Abstract
We investigated the temporal and spatial expression of SK2 in the developing mouse hippocampus using molecular and biochemical techniques, quantitative immunogold electron microscopy and electrophysiology. The mRNA encoding SK2 was expressed in the developing and adult hippocampus. Western blotting and immunohistochemistry showed that SK2 protein increased with age. This was accompanied by a shift in subcellular localization. Early in development (P5), SK2 was predominantly localized to the endoplasmic reticulum in the pyramidal cell layer. But by P30 SK2 was almost exclusively expressed in the dendrites and spines. The level of SK2 at the postsynaptic density (PSD) also increased during development. In the adult, SK2 expression on the spine plasma membrane showed a proximal-to-distal gradient. Consistent with this redistribution and gradient of SK2, the selective SK channel blocker apamin increased evoked excitatory postsynaptic potentials (EPSPs) only in CA1 pyramidal neurons from mice older than P15. However, the effect of apamin on EPSPs was not different between synapses in proximal or distal stratum radiatum or stratum lacunosum-moleculare in adult. These results show a developmental increase and gradient in SK2-containing channel surface expression that underlie their influence on neurotransmission, and that may contribute to increased memory acquisition during early development.
Introduction
CA1 pyramidal neurons are required for hippocampal information processing (Spruston, 2008). They receive excitatory input onto their dendrites from several other classes of pyramidal cells, within the hippocampus as well as from the entorhinal cortex, and they are the primary output of the hippocampus. Synaptic plasticity in CA1 pyramidal neurons is thought to play a major role in hippocampus-dependent memory encoding (Spruston, 2008), and dysfunction of CA1 pyramidal neurons is implicated in pathologies such as epilepsy and Alzheimer’s disease (McCormick and Contreras, 2001; Ondrejcak et al., 2010). These functions depend on electrical signals whose generation, patterns, and propagation are largely determined by synaptic receptors and ion channels.
SK channels are one type of ion channel shaping the synaptic responses of CA1 pyramidal neurons. SK channels are activated by submicromolar concentrations of cytosolic Ca2+ and blocked by the peptide toxin apamin (Kohler et al., 1996; Ngo-Anh et al., 2005; Luján et al., 2009). Blocking SK channels with apamin facilitates the induction of synaptic plasticity and systemic application of apamin facilitates hippocampus-dependent memory encoding (Stackman et al., 2002). Three mammalian SK channel genes (SK1, SK2, and SK3) are expressed in the rodent hippocampus (Stocker and Pedarzani, 2000). SK2 expression in CA1 pyramidal neurons is necessary for the apamin sensitive current measured in voltage clamp (Bond et al., 2004) and overexpression of SK2 diminishes synaptic plasticity and hippocampus-dependent memory encoding (Hammond et al., 2006) suggesting that SK2-containing channels account for the physiological roles of SK channels in CA1 pyramidal neurons. These roles for SK2-containing channels reflect, at least in part, localization to dendritic spines in young adult mice (Lin et al., 2008) where SK2 containing channels are activated by synaptically evoked Ca2+ entry and modulate synaptic responses (Ngo-Anh et al., 2005; Bloodgood and Sabatini, 2007; Lin et al., 2008, 2010). However, the developmental profiles of SK2 expression and channel function, as well as the precise subcellular distribution during development remain unknown. Here we used molecular, biochemical, electron microscopic, and electrophysiological approaches to determine the expression profile, surface distribution, and function of SK2-containing channels during postnatal development. Our findings show that synaptic localization and function are acquired during postnatal development and reveal a gradient of SK2-containing channels along the somato-dendritic domains of CA1 pyramidal neurons in the adult.
Material and Methods
Tissue Preparation
OF-1 mice from the day of birth (P0) to adulthood obtained from the Animal House Facility of the School of Medicine of the University of Castilla-La Mancha were used in this study for qPCR, western blots and pre-embedding immunohistochemical analyses. The care and handling of the animals prior to and during the experimental procedures followed Spanish and European Union regulations, and were approved by the Animal Care and Use Committee of the institution. For each developmental stage, the animals used were from different litters.
For RT-PCR and immunoblotting, animals were deeply anaesthetized by hypothermia (P0-P5) or by intraperitoneal injection of ketamine-xylazine 1:1 (0.1 ml/kg b.w.) and the brains were quickly frozen. For immunohistochemistry, animals were anaesthetized and transcardially perfused with ice-cold fixative containing 4% paraformaldehyde, with or without 0.05% glutaraldehyde and 15% (v/v) saturated picric acid made up in 0.1 M phosphate buffer (PB, pH 7.4). After perfusion, brains were removed and immersed in the same fixative for 2 hours or overnight at 4°C. Tissue blocks were washed thoroughly in 0.1 M PB. Coronal 60 μm thick sections were cut on a Vibratome (Leica V1000).
Antibodies and chemicals
The characteristics and specificity of the antibodies anti-SK2 have been described elsewhere (Lin et al., 2008; Cueni et al., 2008). An affinity-purified polyclonal antibody against GluN1 was raised in rabbit and characterized previously (Watanabe et al., 1999). The monoclonal antibody against α-tubulin was obtained from Calbiochem (Germany). The monoclonal antibody against PSD95 was obtained from Abcam (Cambridge, UK). Antibodies against calretinin and calbindin were from Chemicon (Temecula, CA, USA).
CGP55845, and SR95531 were obtained from Tocris Cookson (Ellisville, MO). Apamin was from Calbiochem (La Jolla, CA). All perfusing solutions were modified from regular aCSF unless otherwise noted.
Real-time quantitative PCR
RNA was extracted using an RNA isolation kit (Qiagen, Duesseldorf, Germany). The integrity of RNA was verified by agarose 1% gel and cDNA was synthesized from 1 μg of total RNA primed with oligo(dT) and random hexamers using M-MuLVRT (Fermentas, Burlington, Ontario, Canada). Real Time PCRs were performed in triplicate. Gene-specific primers were designed with Primer Express 3.0 (Applied Biosystems), as follows. KCNN2 (SK2) (GenBank # NM_080465.2): forward 5′-GTCGCTGTATTCTTTAGCTCTG -3′; reverse 5′-ACGCTCATAAGTCATGGC-3′. GAPDH (GenBank #MM_008084.2): forward 5′-TGTGTCCGTCGTGGATCTGA-3′; reverse 5′-CCTGCTTCACCACCTTCTTGAT-3′. All PCR products span an intron in the genomic DNA. The cDNA was amplified with Fast SYBR® Green Master Mix (Applied Biosystems, Foster City, CA, USA). PCR conditions were: 20 min at 95°C, followed by 40 cycles consisting of 95°C for 15 s and 60°C for 30 s in a StepOne Real-Time PCR System (Applied Biosystems). A melting curve confirmed the specificity of PCR products. The efficiencies of the primer pairs were tested following the equation: E= 10 [−1/slope] (Pfaffl, 2001) where the slope was determined by a standard curve for each transcript. Relative quantification of mRNA levels was performed using the comparative Ct method 2−ΔΔCt. GAPDH, which did not vary during development, was amplified as an internal control. Statistical data for each transcript were determined in triplicate per genotype and each reaction was done in triplicate wells. Significance was determined by 1-way ANOVA of ΔCt values across all genotypes followed by Tukey’s test (GraphPad Prism, La Jolla, CA, USA).
Western blots
Hippocampi were homogenized in 320 mM sacarose, 2 mM EDTA, 10 mM HEPES, pH 7.4, and Protease Inhibitor Cocktail (Sigma-Aldrich) with a pestle motor (Sigma-Aldrich). The homogeneized tissue was centrifuged at 27,000 × g at 4°C and the supernatant was ultracentrifuged at 150,000 × g at 4°C (Beckman Coulter Optima L-90K Ultracentrifuge, CA, USA) using rotor SW40Ti (Beckman). Hundred micrograms of membrane protein was prepared as Western blots and probed with anti-SK2 (1:400). Protein bands were visualized after application of goat anti-rabbit secondary antiserum coupled to horseradish peroxidase (1:3000) using the ECL blotting detection kit (SuperSignal West Dura, Pierce, Rockford, USA). Blots were quantified by densitometry using a LAS4000 MINI (Fujifilm, Japan). A series of primary and secondary antibody dilutions and incubation times were used to optimize the experimental conditions for the linear sensitivity range, confirming that our labeling was well below saturation levels.
