Abstract
Introduction
Factors regulating brain tissue plasminogen activator (tPA) are pertinent for stroke. Recent observations have suggested a role for the phosphodiesterase-4 (PDE4) pathway in stroke pathogenesis, via an uncertain mechanism. We studied PDE4 regulation of tPA expression by human brain microvascular endothelial cells in a variety of conditions, including an in vitro model of ischemia.
Materials and Methods
We analyzed tPA antigen and mRNA of human brain microvascular endothelial cells (HBEC) during normoxia and oxygen-glucose deprivation (OGD) following inhibition of PDE4 and PDE4D, using HBEC monocultures and co-cultures with astrocytes and pericytes, and analyzed relevant signal transduction pathways.
Results
PDE4 inhibitor rolipram enhanced OGD effects on endothelial tPA release in endothelial monocultures and co-cultures with astrocytes; there was a 54±10% (p<0.001) reduction of tPA release in astrocyte-endothelial co-cultures under OGD. PDE4D siRNA reduced endothelial tPA mRNA to 40-55% of control (p<.05). Use of Epac inducer mimicked, while use of Epac siRNA inhibited, these effects.
Conclusions
Inhibition of PDE4 and PDE4D reduced expression of tPA by HBEC via Epac pathway.
Keywords: tPA, endothelium, microcirculation, vascular biology, cell culture
Tissue plasminogen activator (tPA) is the only effective treatment for ischemic stroke [1], and factors that regulate expression of endogenous tPA are of interest from the perspective of stroke pathogenesis [2]. Recently, considerable attention has been given to the phosphodiesterase-4 (PDE4) pathway in stroke, with a focus on single nucleotide polymorphisms of the PDE4D gene [3]. While recent studies continue to link these polymorphisms to stroke [4], the original observations associating PDE4D with ischemic stroke risk remain unconfirmed[5]. In order to define potential pathways linking PDE4 and stroke, we studied in vitro effects of PDE inhibition and report herein relationship between PDE4 inhibition and endothelial tPA expression.
METHODS
Cell Culture Preparation
Human brain microvascular endothelial cells (HBEC) harvested from normal human brain cortical tissue (Applied Cell Biology Research Institute, Kirkland, WA) were cultured in Medium 131 (Cascade Biologics, Portland, OR) containing microvascular growth supplement and 5% fetal bovine serum (FBS). Identification of endothelial cells was confirmed by immunoreactivity for von Willebrand factor antibody (Dako, Carpinteria, CA) and uptake of acetylated LDL labeled with 1-1'-dioctacecyl-1-1-3-3-3'-3'-tetamethyl-indocarbocyanine perchlorate (Biomedical Technologies, Stoughton, MA). HBECs at passages 7 to 11 were used for experiments. Human pericytes were obtained from normal human brain cortical tissue (Applied Cell Biology Research Institute, Kirkland, WA). Pericytes were identified by positive immunoreactivity for α-smooth muscle actin, positive immunoreactivity for vimentin intermediate filaments and nestin, negative immunoreactivity for von Willebrand factor and glial fibrillary acidic protein, and absence of hill and valley morphology. Pericytes at passages 4 to 7 were used for experiments. Human astrocytes were prepared by Cambrex (Walkersville, MD) and maintained on Dulbecco's modified Eagle's medium (DME) containing 10% FBS. Astrocytes were characterized by immunoreactivity for glial fibrillary acidic protein. Astrocytes used for these experiments were taken from passage 3 to 7.
We used rolipram at 10 μM Rolipram (AG Scientific, San Diego CA) to inhibit PDE4 of endothelial cells [6]. We used 8CPT-2’OMe-cAMP (Biolog, Bremen, Germany) at 100 μM to activate Epac pathway [7] and inhibition of PKA pathway was performed using 1 μM KT5720 (Sigma, St. Louis, MO) [8] and 10 μM H89 (Calbiochem, Gibbstown, NJ) [9]. Rolipram, 8CPT-2’OMe-cAMP, and KT5720 were dissolved in DMSO; concentrations of DMSO were the same for the treatment groups. Astrocyte- and pericyte-endothelial interactions were studied using transwell preparations (Corning Costar, Cambridge, MA). Co-cultures were established by transferring confluent pericyte/astrocyte transwell inserts over endothelial cells.
