Abstract
The mammalian ovary is a heterogeneous organ, and contains oocyte-containing follicles at varying stages of development. The most immature follicular stage, the primordial follicle, comprises the ovarian reserve and is a finite number, defined at the time of birth. Depletion of all follicles within the ovary leads to reproductive senescence, known as menopause. A number of chemical classes can destroy follicles thus hastening entry into the menopausal state. The ovarian response to chemical exposure can determine the extent of ovotoxicity that occurs. Enzymes capable of bioactivating as well as detoxifying xenobiotics are expressed in the ovary and their impact on ovotoxicity have been partially characterized for trichloroethylene, 7,12-dimethylbenz[a]anthracene, and 4-vinylcyclohexene. This review will discuss those studies, as well as illustrate where knowledge gaps remain for chemicals that have also been established as ovotoxicants.
Keywords: Ovotoxicant, follicle, steroidogenesis
Introduction
The ovary is the female gonad with two major functions, production of the germ cell and the sex steroid hormones, chiefly 17β-estradiol (E2) and progesterone (P4). At birth, the ovary contains a finite number of primordial follicles, comprised of a meiotically-arrested oocyte surrounded by squamous granulosa cells (Hirshfield, 1991). These follicles comprise the ovarian reserve from which pre-ovulatory follicles are developed. A number of chemical classes can deplete ovarian follicles and alter steroidogenesis leading to impaired ovarian function and infertility, including but not limited to, environmental, industrial, chemotherapeutic and xenoestrogenic chemicals (Hoyer and Sipes, 1996). This review article will describe studies that have investigated the impact of ovarian metabolism on ovotoxicity induced by xenobiotics. Three chemicals for which the majority of knowledge on the impact of ovarian metabolism is available will be first described – Trichloroethylene (TCE), 7,12-dimethylbenz[a]anthracene (DMBA) and 4-vinylcyclohexene (VCH). The remainder of this review will summarize the ovarian effects of chemicals for which less is known on the role of metabolism, and will indicate where knowledge gaps remain.
Biotransformation enzymes
Of the enzymes capable of biotransformation of chemical compounds, certain enzymes that have emerged as having roles in ovarian metabolism are discussed in this review. A brief summary of their function is provided here as an introduction to those studies in which they are found to have roles in determining the extent of ovotoxicity that occurs.
The cytochrome P450 (CYP) enzymes comprise a large family that play critical roles in phase I metabolism of a wide variety of xenobiotics. CYP enzymes are found in all tissues and they can be involved in activation or detoxification, depending on the chemical substrate (Casarett and Doull, 2008). Another group of phase I biotransformation enzymes are the epoxide hydrolases (EH) which catalyze the addition of water to alkene epoxides and arene oxides. There are five forms of EH, including the isoform under discussion in this review, microsomal epoxide hydrolase (mEH). mEH has a wide alkene epoxide and arene oxide substrate range, but has a preference for mono-substituted epoxide structures (Casarett and Doull, 2008).
Glutathione (GSH) is a ubiquitous antioxidant compound, present in all cells, and is composed of glycine, cysteine and glutamic acid. Formation of GSH occurs in two steps catalyzed by γ-glutamylcysteine (γ-Gcl) and glutathione synthetase (Gss). Conjugation of GSH to a chemical generally represents a phase II detoxification modification. GSH xenobiotic conjugation is catalyzed by the glutathione S-transferase (Gst) family of enzymes, which include the isoforms alpha, pi, mu, omega and theta, and comprise about 10% of total cellular protein (Casarett and Doull, 2008).
Trichloroethylene
TCE is a common water contaminant (Davidson and Beliles, 1991). Its lipophilic characteristic together with low boiling point makes it ideal for several industrial processes including metal degreasing and dry cleaning (Weiss, 1996). Numerous commercially used products like wood stains, adhesives, lubricants and paint removers contain TCE (Wu and Berger, 2007). Due to widespread use, human exposure can occur through drinking, inhalation or transdermal absorption (EPA, 1985) and U.S. urban areas have approximately three times more detectable TCE compared to rural areas (Wu and Schaum, 2000).