Immunohistochemistry for light microscopy
Immunohistochemical reactions at the light microscopic level were carried out using the immunoperoxidase method as described earlier (Luján et al., 1996). Briefly, sections were incubated in 10% normal goat serum (NGS) diluted in 50 mM Tris buffer (pH 7.4) containing 0.9% NaCl (TBS), with 0.2% Triton X-100, for 1 h. Sections were incubated in anti-SK2 (1–2 μg/ml diluted in TBS containing 1% NGS), followed by incubation in biotinylated goat anti-rabbit IgG or anti-guinea pig IgG (Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in TBS containing 1% NGS. Sections were then transferred into avidin-biotin-peroxidase complex (ABC kit, Vector Laboratories). Bound peroxidase enzyme activity was revealed using 3, 3′-diaminobenzidine tetrahydrochloride (DAB; 0.05% in TB, pH 7.4) as the chromogen and 0.01% H2O2 as the substrate. Finally, sections were air-dried and mounted prior to observation in a Nikkon photomicroscope (Nikkon, Eclipse 80i) equipped with differential interference contrast optics and a digital imaging camera.
Immunohistochemistry for electron microscopy
Immunohistochemical reactions at the electron microscopic level were carried out using the immunogold methods as described earlier (Luján et al., 1996). Ultrastructural analyses were performed in a Jeol-1010 electron microscope.
Pre-embedding immunogold method
Briefly, free-floating sections were incubated in 10% NGS diluted in TBS. Sections were then incubated in anti-SK2 antibodies (3–5 μg/ml diluted in TBS containing 1% NGS), followed by incubation in goat anti-rabbit IgG or goat anti-guinea pig IgG coupled to 1.4 nm gold (Nanoprobes Inc., Stony Brook, NY, USA) diluted 1:100. Sections were postfixed in 1% glutaraldehyde and washed in double distilled water, followed by silver enhancement of the gold particles with a HQ Silver kit (Nanoprobes Inc.). Sections were then treated with osmium tetraoxide (1% in 0.1 M PB), block-stained with uranyl acetate, dehydrated in graded series of ethanol and flat-embedded on glass slides in Durcupan (Fluka) resin. Regions of interest were cut at 70–90 nm on an ultramicrotome (Reichert Ultracut E, Leica, Austria) and collected on 200-mesh copper grids. Staining was performed on drops of 1% aqueous uranyl acetate followed by Reynolds’s lead citrate.
Post-embedding immunogold method
Briefly, ultrathin sections 80-nm thick from Lowicryl-embedded blocks of hippocampus were picked up on coated nickel grids and incubated on drops of a blocking solution consisting of 2% Human Serum Albumin (HSA) in 0.05 M TBS and 0.03% Triton X-100 (TBST). The grids were incubated with SK2 antibodies or GluN1 antibodies (10 μg/ml in TBST with 2% HSA) at 28°C overnight. The grids were incubated on drops of goat anti-guinea pig IgG or goat anti-rabbit IgG conjugated to 10 nm colloidal gold particles (Nanoprobes) in 2% HSA and 0.5% polyethylene glycol in TBST. The grids were then washed in TBS and counterstained for electron microscopy with saturated aqueous uranyl acetate followed by lead citrate.
SDS-FRL technique
SDS-FRL was performed with some modifications to the original method described by Fujimoto (1995). Animals (C57BL mice) were anesthetized with sodium pentobarbital and subjected to transcardiac perfusion with formaldehyde (0.5%; freshly depolymerized from paraformaldehyde) in 0.1 M sodium phosphate buffer. The hippocampi were dissected, cut into sections 120 μm-thick by a Microslicer (Dosaka, Kyoto, Japan) and replicas were obtained as described (Tarusawa et al., 2009) using a high pressure freezing machine (HPM010, BALTEC) and freeze-fracture machine (BAF060, BALTEC). Replicas were transferred to 2.5% SDS containing 0.0625 M Tris and 10% glycerol (pH 6.8) for 16 h at 80°C with shaking, and then washed and reacted with a mixture of polyclonal guinea-pig antibody for SK2 and monoclonal mouse antibody for PSD-95 at 15°C overnight. Following three washes in 0.1% BSA in TBS and blocking in 5% BSA/TBS, replicas were incubated in a mixture of anti-guinea pig and anti-mouse secondary antibodies coupled to 10 and 5nm gold particles, respectively (British Biocell International, UK) overnight at 4°C. When one of the primary antibodies was omitted, no immunoreactivity for the omitted primary antibody was observed. After immunogold labeling, the replicas were immediately rinsed three times with 0.1% BSA/TBS, washed twice with distilled water, and picked up onto grids coated with pioloform (Agar Scientific, Stansted, Essex, UK). Specificity of SDS-FRL immunolabeling was confirmed in samples from SK2 null mice (Lin et al., 2008).
Semi-quantitative analysis of SK2 channel immunoreactivity during development
To establish the relative abundance of SK2 channel immunoreactivity in CA1 pyramidal cells during development, semi-quantification of immunolabeling was carried out in three different ways:
To determine the abundance of SK2 channel immunoreactivity in different compartment of pyramidal cells during development, we used 60-μm coronal slices processed for pre-embedding immunogold immunohistochemistry. The procedure was similar to that used previously (Luján et al., 1996; Luján and Shigemoto, 2006). Briefly, for each of three animals from different postnatal ages and adult, three samples of tissue were obtained for preparation of embedding blocks (totaling nine blocks for each age). To minimize false negatives, ultrathin sections were cut close to the surface of each block. We estimated the quality of immunolabeling by always selecting areas with optimal gold labeling at approximately the same distance from the cutting surface. Randomly selected areas were then photographed from the selected ultrathin sections at a final magnification of 45,000X. Semi-quantification of immunogold labeling was carried out in reference areas totaling approx. 2,000 μm2 for each age. Immunoparticles identified in each reference area and present along the plasma membrane and intracellular sites in dendritic spines were counted.
To establish the relative abundance of SK2 and GluN1 immunoreactivity at synaptic sites during postnatal development, semi-quantification of immunolabeling at excitatory synapses was performed in the distal part of the stratum radiatum from 80 nm ultrathin sections obtained from Lowicryl-embedded blocks. Only synapses made by axon terminals with CA1 pyramidal cell spines were evaluated for the number of gold particles per synapse (both labeled and unlabelled) or number of gold particles per labeled synapse; labeled synapses had one or more gold particles. Synapses were only included in the analysis if the synaptic cleft was visible.
To establish the density of SK2 at extrasynaptic sites in dendritic spines of CA1 pyramidal cells during postnatal development, semi-quantification of immunolabeling was performed from 60-μm coronal slices processed for pre-embedding immunogold in four different layers: stratum pyramidale, the proximal stratum radiatum (defined as the portion among the 100 μm away from the stratum pyramidale), the distal stratum radiatum (defined as the portion among the 100 μm away from the border of the stratum lacunosum-moleculare) and the stratum lacunosum-moleculare. Dendritic shafts were not included in the analysis. For each of three animals from different postnatal ages and adult, three samples of tissue were obtained. Randomly selected areas were then photographed from the selected ultrathin sections at a final magnification of 45 000X. Semi-quantification of immunogold labeling was carried out in reference areas totaling approx. 2,000 μm2 for each age. Immunoparticles identified in dendritic spines were counted and the cross-sectioned spine area was measured. The data (density of SK2 in spines in each CA1 layer at each postnatal age) was expressed as number of immunoparticles per square micron. We calculated the non-specific labeling density in every reaction in the nuclei of pyramidal cells, a subcellular compartment that should not contain any SK2. The immunoparticle density over the nuclei was 0.05 ± 0.01 immunoparticles/μm2.
Controls
To test method specificity in the procedures for western blots, as well as for both light and electron microscopy, antiserum against SK2 was tested on brain slices of mice lacking the SK2 channel subtype (Lin et al., 2008; Cueni et al., 2008). Furthermore, the primary antibody was either omitted or replaced with 5% (v/v) normal serum of the species of the primary antibody. Under these conditions, no specific labeling was observed. Staining patterns were also compared to those obtained by calretinin and calbindin; only antibodies against SK2 and GluN1 labeled consistently the plasma membrane.