Oxygen-Glucose Deprivation (OGD)
Rolipram 10 μM was added to endothelial wells at the beginning of HBEC monoculture, astrocyte co-culture, or pericyte co-culture. 8CPT-2’OMe-cAMP at 100 μM was added for Epac studies [7]. After 42 hours of co-culture, media was changed and cultures were placed in OGD chambers or 5% CO2 incubator. Growth medium of OGD cultures was exchanged for 5% FBS DME medium without glucose. Cell culture preparations were placed in hypoxia chamber infused with 2% O2, 5% CO2, remainder nitrogen and the chamber placed in 37°C incubator. After 6 hours OGD, conditioned media were collected and stored at -80°C for enzyme immunoassay. Control cultures had media replaced with 5% FBS DME medium with low glucose and were then placed in 5% CO2, 37°C incubator for 6 hrs.
Fibrinolysis Assays
tPA and PAI-1 enzyme immunoassays were performed using commercial kits (American Diagnostica Inc., Greenwich, CT). Briefly, samples or standards were added to wells coated with goat polyclonal anti-human tPA or mouse monoclonal anti-human PAI-1 antibodies. After 1-hour incubation, detection antibody conjugate was added and incubated for an additional 30 minutes or 1 hour for tPA or PAI, respectively. After washing the plates 4 times, substrate was added. After 15 minutes, the reaction was terminated and absorbance was read at 492 nm. tPA activity was measured using Chromolize tPA Assay Kit (Trinity Biotech, Wicklow, Ireland). tPA standards or test samples (100ml each) were added to individual wells. After 20-minute incubation in wells pre-coated with tPA antibody, substrate (50 ml) was added and incubated for an additional 90 minutes, followed by reaction termination and measurement of absorbance.
siRNA mediated gene silencing
We used two siRNAs targeting PDE4D(CCGGCCCTTGACTGTTATCAT and CAGAGTTTATATCAAACACAT) as well as an siRNA targeting LaminA/C (AACTGGACTTCCAGAAGAACA) (Qiagen, Valencia, CA). For Epac1 siRNA transfection, we used two siRNAs targeting Epac1 (SI00698530 and SI03126928, Qiagen, Valencia, CA). For co-transfection experiments, siRNAs targeting PDE4D were co-transfected with one of two siRNAs targeting Epac1 (SI00698530 Qiagen, Valencia, CA) or, as a control, a nonsense siRNA (Qiagen, Valencia, CA ). The day before transfection, 2×105 cells per well of a 6-well plate were seeded and incubated in growth medium containing serum and antibiotics at 37°C and 5% CO2 for 24 hours. 0.4 μg siRNA was transfected into HBECs using HiPerfect Transfection Reagent (Qiagen, Valencia, CA). 48 hours after transfection, cell pellets and conditioned medium were collected. Transfection efficiency was determined by mRNA expression level measured using real-time PCR.
Real-time PCR
RNeasy Mini kit (Qiagen, Valencia, CA) was used for RNA extraction. 200ng total mRNA was added in 20 μl reverse-transcription reaction. The final concentration of each reagent used in 20 μl reverse-transcription reaction was: Taqman RT buffer (1X), 5.5 mM MgCl2, 500μM dNTP mixture, 0.4 U/μl RNase inhibitor, 1.25 U/μl Multiscribe Reverse Transcriptase (Applied Biosystems, Foster City, CA) and 200ng total mRNA. The mixture was incubated 5 minutes at 25 °C, 30 minutes at 98°C, and then 5 minutes at 95°C. cDNAs were stored in -20 °C condition for further use.