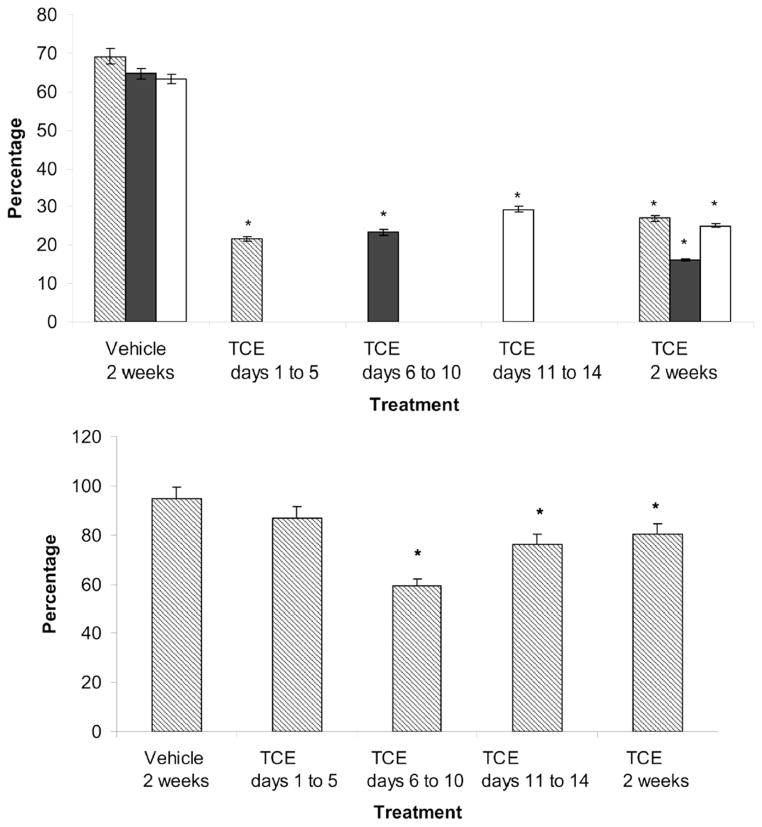
Female rats exposed to TCE via inhalation (1700 ppm, 2h/d, 5 d/wk) display decreased oocyte fertilizability induced by alterations to the oocyte plasma membrane composition (Berger and Horner, 2003). Oocytes from female rats exposed to TCE orally (0.45% TCE in 3% Tween) for 4, 5, or 14 days also showed less capacity for binding of and fertilization by unexposed rat sperm compared to oocytes from vehicle control exposed females (Wu and Berger, 2007) (Figure 1).
Figure 1.
(A) Decreased fertilizability of oocytes from TCE-exposed (0.45% TCE (v/v) in 3% Tween) rats compared with oocytes from vehicle-control (3% Tween) rats. Fertilization was assessed by the presence of decondensed sperm heads. Values represent the least squares means from three replicates. * indicates P < 0.05 compared with the vehicle control. (B) Oocytes with bound sperm. Bars represent the percentage of total oocytes (fertilized and unfertilized) that bound sperm. Oocytes from females treated with TCE on d 6 to 10, d 11 to 14 and for 2 wks bound fewer sperm compared with vehicle-controls. * indicates P < 0.05 compared with the vehicle control (Adapted from Wu and Berger, 2007 with copyright permission).
Hepatic TCE metabolism primarily occurs in two ways. The first is through CYP enzyme-mediated oxidation (Nakajima et al., 1988; Guengerich et al., 1991a), resulting in formation of trichloroethanol (TCOH), which can further be metabolized to TCOH glucuronide (Cummings and Lash, 2000). TCE can also be metabolized to trichloroacetic acid (TCAA), dichloroacetic acid (DCA) and monochloroacetic acid (Cummings and Lash, 2000). The CYP isoforms involved in TCE metabolism are CYP1A1/2, CYP2B1/2, CYP2C11/6 and CYP2E1 (Nakajima et al., 1988; Nakajima et al., 1990; Guengerich et al., 1991a; Guengerich and Turvy, 1991b; Nakajima et al., 1992a; Nakajima et al., 1992b; Nakajima et al., 1993). Of these isoforms CYP2E1 has the highest affinity for TCE (Nakajima et al., 1990; Guengerich et al., 1991a; Guengerich and Turvy, 1991b; Cummings and Lash, 2000). In vitro exposure of mouse oocytes to TCAA, DCA and TCOH also reduce fertilization rates (Cosby and Dukelow, 1992) and have been detected in the rat ovary following TCE exposure through drinking water (0.45% for 2 wk; Wu and Berger, 2007). Additionally, increased ovarian CYP2E1 activity was demonstrated in these rats suggesting ovarian TCE metabolism through the CYP-dependent oxidative pathway (Reinke and Moyer, 1985).
