)01041-2. [DOI] [PubMed] [Google Scholar]
- 24.Mackie AS, Gauvreau K, Newburger JW, Mayer JE, Erickson LC. Risk factors for readmission after neonatal cardiac surgery. Ann Thorac Surg. 2004 Dec;78(6):1972–1978. doi: 10.1016/j.athoracsur.2004.05.047. discussion 8. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins PC, Flanagan MF, Jenkins KJ, Sargent JD, Canter CE, Chinnock RE, et al. Survival analysis and risk factors for mortality in transplantation and staged surgery for hypoplastic left heart syndrome. J Am Coll Cardiol. 2000 Oct;36(4):1178–1185. doi: 10.1016/s0735-1097(00)00855-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.