TaqMan real-time quantitative PCR was performed in a GeneAmp 7300 sequence detector. The PCR reaction, performed in triplicate in a 25 μl total volume, contained 12.5 μl TaqMan Universal PCR Master Mix and 2.5μl (0.5μM) cDNA, primers, and probes. The PCR reaction was carried out at 50°C for 2 min to activate uracil-N-glycosylase (UNG), 95°C for 10 minutes to activate Taq polymerase, followed by 40 cycles of denaturation at 95°C for 15 seconds and primer annealing-extension at 60°C for 1 minute. A standard curve, generated from four-fold serial dilutions of hHBEC cDNA was used to determine target gene and β-actin concentrations. Quantity of the gene expression was adjusted to β-actin levels. The primers and probes were: tPA Forward: 5’-GGCCTTGTCTCCTTTCTATTCG-3’ , Reverse: 5’-GCGGCTGGATGGGTACAGT-3’ Probe: FAM-TGACATGAGCCTCCTTCAGCCGCT-TAMRA; β-actin Forward: 5’-CCTGGCACCCAGCACAAT-3’, Reverse: 5’-GCCGATCCACACGGAGTACT-3’, Probe: FAM-ATCAAGATCATTGCTCCTCCTGAGCGC-TAMRA; PDE4D Forward: 5’-GGACGGACCGGATAATGGA-3’, Reverse: 5’-CGGAAGCATTGTGCTTGTCA-3’ Probe: FAM-AGACCGAGAGAGGGAACGTGGCATG–TAMRA.
Statistical Analysis
Data are presented as percent of mean control value. Differences among the means of the treatment groups were tested using analysis of variance and Tukey's two-tailed t-test, testing if any treatments are significantly different from the control group. Differences were considered statistically significant if p<0.05.
RESULTS
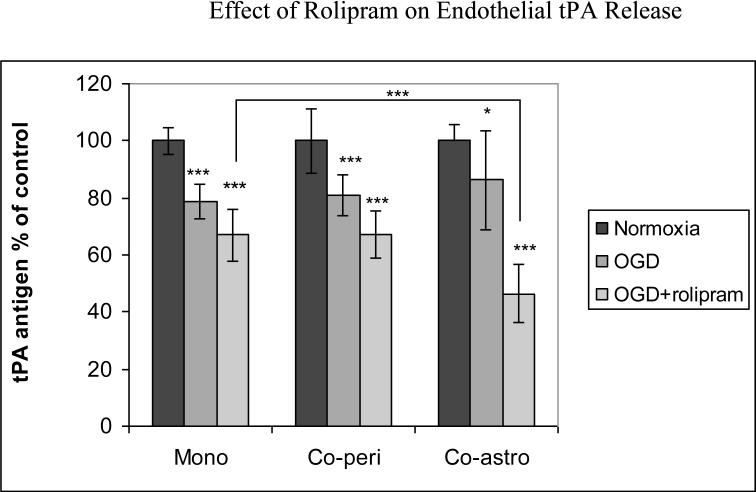
HBEC were cultured alone or co-cultured with pericytes or astrocytes for 42 h before being subjected to 6 h of OGD with or without pretreatment of rolipram (10uM). For HBEC monocultures subjected to OGD, rolipram enhanced the OGD effects on tPA release, a reduction of 33±9% below control (p<0.001) (Fig 1a). For HBEC-astrocyte co-cultures, rolipram reduced tPA release 54±10% below control (p<0.001). Pericyte-endothelial cell co-cultures showed response to OGD similar to mono-cultures (Fig 1a). Under normoxic conditions, rolipram reduced tPA release in monocultures to 70±4.7% of control (p<0.01); in co-cultures, tPA release was 90± 3.9% of control in pericyte-endothelial co-cultures (p<0.001) and 90±10% of control in astrocyte-endothelial co-cultures. Rolipram exhibited dose-dependent effects, with 1uM rolipram reducing tPA to 84%±4% of control (61±1 ng/ml) (p<0.05) in endothelial monocultures under normoxic conditions. Rolipram did not induce significant change in PAI-1 levels, and tPA activity following rolipram treatment showed a non-significant decline (data not shown). Neither rolipram nor OGD, alone or together, had significant effect on either endothelial cell count or endothelial cell viability by trypan blue exclusion (data not shown).
Figure 1a.