In extra-ovarian tissues, the second route of TCE metabolism of TCE is through GSH conjugation resulting in S-(1,2-dichlorovinyl)glutathione (DCVG) formation (Lash et al., 1995; Lash et al., 1998). Dipeptidase and γ–glutamyltransferase (GTT) further metabolize DCVG to S-(1,2-dichlorovinyl)-L-cysteine (DCVC). This product can then be metabolized either by cysteine S-conjugate N-acetyl-S-transferase (NAT) to form N-acetyl-DCVC (NAcDCVC). DCVC can also be converted by the cysteine conjugate β–lyase (β-lyase) to form a reactive thiol compound (Lash et al., 1995; Lash et al., 1998). NAcDCVC has been detected in both rats and human urine after TCE exposure (Commandeur and Vermeulen, 1990; Birner et al., 1993). In vitro exposure of rat oocytes to DCVC (5 mM; 4 h) reduced zona pellucida-free oocyte fertilizability (Wu and Berger, 2008), which is interesting since GSH conjugation usually results in less reactivity compared to the parent compound. In summary, TCE induces ovotoxicity through bioactivation by the CYP-dependent and GSH-conjugating pathways: whether these biotransformation events occur in ovarian tissue, however, remains unclear.
7,12-dimethylbenz(a)anthracene
7,12-dimethylbenz(a)anthracene (DMBA) is a polycyclic aromatic hydrocarbon (PAH) and is a model carcinogenic chemical for its ability to induce ovarian (Kanter et al., 2006), skin (Diagaradjane et al., 2006) and mammary (Russo and Russo, 1996) tumors in rodents. Humans are exposed to this chemical through burning of organic materials, thus exposure can come from cigarette smoke and car exhaust fumes (Lawther and Waller, 1976). DMBA destroys all follicle stages including corpora lutea in a dose-dependent manner in mice and rats (Mattison, 1980; Hoyer, 2001; Rajapaksa et al., 2007a; Igawa et al., 2009). A decrease in ovarian volume typically results from lack of ovarian follicles (Mattison and Schulman, 1980; Weitzman et al., 1992).
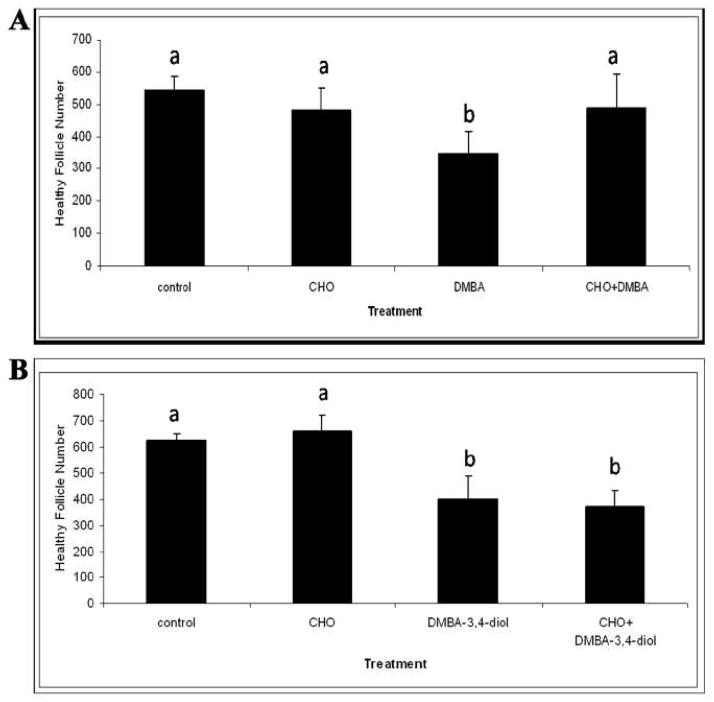
DMBA is metabolized to DMBA-3,4-epoxide by CYP1B1, which is hydrolyzed to DMBA-3,4-diol by the action of mEH. DMBA-3,4-diol then undergoes epoxidation by CYP1A1 or CYP1B1 to form the ultimate ovotoxic and carcinogenic compound, DMBA-3,4-diol-1,2-epoxide (Miyata et al., 1999). CYP1A1, CYP1B1 and mEH enzymes are induced at mRNA and protein levels in mouse and rat ovaries exposed to DMBA (Cannady et al., 2002; Shimada et al., 2003; Rajapaksa et al., 2007a; Igawa et al., 2009). In cultured post natal day 4 (PND4) rat ovaries meh mRNA and protein are increased during DMBA (1 μM) exposure at a time point prior to follicle depletion (Rajapaksa et al., 2007b; Igawa et al., 2009). Use of the competitive mEH inhibitor, cyclohexene oxide prevented DMBA-induced primordial follicle loss, however, the DMBA-3,4-diol metabolite was unaffected by mEH inhibition (Figure 2). These results support that mEH is involved in ovarian DMBA bioactivation (Rajapaksa et al., 2007b; Igawa et al., 2009).