Slice preparation
All procedures were performed in accordance with the guidelines of Oregon Health & Science University and University of Castilla-La Mancha, Albacete, Spain. Hippocampal slices were prepared from C57BL/6J male or female mice, as previously described (Lin et al., 2008; Wu et al., 2008). Briefly, animals were anesthetized with isoflurane and decapitated. The cerebral hemispheres were quickly removed and placed into cold artificial CSF and equilibrated with carbogen (95%O2/5%CO2). Hippocampi and cortex were removed, placed onto an agar block, and transferred into a slicing chamber containing sucrose-ACSF (in mM): 70 sucrose, 80 NaCl, 2.5 KCl, 21.4 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 1.3 ascorbic acid, 20 glucose and equilibrated with carbogen. Transverse hippocampal slices (300–350 μm) were cut with Leica VT1000s or VT1200s (Leica Instruments, Nussloch, Germany) and transferred into a holding chamber containing regular ACSF (in mM): 125 NaCl, 2.5 KCl, 21.5 NaHCO3, 1.25 NaH2PO4, 2.0 CaCl2, 1.0 MgCl2, 15 glucose and equilibrated with carbogen. Slices were incubated at 34°C for 30 min and then at room temperature for ≥1 hrs before recordings were performed.
Electrophysiology
For synaptically evoked recordings, CA1 pyramidal cells were visualized with IR/DIC optics (Leica DMLFS) and a CCD camera. Whole-cell patch-clamp recordings were obtained from CA1 pyramidal cells using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), digitized using an ITC-16 analog-to-digital converter (Heka Instruments Inc., Bellmore, NY) and transferred to a computer using Pulse software (Heka Instruments Inc., Bellmore, NY). Patch pipettes (open pipette resistance, 2–4 MΩ) were filled with a solution containing (in mM): 130 K-gluconate, 8 NaCl, 1 MgCl2, 10 HEPES, 4 ATP, 0.3 GTP, and 10 phosphocreatine, pH 7.26. Series resistance was not electronically compensated and recordings with series resistance that changed more than 20% during the experiment were discarded. Electrophysiological records were filtered at 5 kHz and sampled at 20 kHz. The input and series resistance was determined from a ~30 pA (500 ms) hyperpolarizing current injection pulse interspersed between events. All recordings were from cells with a resting membrane potential between −70 and −50 mV, and a stable input resistance. For EPSP measurements in current clamp mode, a bias current was applied to maintain the membrane potential at −60 mV. All electrophysiological recordings were performed at room temperature (20–21°C).
Synaptic stimulation
Excitatory postsynaptic potentials (EPSPs) were recorded in whole-cell mode. Capillary glass pipettes filled with ACSF, with a tip diameter of ~5 μm, connected to an Iso-Flex stimulus isolation unit (A.M.P.I., Israel) were used to stimulate presynaptic axons in stratum radiatum as described in results. For distance dependence studies (Fig. 10), stimulating electrode was placed at 10–20 μm, and ~100–120 μm away from the soma to stimulate pSR and dSR, respectively. In DIC, the color reflection of hippocampal slices changes from SR to SLM regions and stimulating electrodes were placed in the darker SLM region to stimulate the perforant pathway, ~200 μm away from the soma. Stimulus intensity of ~5–90 μA was used to evoke ~1–3 mV EPSPs. Time constants for the rising phase of the EPSP were determined from fits of a sum of a rising and decaying exponential function. SR95531 (2 μM) and CGP55845 (1 μM) were present to reduce GABAA and GABAB receptor contributions, respectively. To prevent epileptic discharges in the presence of GABAergic blockers, the CA3 region was microdissected out before recording.
Data analysis
Statistical analyses for morphological data were performed using SigmaStat Pro (Jandel Scientific) and data were presented as mean ± SEM. Statistical significance was defined as P < 0.05, as determined using ANOVA followed by the Bonferroni test for multiple comparisons. For the electron microscopic data, statistical significance in the distribution of gold particles among samples was assessed with the Kolmogorov–Smirnov non-parametric test.
Electrophysiological data were analyzed using IGOR (WaveMetrics, Lake Oswego, OR, USA). Data are expressed as mean ± SEM. Paired two sample t-tests or non-parametric Wilcoxon signed rank test were used to determine significance of data; P < 0.05 was considered significant.
Results
Developmental profile of SK2 mRNA and protein in the hippocampus
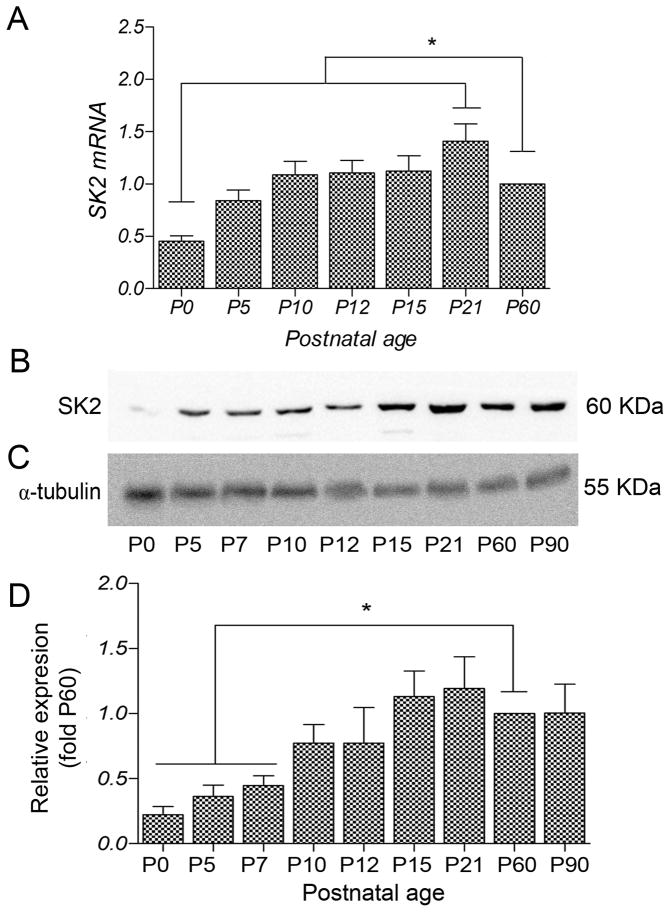
The steady state expression profile for SK2 mRNA was determined during postnatal development in the hippocampus using quantitative real-time PCR (qRT-PCR). The results showed that SK2 mRNA increased from its lowest at P0 to a peak at P21 and then declined slightly by P60 (Fig. 1A).
Figure 1.
Expression profile of SK2 mRNA (A) and quantitative immunoblot analysis of SK2 protein expression (B-D) in mouse hippocampus during postnatal development. The qPCR measurements from three animals were averaged to compare the mRNA level for each age. (A) SK2 transcripts were expressed at low levels at P0 and then progressively increased during development. Error bars indicate SEM; *, p < 0.05 compared to P60. (B) Using western blots, the SK2 antibody recognized a band of ~50 kDa. (C) The band for α tubulin was used as a control for normalizing SK2 expression at different developmental ages. (D) During postnatal development, SK2 protein was detected at birth, increased 3-fold between P10 and P15, and then reached a plateau. Densitometry measurements from nine independent experiments were averaged for each developmental age and normalized to P60. Error bars indicate SEM. *, p < 0.05.
To determine the developmental profile of SK2 protein expression Western blots were prepared using equal amounts of membrane protein obtained from hippocampi of individual mice at different developmental ages. Probing the blots with SK2 antibody labeled a band of ~50 KDa at each developmental age (Fig. 1B), consistent with the size of SK2 (Strassmaier et al., 2005) and showed that SK2 expression increases after birth, reaching steady state by P15 (Fig. 1B,D). Densitometry measurements from six different experiments were averaged to compare protein expression at each age and revealed a 2-fold increase in SK2 protein expression between ages P7 and P60 and a 2.5-fold increase in protein expression between ages P10 and P15. In the adult (P60-P90), SK2 expression was similar to that detected at P15-P21 (Fig. 1D). Statistical comparisons and significance (p < 0.05) between postnatal ages are shown in Table 2.
Table 2.