Effect of rolipram on tPA protein release by HBECs, monocultured or co-cultured with astrocytes or pericyte for 42 hrs and then subjected to 6 hours OGD. Pooled data from three independent experiments performed in triplicate. Mean with error bars representing standard deviation. * p<0.05, *** p<0.001
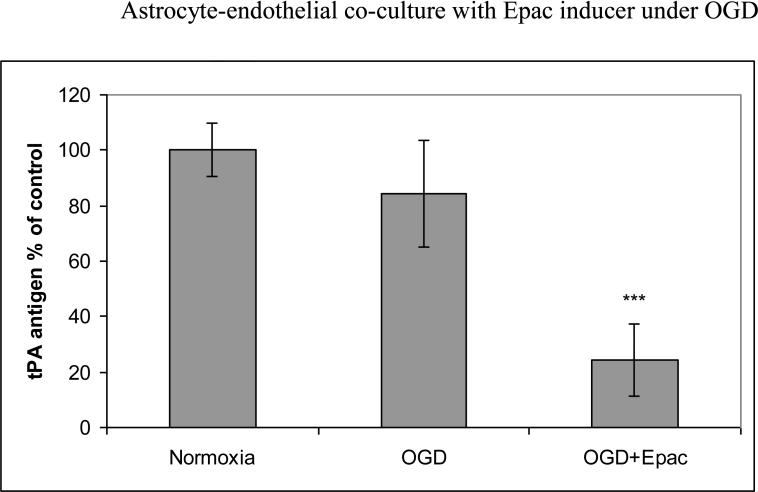
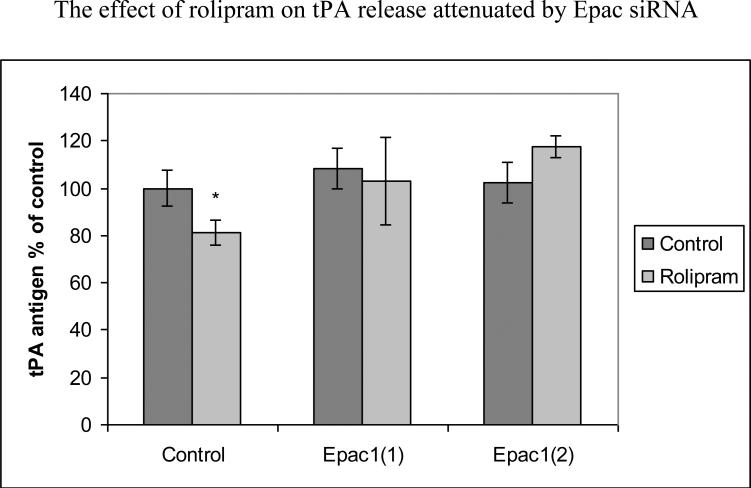
We also pretreated HBEC with Epac inducer 8CPT-2’OMe-cAMP before OGD. Addition of Epac inducer resulted in 76±13% reduction compare to normoxia control (p<0.001) or 71±13% (p<0.001) reduction compared to OGD alone (Fig. 1b). We then transfected HBECs with Epac1 siRNA before treatment with rolipram. For Epac1 transfection, efficiency was 44%±7% of control (p<0.001) for siRNA1 and 42%±13% of control (p<0.001) for siRNA2. As shown in figure 1c, after 48-hour incubation with rolipram, HBECs transfected with nonsense siRNA reduced tPA release to 81±5% of control (p<0.05) while those tranfected with Epac1 siRNA showed no significant reduction of tPA release. Co-incubation of either PKA inhibitor KT5720 or H89 with rolipram did not inhibit effects on tPA release by rolipram; effectiveness of these PKA inhibitors was confirmed by Western blot indicating decreased expression of forskolin-induced endothelial phosphorylated CREB (data not shown) [10].
Fig 1b.
Effect of Epac inducer on tPA release by HBECs co-cultured with astrocytes for 42 hrs and then subjected to 6 hours of OGD. Treatment was with Epac inducer 8CPT-2'OME-cAMP. Pooled data from three independent experiments performed in triplicate. Mean with error bars representing standard deviation. *** p<0.001
Fig 1c.