Figure 2. Effect of CHO (mEH inhibitor) on DMBA- or DMBA-3,4-diol-induced follicle loss.
Ovaries from PND4 Fischer 344 rats were cultured with (A) vehicle control or media containing DMBA (1 μM) ± CHO (2 mM) for 4 d; or (B) vehicle control or media containing DMBA-3,4-diol (75 nM) ± CHO (2 mM) for 4 d. Following incubation, ovaries were collected, processed for histological evaluation and healthy follicles were counted. Values are mean ± SE total follicles counted/ovary, n=5. Different letters indicate significant difference between follicle number (Adapted from Igawa et al., 2009 with copyright permission).
Cultured PND4 mouse ovarian primordial follicle oocytes have increased expression of the pro-apoptotic protein, BAX, following DMBA (1μM) exposure, mediated through the action of the aryl hydrocarbon receptor (AHR; Matikainen et al., 2001). Use of an AHR antagonist, alpha-naphthoflavone (ANF) prevented BAX-induced follicle loss caused by DMBA (Matikainen et al., 2001). Also, intra-ovarian ANF treatment (80mg/kg) during DMBA exposure (10 μg) prevented follicle destruction in mice (Shiromizu and Mattison, 1985). Further, Bax- and Ahr-deficient mice are resistant to DMBA-induced primordial follicle destruction (Matikainen et al., 2001). Although the mechanisms behind protection are not understood, DMBA-induced oocyte destruction is preventable by co-treatment with the E2 agonist Tamoxifen (TAM) in vivo (Ting and Petroff, 2010).
While DMBA bioactivation is at least partially characterized, less is known about DMBA detoxification. GSH supplementation (glutathione ethyl ester; GEE; 5 mM) protects ovaries from DMBA-induced follicle loss in cultured pre-ovulatory follicles from Sprague-Dawley rats (Tsai-Turton et al., 2007a). Also, Gstp-null mice have increased DMBA-induced skin tumor formation compared to wild type littermates (Henderson et al., 1998). PND4 cultured rat ovaries exposed to DMBA (1 μM) have increased Gstp mRNA and protein expression (Bhattacharya et al., 2012) at a time-point prior to DMBA-induced follicle loss (Igawa et al., 2009). The increased GSTP is associated with negative regulation of pro-apoptotic c-Jun N-terminal kinase (JNK), indicating a protective role for GSTP within the ovary (Bhattacharya and Keating, 2012). Furthermore, DMBA exposure increased Ahr mRNA and protein, and Nuclear factor erythroid-related factor 2 (Nrf2) protein suggesting that these transcription factors play role(s) in ovarian xenobiotic biotransformation enzyme activation during DMBA exposure (Bhattacharya and Keating, 2012).
PND4 mouse ovaries exposed to DMBA (50 nM) had altered mRNA expression of a number of genes involved in primordial follicle activation, cell survival and proliferation (Sobinoff et al., 2011). One pathway that was identified was the phosphatidylinositol-3 kinase (PI3K) pathway. PI3K is essential for oocyte viability as well as regulation of the rate by which primordial follicles are recruited into the growing follicular pool (Castrillon et al., 2003; Reddy et al., 2005; Liu et al., 2006). Activation of this pathway occurs when granulosa-expressed Kit Ligand (KITL) binds to the oocyte-expressed receptor, c-KIT (Manova et al., 1990; Orr-Urtreger et al., 1990; Horie et al., 1991). c-KIT undergoes auto-phosphorylation and the PI3K components are activated (Serve et al., 1994). PI3K converts PIP2 to PIP3, which acts as a second messenger eventually resulting in phosphorylation (activation) of AKT (Engelman, 2009). Activated AKT can promote expression of a number of pro-survival genes, and can also negatively regulate pro-apoptotic genes, including the Forkhead transcription factor isoform 3a (Foxo3a; Brunet et al., 1999). Increased AKT phosphorylation with a concomitant decrease in FOXO3A phosphorylation, along with activation of the PI3K-regulated protein, mammalian target of rapamycin (mTOR) was observed in DMBA-treated primordial follicle oocytes (Sobinoff et al., 2011). Inhibition of PI3K signaling accelerated DMBA-induced loss of all follicle stages in cultured PND4 rat ovaries (Keating et al., 2009), thus a role for PI3K signaling during DMBA-induced ovotoxicity is supported.