Summary of immunogold labeling for synaptic SK2 channels and NMDARs during postnatal development.
| P5 | P15 | P30 | ||
|---|---|---|---|---|
| SK2 | Number of immunoparticles | 20 | 55 | 90 |
| Number of synapses | 97 | 99 | 102 | |
| % Synapses labeled | 20% | 39% | 52% | |
| Immunoparticles/labeled synapse# | 1.1 ± 0.1 | 1.4 ± 0.1 | 1.7± 0.1 | |
| GluN1 | Number of immunoparticles | 98 | 116 | 124 |
| Number of synapses | 102 | 103 | 105 | |
| % Synapses labeled | 57% | 58% | 63% | |
| Immunoparticles/labeled synapse | 1.7 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 |
3-way comparison shows each age significantly different from each other age
Developmental shift in the subcellular localization of SK2
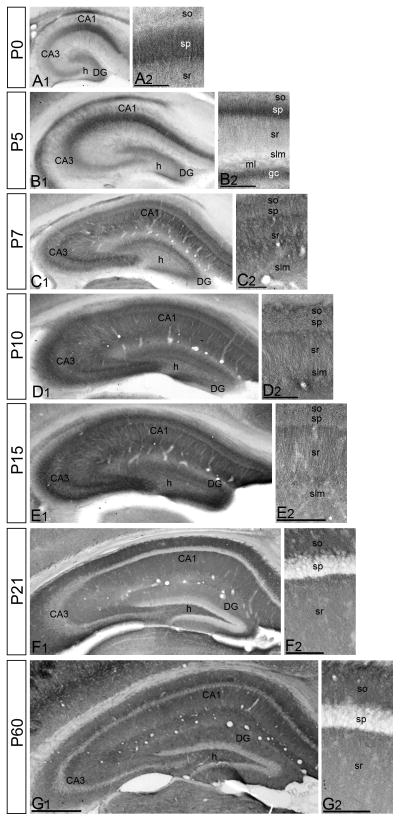
To determine the subcellular profile of SK2 expression during development immunohistochemical studies were performed (Fig. 2). At birth (P0) and at P5, SK2 was expressed in the pyramidal neurons, while very weak labeling was detected in the dendritic layers (Fig. 2A,B). At P7, the distribution pattern of SK2 changed dramatically, showing a decrease in the pyramidal cell layer and strong expression in the neuropile of all dendritic layers (Fig. 2C). During the second (P10) and beginning of the third (P15) postnatal week, the pattern of SK2 immunolabeling was similar but stronger (Fig. 2D,E) compared to P7. However, during the fourth postnatal week (P21), there was a decrease in SK2 immunolabeling throughout the hippocampus (Fig. 2F). Nevertheless, the overall distribution of SK2 did not change from P21 to adulthood (P60). Thus, after the second postnatal week, there was very weak labeling in the pyramidal cell layer of area CA1, but intense labeling in the dendritic layers including the strata oriens, radiatum and lacunosum-moleculare (Fig. 2G).
Figure 2.
Immunoreactivity for SK2 in the hippocampus during postnatal development using a pre-embedding immunoperoxidase method. (A-B) At P0 and P5, immunoreactivity for SK2 was strong in the principal cell layers of all hippocampal areas, and very weak in the dendritic layers. (C) At P7, the distribution pattern of SK2 changed, showing weaker immunoreactivity in the pyramidal cell layer and stronger in the neuropile of all dendritic layers, including the strata oriens (so), radiatum (sr) and lacunosum-moleculare (slm). (D,E) At P10 and P15, a similar distribution for SK2 as at P7 was observed but showing stronger neuropilar labeling in all dendritic layers. (F,G) At 21 and P60, immunoreactivity for SK2 was very week labeling in the pyramidal cell layer (sp) and intense in the neuropile of strata oriens (so), radiatum (sr) and lacunosum-moleculare (slm). Scale bar: A1-G1, 0.5 μm; A2-G2, 0.1 μm.
To investigate in more detail changes in the subcellular localization of SK2 in CA1 pyramidal neurons at different times in postnatal development (P5, P15 and P30) we carried out electron microscopic studies using the pre-embedding and post-embedding immunogold techniques, which allowed us to determine the subcellular localization at extrasynaptic and synaptic sites, respectively.
Extrasynaptic SK2-containing channels
In this pre-embedding study we restricted quantitative analysis to somata and dendritic spines. In spines, this includes all particles not localized within the main body of the postsynaptic density (PSD), but only from its edge to the extrasynaptic membrane. Using the pre-embedding immunogold technique, we verified that SK2 was expressed early in pyramidal cells and that the subcellular localization pattern changes significantly during postnatal development. Distribution in the soma and in spines was quantified at P5, P15 and P30 (Table 1).
Table 1.
Summary of immunogold labeling for extrasynaptic SK2 during postnatal development.
| P5 | PM gold | % PM | Intra gold | % Intra |
|---|---|---|---|---|
| SLM | 13 | 10 | 117 | 90 |
| SR-D | 22 | 15 | 121 | 85 |
| SR-P | 18 | 3 | 126 | 97 |
| Soma | 54 | 2 | 3152 (rER) | 98 |
| P15 | PM gold | % PM | Intra gold | % Intra |
| SLM | 131 | 38 | 217 | 62 |
| SR-D | 133 | 38 | 222 | 62 |
| SR-P | 114 | 33 | 232 | 67 |
| Soma | 74 | 4 | 1921 (rER) | 96 |
| P30 | PM gold | % PM | Intra gold | % Intra |
| SLM | 452 | 64 | 258 | 36 |
| SR-D | 247 | 52 | 233 | 48 |
| SR-P | 129 | 32 | 275 | 68 |
| Soma | 37 | 7 | 477 (rER) | 93 |
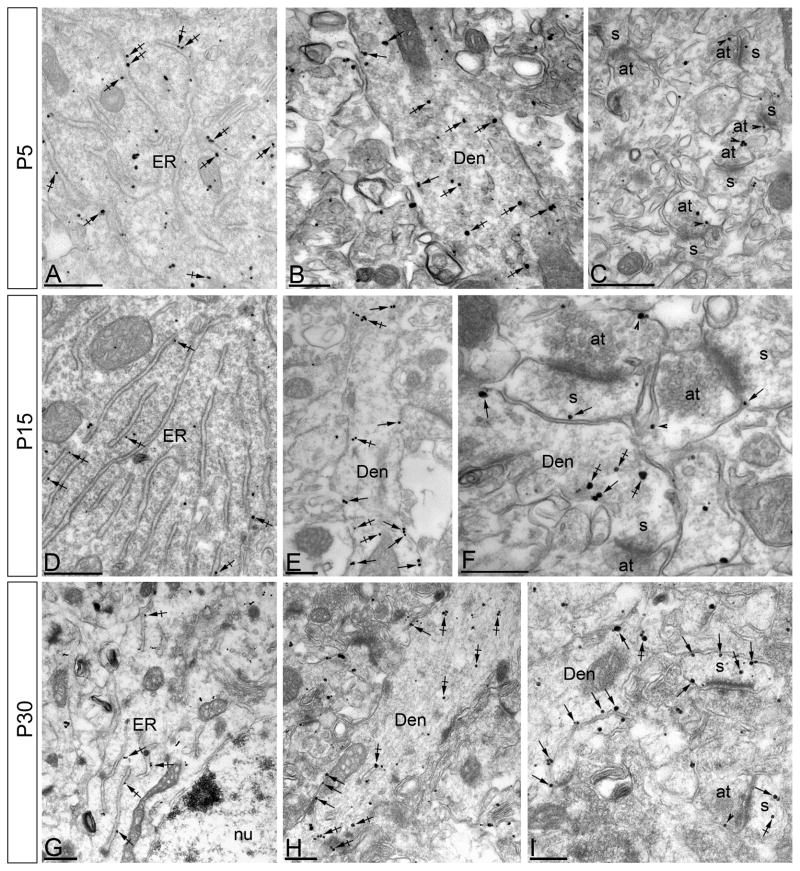
At P5 the majority of immunoparticles were in the soma (88%) and almost all of those (98%) were associated with the rough endoplasmic reticulum (rER) with a few (2%) residing on the plasma membrane (PM) (Fig. 3A). Of the immunoparticles in spines on the apical dendrite in stratum radiatum (SR) (8% of all immunoparticles), most were detected at intracellular sites (86%) and some (14%) were found along the PM of the spines, as well as on the dendritic shafts. Similarly, in spines in stratum lacunosum-moleculare (SLM; 4% of all immunoparticles), most were intracellular (90%) with some gold particles on the spine PM (10%) (Fig. 3B,C). Although relatively few immunoparticles were on the PM (3% of all immunoparticles), 50% of those were on the somatic PM, while 37% (SR) and 13% (SLM) were on the spine PM. At P15, 66% of immunoparticles were in the soma, with 93% of those associated with the rER and 7% on the PM (Fig. 3D). SK2 immmunoparticles were also found on the PM of the apical dendrite (Fig. 3E) and in SR where 23% of all immunoparticles were in spines and 35% of those were on the PM. Similarly, in spines in SLM (11% of all immunoparticles), 38% were on the PM (Fig. 3F). By P30, only 24% of all immunoparticles were in the soma and of those only 7% were on the PM (Fig. 3G). In SR (42% of all immunoparticles), 43% were on the spine PM, while in SLM (34% of all immunoparticles) 64% were on the spine PM (Fig. 3H,I; Table 1).