The reduction of tPA release produced by rolipram was mediated by Epac pathway. HBECs were transfected with Epac1 siRNA and then treated with rolipram , Pooled data from three independent experiments. Mean with error bars representing standard deviation. *p<0.05
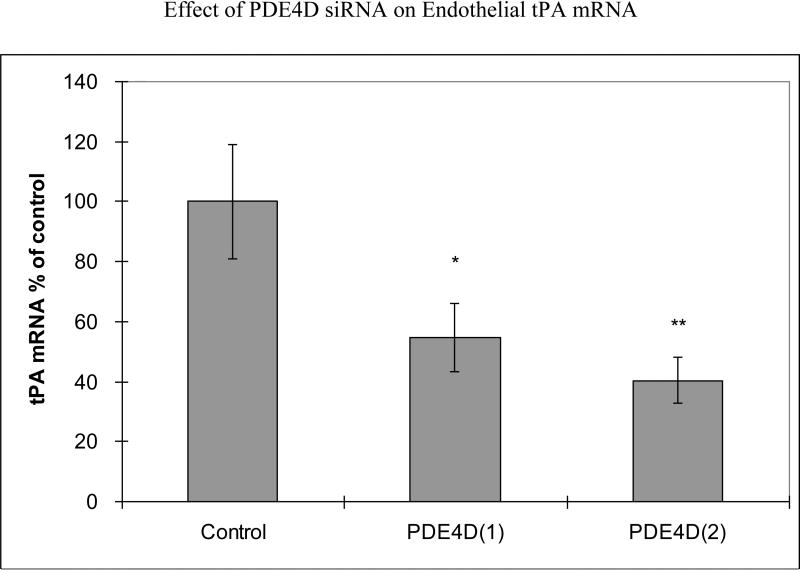
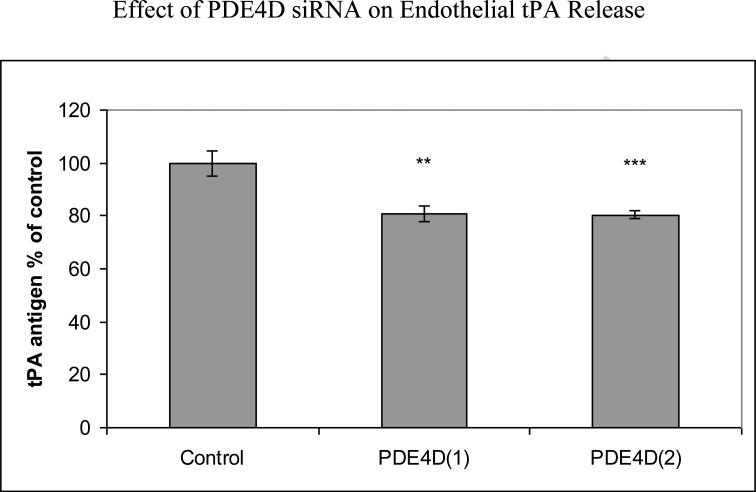
We performed additional studies to analyze effects of specific inhibition of PDE4D gene on tPA expression. Transfection with two different PDE4D siRNAs produced PDE4D mRNA 31%±6% of control (a nonsense siRNA) (p<0.001) for siRNA1 and 30%±3% of control (p<0.001) for siRNA2. 48 hours following PDE4D siRNA transfection of HBECs, tPA mRNA was reduced to 55%±11% of control (p<0.05) for siRNA1 and to 40%±8% of control (p<0.01) for siRNA2 (Fig 2a). Using lamin siRNA had no effect on tPA expression (data not shown). PDE4D siRNA transfection significantly decreased tPA protein release to 81%±3% of control for siRNA1 (p<0.01) and 80%±2% of control for siRNA2 (p<0.001) (Fig 2b).
Fig 2a.
Effects of PDE4D siRNA transfection on tPA mRNA. HBECs were transfected with PDE4D siRNAs for 48 hours. Pooled data from three independent experiments. Mean with error bars representing standard deviation. *p<0.05, **p<0.01
Fig 2b.