4-Vinylcyclohexene
4-vinylcyclohexene (VCH) is produced as a by-product of the pesticide, rubber, plastic and flame retardant industries (Rappaport and Fraser, 1977). Human exposures are through dermal contact, oral intake (NTP, 1989) and inhalation (Bevan et al., 1996). VCD selectively destroys primordial and small primary follicles leading to premature ovarian failure in mice and rats (Smith et al., 1990; Hooser et al., 1994; Mayer et al., 2002).
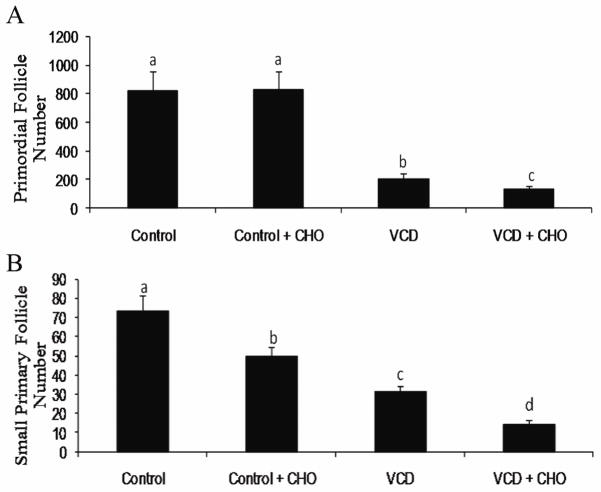
VCH is bioactivated in the ovary to the ultimate diepoxide ovotoxic metabolite, VCD, through the action of CYP2E1 (Rajapaksa et al., 2007b). Cyp2e1-null mice had less follicle loss relative to their wild type littermates (Rajapaksa et al., 2007b), thus CYP2E1 is involved in VCH bioactivation to VCD. Detoxification of VCD is thought to occur through the action of ovarian expressed mEH. VCD induces meh mRNA and protein expression both in vivo (Cannady et al., 2002) and in vitro (Keating et al., 2008; Bhattacharya et al., 2012), prior to follicle loss in mice and rats. Inhibition of mEH using CHO in the presence of VCD caused more follicle loss relative to VCD-treated ovaries, supporting a detoxification role for mEH during VCD-induced ovotoxicity (Bhattacharya et al., 2012; Figure 3).
Figure 3. Effect of mEH inhibition on VCD-induced follicle loss.
PND4 F344 rat ovaries were cultured in media containing vehicle control or VCD (30 μM), ± CHO (2 mM) for 8 d. Ovaries were processed for histological evaluation and healthy follicles were classified and counted as described in methods. Values are expressed as mean ± SE total follicles counted/ovary, n=5. Different letters indicate significant difference; P < 0.05 (Adapted from Bhattacharya et al., 2012 with copyright permission).
A protective role for the ovarian Gstp isoform has also been reported during VCD exposure (Keating et al., 2010). Gstp mRNA and protein are increased in response to VCD, and in a similar manner to that during DMBA-induced ovotoxicity, GSTP forms a protein complex with JNK, and inhibits JNK action, as evidenced by reduced phosphorylation of the JNK target, c-Jun (Keating et al., 2010). GSH conjugates of VCD have also been detected in media from VCD-treated cultured PND4 mouse ovaries (Rajapaksa, 2007), indicating that GSH-conjugation to VCD is a likely detoxification mechanism during VCD exposure, however, the role for GSTP in this conjugation remains unclear.