Figure 3.
Electron micrographs of the CA1 region of the hippocampus showing immunogold particles for SK2 during postnatal development, as detected using a pre-embedding method. (A-C) At P5, immunoparticles for SK2 were mainly associated with the endoplasmic reticulum (ER) in the cytoplasm (crossed arrows) of pyramidal cells. In the stratum radiatum (panels B and C) immunoparticles for SK2 were mainly detected at intracellular sites in the dendritic shafts (Den) of pyramidal cells, and only a few immunoparticles were found along the plasma membrane. (D-F) At P15, fewer immunoparticles for SK2 were mainly detected in the endoplasmic reticulum (ER) (crossed arrows) of pyramidal cells. In the stratum radiatum (panels E and F) immunoparticles for SK2 were mainly detected along the plasma membrane of dendritic shafts (Den, arrows), and only a few along the extrasynaptic plasma membrane (arrows) of dendrites spines (s) but far from the PSD. (G-I) At P30, immunoparticles for SK2 were mainly detected in the endoplasmic reticulum (ER) (crossed arrows) of pyramidal cells, with very few of them being localized along the somatic plasma membrane. In the stratum radiatum (panels H and I) immunoparticles for SK2 were localized along the plasma membrane and intracellular sites of dendritic shafts (Den), but mainly concentrated along the whole extrasynaptic plasma membrane of dendritic spines (s). at, axon terminal; arrowheads indicate immunoparticles for SK2 at presynaptic sites Scale bars: A,C,D, 0.5 μm; B,E-I, 0.2 μm.
Synaptic SK2-containing channels
To determine specifically the developmental expression profile of synaptic SK2-containing channels, we performed quantitative post-embedding immunogold labeling, and examined SK2 at asymmetric, excitatory synapses of CA1 pyramidal neurons in the distal SR during postnatal development (Table 2).
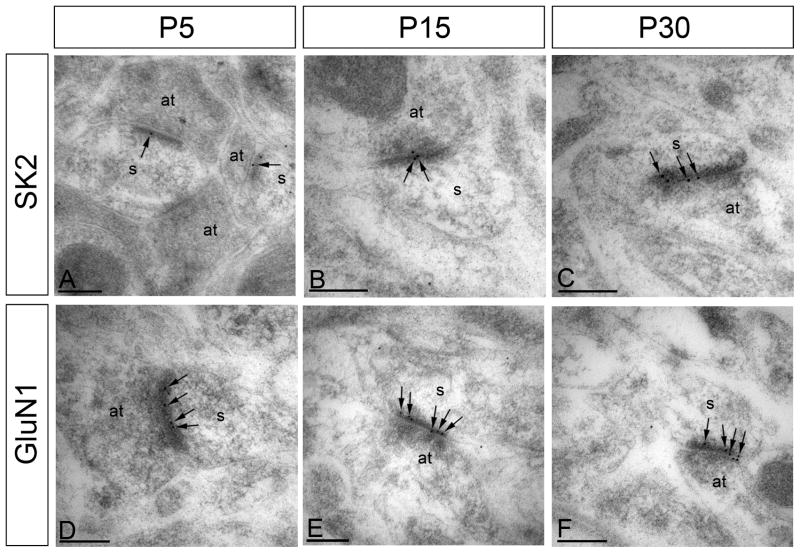
At P5, SK2 immunoparticles were detected at low levels along the PSD (1.1 ± 0.1 immunoparticles/labeled synapse; Fig. 4A; Table 2). Synaptic SK2 immunolabeling increased at P15 (1.4 ± 0.1 immunoparticles/labeled synapse, p < 0.05; Fig. 4B) and was highest at P30 (1.7 ± 0.1 immunoparticles per labeled synapse, p < 0.05; Fig. 4C). Although labeling efficiency is not 100% - some synapses that contain SK2 will not be labeled (see discussion) - the percentage of synapses containing SK2 immunoparticles increased with development, from 20% at P5 to 39% at P15, and to 52% at P30; Table 2). In contrast to SK2 channels, immunolabeling for GluN1, the obligate subunit of NMDARs, did not show significant changes over the ages examined; there were a similar number of gold particles per synapse and percentage of synapses labeled at all ages studied (Fig. 4D–F, Table 2).
Figure 4.
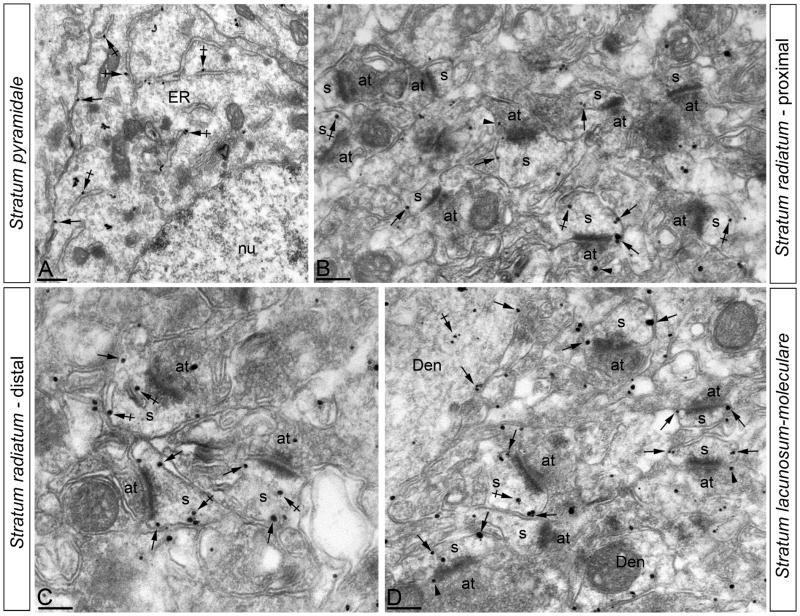
Electron micrographs of the CA1 region of the hippocampus showing immunoparticles for SK2 and GluN1 in the stratum radiatum, as detected using a post-embedding immunogold method at P5, P15 and P30. SK2 and GluN1 were detected along the PSD of dendritic spines (s) of CA1 pyramidal cells establishing asymmetrical synapses with axon terminal (at), likely Schaffer collaterals. (A-C) Immunoreactivity for SK2 at the PSD (arrows) was low at P5, increased at P15 and was high at P30. (D-F) Immunoreactivity for GluN1 at the PSD (arrows) was similar throughout postnatal development. Scale bars: 0.2 μm.
Somato-dendritic gradient of SK2 distribution in pyramidal cells
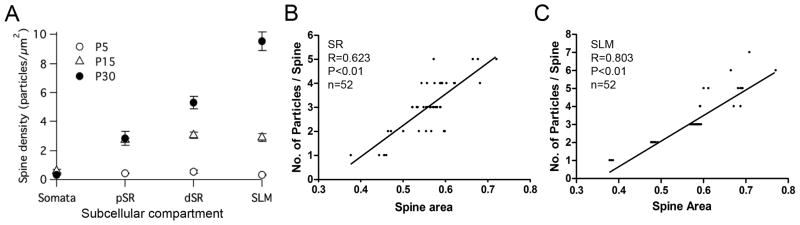
The cell bodies of CA1 pyramidal neurons are arranged in a single layer and the dendrites and dendritic spines of pyramidal neurons in a given portion of the SR or SLM are approximately the same distance away from the parent somata. This laminar arrangement allowed us to investigate changes in the density of SK2 distribution as a function of distance from the soma during postnatal development. Therefore, we analyzed SK2 PM distribution in spines as a function of distance from the soma at P5, P15, and P30 (immunoparticles/μm2; Table 3, Figs. 5 and 6A). At P5, the density of SK2 was low in the soma and spines, and without a gradient of expression. In contrast, at P15, the density of SK2 in the soma remained low but increased significantly in the spines in proximal and distal SR as well as in the SLM, showing similar values among them. In mature (P30) CA1 neurons, the density of SK2 on the soma remained low (Fig. 5A) but in the SR, the density of SK2 was higher and increased as a function of the distance from the proximal portion (Fig. 5B) to the more distal portion of SR (Fig. 5C). This increasing expression with distance from the soma continued in SLM where the density of immunoparticles was highest (Fig. 5D). Therefore, the density of SK2 immunoparticles is low and uniform at P5, becomes higher but remains uniform in spines at P15. In the adult SK2 is concentrated in the dendritic spines, and SK2 expression increases with distance from the soma (Fig 6A). The difference in density of SK2 was statistically significant (p < 0.05) between the different times points and all hippocampal subfields, with the only exception of density in somata and in the dSR for P15 and P30 (Fig 6A).