Reduction of tPA release produced by PDE4D siRNA transfection. HBECs were transfected with PDE4D siRNAs for 48 hours. Pooled data from three independent experiments. Mean with error bars representing standard deviation **p<0.01, ***p<0.001
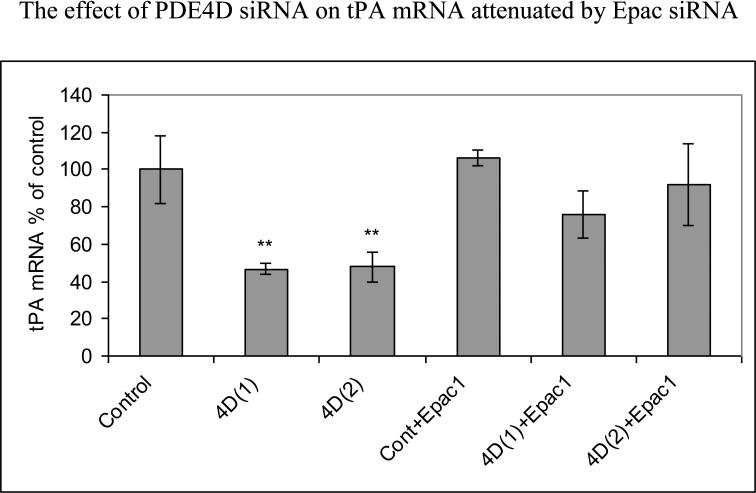
To study mechanisms of tPA mRNA inhibition, PDE4D-siRNA-transfected HBECs were co-transfected with Epac1 siRNA. Initial transfection efficiency studies using two different PDE4D siRNAs showed that PDE4D(1) and Epac1 co-transfection produced PDE4D mRNA 36%±19% of control (p<0.001) and Epac1 mRNA 44%±6% of control (p<0.001); in addition, PDE4D(2) and Epac1 co-transfection produced PDE4D mRNA 37%±11% of control (p<0.001) and Epac1 mRNA 52%±9% of control (p<0.001). This co-transfection substantially attenuated effects of PDE4D siRNA: 48 hours following transfection, tPA mRNA was reduced to 47%±3% of control (p<0.01) for PDE4D(1) siRNA transfection and to 48%±8% of control (p<0.01) for PDE4D(2) siRNA transfection. When co-transfected with Epac1 siRNA and two PDE4D siRNAs, there was no significant change of tPA mRNA compared to control (Fig 2c).
Fig 2c.
The reduction of tPA mRNA produced by PDE4D siRNA was mediated by Epac1 pathway. HBECs were transfected with PDE4D siRNAs and co-transfected with Epac1 siRNAfor 48 hours. PDE4D siRNA transfection alone showed significant reduction of tPA mRNA while PDE4D and Epac1 siRNA co-transfection showed no significant change of tPA mRNA. Pooled data from three independent experiments. Mean with error bars representing standard deviation. **p<0.01
DISCUSSION
We demonstrated that the PDE4 inhibitor rolipram decreased endothelial tPA release in an in vitro ischemia model. Moreover, inhibition of PDE4D isoform reduced endothelial tPA expression. Furthermore, activating Epac mimicked the effect of rolipram, while inhibiting Epac attenuated effects of rolipram and PDE4D siRNA. This suggests regulation of brain microvascular tPA expression by PDE4/PDE4D is mediated by Epac.
Epac is a cAMP effector that regulates a wide range of biological processes, including endothelial barrier properties and vascular permeability [7, 11]. Epac exists in two isoforms, Epac1 and Epac2. Epac1 is the isoform most abundant in vascular endothelium [7] and was targeted by our siRNA. We also used cAMP analogue 8CPT-2’OMe-cAMP to activate Epac; this analogue is known to activate Epac1 [7], with uncertain effects on Epac2. Previous work has shown Epac1 activation reduces permeability in human umbilical vein endothelial cells, possibly by modifying cytoskeleton and cadherin junctions [12, 13]. Our study suggests a new role for Epac, mediating and regulating brain endothelial tPA expression.
Studies on regulation of fibrinolysis by cAMP modifying agents are limited and yielded inconsistent results. This is possibly due to the different cell lines, species, timeframes, and the cAMP modifying agents used in those studies. For example, forskolin (elevating global cAMP levels) increased tPA release within 30 minutes in human umbilical vein endothelial cells [14], but decreased fibrinolytic activity in human thyroid follicular cells between 7 and 11 days [15]. It is understood that A-kinase anchoring proteins (AKAPs) facilitate the formation of cAMP signaling complex by interacting with PDE, PKA, and Epac [7, 11, 16]. This allows for local cAMP regulation, resulting in subcellular compartmentalization of and distinct signal transduction by cAMP [7, 11, 16]. We used rolipram, a specific PDE4 inhibitor, and PDE4D specific siRNA. Thus, our study focused on PDE4 inhibition rather than global cAMP change induced by forskolin. In addition, our study focused on relatively long-term, human brain regulation of fibrinolysis rather than acute tPA release. Although limited by in vitro conditions which are inherently different from those in vivo, our study used co-culture systems to simulate cell-cell interactions in vivo. Moreover, most cAMP analogues, including Epac inducer 8CPT-2’OMe-cAMP, have multiple targets [17]. Incorporation of Epac1 siRNA into the experimental paradigm supports the specificity of our findings.