Table 3.
Summary of SK2 immunoparticle density in different subcellular compartments during postnatal development.
| P5 | P15 | P30 | ||
|---|---|---|---|---|
| Subcellular Compartment | Extrasynaptic density (immunoparticles/μm2) |
Extrasynaptic density (immunoparticles/μm2) |
Extrasynaptic density (immunoparticles/μm2) |
Synaptic density (immunoparticles/μm2) |
| Spines (SLM) | 0.3 ± 0.1 | 2.9 ± 0.3 | 9.5 ± 0.6 | 1.9 ± 0.1^ |
| Spines (SR, distal) | 0.6 ± 0.1 | 3.0 ± 0.2 | 5.3 ± 0.4***# | 1.7 ± 0.1^ |
| Spines (SR, proximal) | 0.4 | 2.7 ± 0.1 | 2.8 ± 0.5*** | 1.4 ± 0.1^ |
| Somata | 0.5 | 0.6 ± 0.1* | 0.3** | 0 |
P <0.001 for somata compared to spines
P <0.001 for somata compared to spines
P <0.001 for proximal and distal SR compared to SLM
P <0.001 for proximal SR compared to distal SR
P <0.05 each subfield
Figure 5.
Change in the density of SK2 in CA1 pyramidal cells as a function of distance from the soma. Electron micrographs of the CA1 region showing immunogold particles for SK2 in different subfields, as detected using a pre-embedding immunogold method in the adult (P60). (A) In the stratum pyramidale, few immunoparticles for SK2 were detected along the somatic plasma membrane (arrows), but mainly associated with the endoplasmic reticulum (ER) of pyramidal cells (crossed arrows). (B,C) In the proximal part (panel B) and distal part (panel C) of the stratum radiatum, immunoparticles for SK2 were detected along extrasynaptic plasma membrane (arrows) of dendritic spines (s), showing higher numbers of immunoparticles and spines in the distal part. (D) The stratum lacunosum-moleculare was the subfield showing the highest number of immunoparticles for SK2 in dendritic spines (s) of pyramidal cells. To a lesser, SK2 immunoparticles were observed intracellularly associated with intracellular membranes (crossed arrows) of the dendritic spines in all subfields. at, axon terminal; nu, nucleus of pyramidal cells. Scale bars: A-D, 0.2 μm.
Figure 6.
Extrasynaptic SK2 immunoparticles at pyramidal cell spines. (A) Graph showing density of SK2 along different compartment of pyramidal cells during postnatal development. The density of SK2 (immunoparticles/μm2) is low and uniform at P5, becomes higher but remains uniform in spines at P15, and at P30 SK2 expression increases significantly with distance from the soma. (B,C) There is a positive correlation in the stratum radiatum (SR, p < 0.01, Spearman rank correlation; r = 0.79; n = 51) and in the stratum lacunosum-moleculare (SLM, p < 0.01, Spearman rank correlation; r = 0.79; n = 51) between the size of pyramidal cell spines and SK2 immunoparticle content of spines.
In addition to the extrasynaptic somato-dendritic gradient, we also found a synaptic gradient in mature (P30) CA1 neurons (Table 3). Synaptic SK2 immunolabeling in the proximal portion of the SR was low (1.4 ± 0.1 immunoparticles/labeled synapse, p < 0.05), increased in the distal portion of the SR (1.7 ± 0.1 immunoparticles/labeled synapse, p < 0.05) and was highest in the SLM (1.9 ± 0.1 immunoparticles per labeled synapse, p < 0.05).
In the adult, a large fraction of SK2 immunoparticles was associated with dendritic spines (see above). Therefore we analyzed the relation between the SK2 labeling in spines, determined by pre-embedding, and spine size. When the proportion of membrane-bound immunoparticles was examined in relation to the spine size, a positive correlation was observed (Fig. 6B,C) in both the SR (R = 0.623, P < 0.001, n = 54) and SLM (R = 0.803, P < 0.001, n = 81). These data show that larger spines contain more SK2-containing channels.
Clusters of SK2-containing channels along the surface of adult CA1 pyramidal neurons
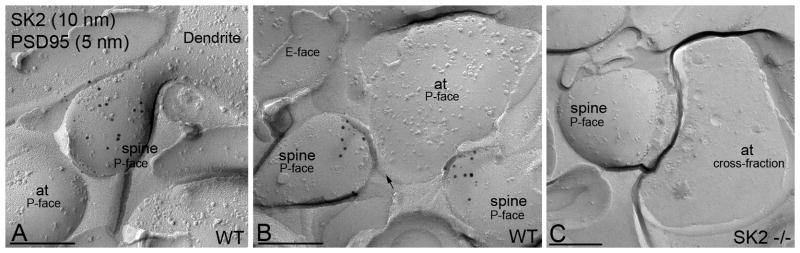
To investigate more precisely the location of SK2 along the postsynaptic PM of CA1 spines in adult, we performed immunogold EM using the SDS-digested freeze-fracture replica labeling (SDS-FRL) technique. SDS-FRL can visualize the membrane protein patterns with high resolution and high sensitivity, beyond the limitations of standard thin-section EM (Fujimoto, 1995). To define the PSD, immunogold labeling for PSD-95, a synaptic scaffolding protein, was performed in concert with SK2 immunogold labeling. Both sets of gold particles were localized to the P-face (protoplasmic face; Fig. 7), reflecting the location of the epitopes for the two proteins. Consistent with the results of pre-embedding experiments at P30, immunolabeling revealed co-clustering of SK2 and PSD-95 immunoparticles, demonstrating synaptic SK2 expression (Fig 7A-B). In addition to co-clustered immunoparticles, there were also segregated populations of SK2, not associated with PSD-95, showing that there are extrasynaptic SK2-containing channels in spines (Fig. 7A–B). Specificity of immunolabeling using the SDS-FRL technique was confirmed in samples of SK2 null mice (Fig. 7C). Thus, SK2 channels are expressed in the PSD as well as on the extrasynaptic spine membrane.
Figure 7.
Localization of SK2 channels along the surface of CA1 pyramidal cells using the SDS-digested freeze-fracture replica labeling (SDS-FRL) technique. Freeze-fracture replicas prepared from mouse hippocampus were labeled with 5-nm (small black dots, arrows) and 10-nm (bold black dots) immunoparticles to detect PSD-95 and SK2, respectively. SK2 is immunolabeled with an antibody directed against an epitope at the intracellular C-terminus, so the two proteins can be detected at the P-face of the plasma membrane. The E-face is free of any immunolabeling. (A-B) Clusters of immunoparticles for SK2 were detected in dendritic spines in the proximal radiatum (panel A) and distal radiatum (panel B) in close proximity or intermingled with PSD-95. (C) Antibody specificity is controlled and confirmed in replicas of SK2 null mice that were free of any immunolabeling. Scale bars: A-C, 0.2 μm.
Development and function of SK2-containing channels
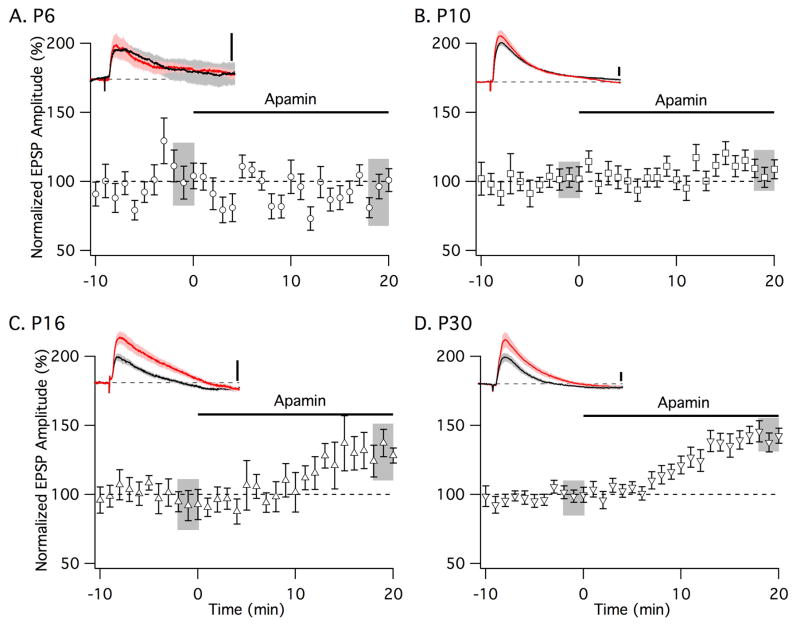
The anatomical results presented above predict that SK2-containing channels would contribute minimal function in spines at early developmental times (P5). In contrast, SK2 spine expression increased by P15, which should contribute to shaping neurotransmission as they do in adult (Ngo-Anh et al., 2005). To test this, whole cell recordings were performed in freshly prepared brain slices from P6, P10, P16, and P30 mice. Subthreshold EPSPs were evoked every 30 s by stimulation of Schaffer collateral axons in the SR ~100 μm from the somata. Following 10 min of stable baseline recordings, apamin (100 nM) was added to block SK channels and the EPSP amplitude, normalized to baseline, was measured after 18–20 min. Apamin had no significant effect on EPSPs recorded from P6 (n = 8) or P10 (n = 12) CA1 neurons (Fig. 8A,B). In contrast, at P16 and P30 apamin increased EPSPs by 39 ± 4% (p < 0.05; n = 10) and 42 ± 2% (p < 0.05; n = 11), respectively (Fig. 8C,D). There was no difference in membrane properties between the different age groups (RMP = resting membrane potential, Rinp = input resistance, Raccess = access resistance), P6 (RMP = −59.3 ± 1.6 mV, Rinp = 193.5 ± 57.7 MΩ, Raccess = 8.2 ± 1.5 MΩ), P10 (RMP = −58.4 ± 3.0 mV, Rinp = 148.0 ± 23.3 MΩ, Raccess = 6.4 ± 0.7 MΩ), P16 (RMP = −60.2 ± 0.3 mV, Rinp 167.2 ± 27.5 MΩ, Raccess = 7.3 ± 1.9 MΩ), and P30 (RMP = −58.9 ± 1.8 mV, Rinp 150.1 ± 24.7 MΩ, Raccess = 9.8 ± 1.5 MΩ) mice
Figure 8.
Developmental expression of functional synaptic SK2 channels in hippocampal CA1 neurons. Time course of the normalized EPSP amplitude (mean ± SEM) from (A) P6, (B) P10, (C) P16, and (D) P30 mice. Left insets show representative average of five EPSPs, mean ± SEM. (shaded area) for baseline (black trace) and after apamin application (red trace). Vertical scale bars: 1 mV. Insets show 200 ms of recordings.
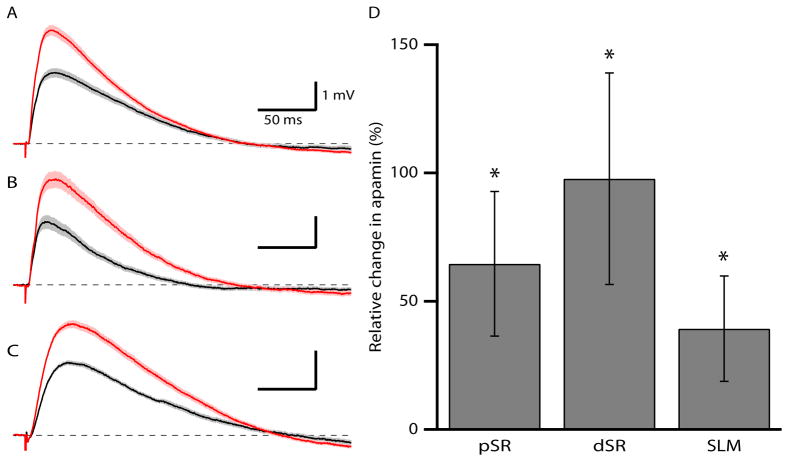
The gradient of SK2 expression in the adult suggests that the effects of apamin on EPSPs may also increase with distance from the soma. Therefore EPSPs were recorded following afferent stimulation in the proximal SR, distal SR, and SLM from P30-35 mice. In all three locations, apamin significantly increased EPSPs (pSR: 77 ± 20%, n = 12; dSR: 84 ± 27%, n = 12; SLM: 47 ± 16%, n = 13, p < 0.05 for effect of apamin in each pathway) (Fig. 9), however the effect of apamin was not different among the three sets of synapses. Consistent with an increase in dendritic filtering as a result of the increased distance from the soma, the rise time of EPSPs (see methods) increased significantly from pSR to SLM (5.8 ± 0.5 ms, 7.5 ± 0.6 ms, 12.8 ± 0.9 ms for pSR, dSR and SLM, respectively).
Figure 9.
SK2 contribution to synaptic responses is maintained with somato-dendritic distance. (A-C) Representative average of ~25 EPSPs, mean ± SEM (shaded area) for baseline (black traces) and after apamin application (red traces) recorded from afferent stimulation in (A) pSR, (B), dSR, and (C) SLM. (D) Summary graph showing the relative increase of EPSPs after apamin application, mean ± SEM, from afferent stimulations in pSR, dSR, and SLM. * shows significance compared to baseline responses.
Discussion
In the hippocampus, SK channels regulate neurotransmission, synaptic plasticity and learning (Behnisch and Reymann, 1998; Stackman et al., 2002; Kramar et al., 2004; Hammond et al., 2006; Lin et al., 2008). The mRNAs for three SK subunits, SK1, SK2, and SK3 are expressed in the adult hippocampus (Stocker and Pedarzani, 2000; Bond et al., 2004). However, SK2-containing channels have been most studied. The results show that SK2-containing channels regulate synaptic responses, plasticity, and hippocampus-dependent memory encoding. In addition, SK2 channels contribute to the expression and maintenance of LTP (Bond et al., 2004, Hammond et al., 2006, Lin et al., 2008, 2010). High-resolution immunoelectron microscopy revealed that during the first week of postnatal development, SK2 was largely expressed in the somata of CA1 pyramidal neurons, almost entirely intracellular in association with the rough ER. This early postnatal distribution of SK2 is distinct from later stages when SK2 migrates into dendritic shafts and spines of CA1 pyramidal neurons, and in the adult SK2 is mainly localized to spines.
During postnatal development, SK2 expression increases at extrasynaptic and synaptic sites in CA1 spines. At synaptic sites, we found that excitatory synapses undergo significant changes in the abundance of SK2 during postnatal development, different from the GluN1 subunit of NMDARs that does not appreciably change, in agreement with previous studies (Somogyi et al., 1998; Petralia et al., 1999; Racca et al., 2000). In the adult 52% of synapses contained SK2 immunoparticles and 63% contained GluN1 immunoparticles. However, we cannot conclude from the anatomical data that there are synapses that lack either SK2-containing channels or NMDARs, or ones that contain one but not the other. This is because post-embedding immunogold EM only detects a fraction of target proteins along the membrane exposed on the surface of ultrathin sections and it is possible that most or all synapses have SK2-containing channels and NMDARs, but because of low detection efficiency some PSDs fail to show immunoreactivity. This technical limitation has also been recognized in other studies on synaptic NMDAR and AMPAR expression (Kharazia et al., 1996; Petralia et al., 1999). In this study, we took advantages of various immunoelectron microscopic techniques namely, pre-embedding and post-embedding immunogold labeling and SDS-digested freeze-fracture replica labeling (SDS-FRL). To reveal SK2 localization in extrasynaptic plasma membrane and endoplasmic reticulum, the pre-embedding immunogold labeling is most suitable and this technique reveals the distribution of immunoreactivity in ultrastructures comparable to that obtained by light microscopy. However, this method often does not reveal antigens expressed in specialized membrane subdomains which are undercoated with dense protein matrixes such as postsynaptic densities of excitatory synapses, presynaptic active zones, and axon initial segments (Watanabe et al., 1998). Thus we employed post-embedding immunolabeling for SK2, by which synaptically expressed antigens are detectable on the surface of ultrathin sections. Indeed, this approach revealed expression of SK2 in excitatory synapses that was not evident with the pre-embedding immunolabeling.