The cAMP pathway has a major impact on blood-brain barrier formation [18] and inhibition of PDE4 adversely impacts outcome in experimental stroke [19]. A relationship between modulation of PDE4 pathways and stroke risk has nevertheless remained obscure. The association described herein between tPA expression and PDE4 inhibition may help shed light on this relationship and, given the established role of tPA pharmacotherapy in stroke treatment, its importance in stroke prevention. In view of its relatively small effects on in vitro tPA release, inhibition of PDE4 is more likely to have an incremental rather than transformative effect on stroke pathogenesis.
In conclusion, PDE4/PDE4D regulates brain microvascular tPA expression and release in vitro. This regulation is mediated by Epac. Targeting PDE4/PDE4D or Epac may represent a novel strategy for modifying brain-specific microvascular fibrinolysis.
ACKNOWLEDGMENT
This research was supported by National Institutes of Health research grant NS20989.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Macko RF, Ameriso SF, Gruber A, Griffin JH, Fernandez JA, Barndt R, et al. Impairments of the protein C system and fibrinolysis in infection-associated stroke. Stroke. 1996;27:2005–11. doi: 10.1161/01.str.27.11.2005. [DOI] [PubMed] [Google Scholar]
- 3.Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet. 2003;35:131–8. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- 4.Liao YC, Lin HF, Guo YC, Yu ML, Liu CK, Juo SH. Sex-differential genetic effect of phosphodiesterase 4D (PDE4D) on carotid atherosclerosis. BMC Med Genet. 2010;11:93. doi: 10.1186/1471-2350-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meschia JF. New information on the genetics of stroke. Curr Neurol Neurosci Rep. 2011;11:35–41. doi: 10.1007/s11910-010-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumer Y, Spindler V, Werthmann RC, Bunemann M, Waschke J. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol. 2009;220:716–26. doi: 10.1002/jcp.21819. [DOI] [PubMed] [Google Scholar]
- 7.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–75. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 8.Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol. 2008;87:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Lee DH, Lee HR, Shin HK, Park SY, Hong KW, Kim EK, et al. Cilostazol enhances integrin-dependent homing of progenitor cells by activation of cAMP-dependent protein kinase in synergy with Epac1. J Neurosci Res. 2011;89:650–660. doi: 10.1002/jnr.22558. [DOI] [PubMed] [Google Scholar]
- 10.Sharma N, Lopez DI, Nyborg JK. DNA binding and phosphorylation induce conformational alterations in the kinase-inducible domain of CREB. Implications for the mechanism of transcription function. J Biol Chem. 2007;282:19872–83. doi: 10.1074/jbc.M701435200. [DOI] [PubMed] [Google Scholar]
- 11.Grandoch M, Roscioni SS, Schmidt M. The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br J Pharmacol. 2010;159:265–284. doi: 10.1111/j.1476-5381.2009.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehrawat S, Cullere X, Patel S, Italiano J, Jr, Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19:1261–1270. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 14.Heggman R, van den Eijnden-Schrauwen Y, Emeis J. Adenosine 3':5'-cyclic monophosphate induces regulated secretion of tissue-type plasminogen activator and von Willebrand factor from cultured human endothelial cells. Thromb Haemost. 1998;79:853–858. [PubMed] [Google Scholar]
- 15.Susarla R, Watkinson J, Eggo M. Regulation of plasminogen activators in human thyroid follicular cells and their relationship to differentiated function. J Cell Physiol. 2007;212:643–54. doi: 10.1002/jcp.21060. [DOI] [PubMed] [Google Scholar]
- 16.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 18.Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, et al. A cell culture model of the blood-brain barrier. J.Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukada H, Fukumoto D, Nishiyama S, Sato K, Kakiuchi T. Transient focal ischemia affects the cAMP second messenger system and coupled dopamine D1 and 5-HT1A receptors in the living monkey brain: a positron emission tomography study using microdialysis. J Cereb Blood Flow Metab 2004. 2004;24:898–906. doi: 10.1097/01.WCB.0000126974.07553.86. [DOI] [PubMed] [Google Scholar]