To address the relative expression levels of SK2 between synaptic and extrasynaptic spine plasma membranes, we took advantage of SDS-FRL that reveals two-dimensional molecular localization over a large area of the plasma membrane. This feature is unique because the pre- and post-embedding immunolabelings demonstrate immunoreactivity only in 70 nm-thick profiles of plasma membrane. In addition, SDS-FRL provides uniform immunolabeling efficiency for target antigens expressed over different membrane subdomains including synaptic and extrasynaptic sites (Masugi-Tokita and Shigemoto, 2007). This is achieved by the treatment of freeze-fracture replicas with SDS, which dissolves tissue and molecules that are not integrated in replicated membranes and exposes most membrane proteins immobilized on the replica. Thus, the target membrane proteins are readily and evenly accessible to the antibodies and give a high sensitivity labeling (Masugi-Tokita and Shigemoto, 2007). In this study, we found a comparable density of SK2 in synaptic and extrasynaptic sites with SDS-FRL.
Interestingly, there is an increase in GluN2A protein expression during postnatal development of the hippocampus (Sans et al., 2002) that is very similar to the pattern of SK2 expression reported here. The developmental acquisition of synaptic SK2 expression suggests that in young animals the lack of synaptic SK2 expression will permit relatively unrestricted Ca2+ influx into the spine and facilitate synaptic plasticity. As synaptic SK2 expression increases, Ca2+ influx will decrease and the activity threshold for the induction of synaptic plasticity will be raised (Stackman et al., 2002, Hammond et al., 2006). During development there are changes in the modifications of synaptic strength that are thought to strengthen synaptic connections and establish neuronal networks important for behavior (Durand et al., 1996; Dudek and Bear, 1993; Dumas, 2005). Our results suggest that SK2 expression may influence this process.
Parallel to the developmental increase in the number of excitatory synapses showing immunoreactivity for SK2 and the increase of immunoparticles for SK2 at individual synapses, electrophysiological recording showed a developmental acquisition of synaptic SK2-containing channel function; apamin increased EPSPs once SK2 immunoparticles were detected at the PSD supporting the view that the developmental increase in the contribution made by SK2-containing channels to neurotransmission is due to an increase in the number of SK2-containing channels at synapses.
Subcellular localization and density are important determinants of the functional impact of an ion channel. A number of studies using LM and EM immunohistochemical approaches have shown that some ion channel subunits can be selectively targeted to specific membrane compartments of hippocampal neurons, thus showing a non-uniform subcellular localization along the neuronal surface (Cooper et al., 1998; Misonou et al., 2004; Jinno et al., 2005; Kollo et al., 2006, Bourdeau et al., 2007). For example, in CA1 pyramidal neurons AMPARs and HCN channels show increasing somato-dendritic expression (Andrasfalvy and Magee, 2001; Lorincz et al., 2002; Nicholson et al., 2006). Similarly, a quantitative comparison of extrasynaptic SK2 immunogold densities in the adult showed a 32-fold increase from somatic to distal apical spine membranes. Somata had very low density of SK2, whereas distal spines had 3 times more SK2 labeling than proximal spines of similar size. Indeed, even within the SR, there was higher SK2 expression in distal spines than in proximal spines. However, this gradient of SK2 expression was not reflected by an increased affect of apamin on EPSPs. There was no difference in the apamin-induced increase in EPSP amplitude following afferent stimulation in pSR, dSR and SLM. Indeed, the number of postsynaptic AMPARs per spine increases with distance (Andrasfalvy and Magee, 2001; Smith MA et al., 2003). Therefore, the increased AMPA-mediated depolarization with distance will drive more synaptic Ca influx and activate more SK2 channels that also increase with distance, normalizing the synaptic response that reaches the soma.
In summary, the developmental profile of SK2 expression reflects transcriptional and posttranslational regulation of expression levels and differential subcellular distribution of SK2-containing channels in CA1 neurons that influences neurotransmission, synaptic plasticity, and hippocampus-dependent learning.
Acknowledgments
We thank Mrs. Mercedes Gil for excellent technical assistance. This work was supported by grants from the Spanish Ministry of Education and Science (BFU-2009-08404/BFI) and CONSOLIDER (CSD2008-00005), and from Consejería de Educación y Ciencia, Junta de Comunidades de Castilla-La Mancha (PAI08-0174-6967) to R.L., and NIH grants NS038880 (JPA) and MH081860-01 (JM).
References
- Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnisch T, Reymann KG. Inhibition of apamin-sensitive calcium dependent potassium channels facilitate the induction of long-term potentiation in the CA1 region of rat hippocampus in vitro. Neurosci Lett. 1998;253:91–94. doi: 10.1016/s0304-3940(98)00612-0. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent after hyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau ML, Morin F, Laurent CE, Azzi M, Lacaille JC. Kv4.3-mediated A-type K+ currents underlie rhythmic activity in hippocampal interneurons. J Neurosci. 2007;27:1942–1953. doi: 10.1523/JNEUROSCI.3208-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–60. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J Neurosci. 1998;18:965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni L, Canepari M, Luján R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Lüthi A. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol. 2005;76:189–211. doi: 10.1016/j.pneurobio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–72. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443–3449. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Jeromin A, Kosaka T. Postsynaptic and extrasynaptic localization of Kv4.2 channels in the mouse hippocampal region, with special reference to targeted clustering at gabaergic synapses. Neuroscience. 2005;134:483–494. doi: 10.1016/j.neuroscience.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Phend KD, Rustioni A, Weinberg RJ. EM colocalization of AMPA and NMDA receptor subunits at synapses in rat cerebral cortex. Neurosci Lett. 1996;210:37–40. doi: 10.1016/0304-3940(96)12658-6. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Kollo M, Holderith NB, Nusser Z. Novel subcellular distribution pattern of A- type K+ channels on neuronal surface. J Neurosci. 2006;26:2684–2691. doi: 10.1523/JNEUROSCI.5257-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of Θ-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Luján R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Luján R, Watanabe M, Frerking M, Maylie J, Adelman JP. Coupled activity-dependent trafficking of synaptic SK2 channels and AMPA receptors. J Neurosci. 2010;30:11726–11734. doi: 10.1523/JNEUROSCI.1411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A, Notomi T, Tamás G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Luján R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Luján R, Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur J Neurosci. 2006;23:1479–1490. doi: 10.1111/j.1460-9568.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- Lujan R, Maylie J, Adelman JP. New sites of action for GIRK and SK channels. Nat Rev Neurosci. 2009;10:475–480. doi: 10.1038/nrn2668. [DOI] [PubMed] [Google Scholar]
- Masugi-Tokita M, Shigemoto R. High-resolution quantitative visualization of glutamate and GABA receptors at central synapses. Curr Opin Neurobiol. 2007;17:387–393. doi: 10.1016/j.conb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Trana R, Katz Y, Kath WL, Spruston N, Geinisman Y. Distance-dependent differences in synapse number and AMPA receptor expression in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:431–442. doi: 10.1016/j.neuron.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Ondrejcak T, Klyubin I, Hu NW, Barry AE, Cullen WK, Rowan MJ. Alzheimer’s disease amyloid beta-protein and synaptic function. Neuromolecular Med. 2010;12:13–26. doi: 10.1007/s12017-009-8091-0. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Strassmaier T, Bond CT, Sailer CA, Knaus HG, Maylie J, Adelman JP. A novel isoform of SK2 assembles with other SK subunits in mouse brain. J Biol Chem. 2005;280:21231–21236. doi: 10.1074/jbc.M413125200. [DOI] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnár E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J Neurosci. 2009;29:12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci. 1998;10:478–487. doi: 10.1046/j.1460-9568.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- Wu WW, Chan CS, Surmeier DJ, Disterhoft JF. Coupling of L-type Ca2+ channels to Kv7/KCNQ channels creates a novel, activity-dependent, homeostatic intrinsic plasticity. J Neurophysiol. 2008;100:1897–1908. doi: 10.1152/jn.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]