VCD reduces PI3K signaling in primordial and small primary oocytes (Keating et al., 2011). Decreased phosphorylation of c-KIT (Mark-Kappeler et al., 2011) and oocyte-expressed AKT (Keating et al., 2011) occur rapidly after VCD exposure in PND4 cultured rat ovaries. Additionally, there is a decrease in oocyte FOXO3a in the VCD target follicles (Keating et al., 2011). In an apparent protective response by the ovary, Kitl mRNA is up-regulated in response to VCD, and, in fact, exogenous addition of KITL during VCD exposure partially attenuates VCD-induced follicle loss (Fernandez et al., 2008). Further, PI3K inhibition prevents VCD-induced primordial but not small primary follicle loss. It is hypothesized that VCD may accelerate the entry of primordial follicles into the growing pool to replace those small primary follicles destroyed (Keating et al., 2009) through classic apoptotic pathways (Springer et al., 1996a; Springer et al., 1996b; Hu et al, 2001).
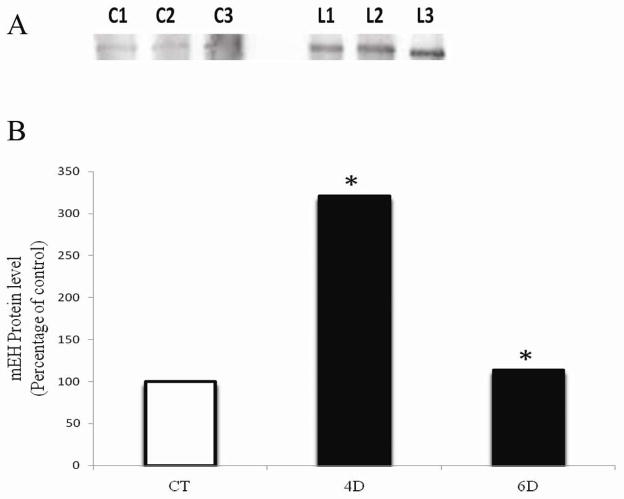
An interesting role for PI3K signaling in regulation of xenobiotic metabolism enzyme gene expression is emerging. The ovotoxic outcomes of DMBA and VCD exposures are altered when PI3K is inhibited – follicle loss by DMBA is accelerated, while that of VCD is lessened (Keating et al., 2009). Since mEH bioactivates DMBA but detoxifies VCD, it is logical to consider that altered mEH expression may occur during PI3K inhibition. Furthermore, it is known that the transcription factors C/EBPα and C/EBPβ are responsible for mEH induction through PI3K signaling (Ki and Kim, 2008). It has recently been demonstrated that mEH is indeed increased during PI3K inhibition (Bhattacharya et al., 2012), providing explanation for the ovotoxic outcomes observed with DMBA and VCD when PI3K is inhibited (Figure 4). Also, inhibition of PI3K signaling has been demonstrated to impact expression of AhR, Nrf2, Gstp, Gstm at the mRNA, protein level or both (Bhattacharya and Keating, 2012). New roles for this pathway are emerging due to manipulation of this pathway through chemical means which may allow for future therapeutic interventions.
Figure 4. Temporal effect of PI3K inhibition on mEH protein.
PND4 F344 rat ovaries were cultured in media containing vehicle control (Agency), ± 20 μM LY294002 for 4 or 6 d. Total protein was isolated and Western blotting was performed to detect mEH protein. (A) Representative Western blot is shown on day 4; Control = C; LY294002 = L. (B) Values are expressed as a percentage of control mean ± SE; n=3 (10 ovaries per pool). * P < 0.05; different from control (Adapted from Bhattacharya et al., 2012 with copyright permission).
Methoxychlor
Methoxychlor (MXC) is an organochlorine pesticide and insecticide which was used as a replacement for dichlorodiphenyltrichloroethane (DDT; DHHS., 2002). MXC acts as an endocrine disrupting chemical (EDC) and has several adverse effects on reproductive function in female mice including persistent estrus (Martinez and Swartz, 1991), reduced fertility (Cummings and Gray, 1989), ovarian atrophy (Martinez and Swartz, 1991; Martinez and Swartz, 1992; Eroschenko et al., 1997; Gupta et al., 2006) and dose-dependent follicular atresia (Martinez and Swartz, 1991; Eroschenko et al., 1997). Cultured antral follicles from mice exposed to MXC (100 μg/μl/96 h) demonstrate decreased antral follicle growth and increased antral follicle atresia (Miller et al., 2005; Gupta et al., 2006; Miller et al., 2006).
MXC’s role as an EDC has been demonstrated by several in utero exposure studies. Pregnant mice exposed to MXC (5.0 mg) via oral gavage from gestation day (GD) 6 to 15 had accelerated vaginal opening in offspring (Swartz and Corkern, 1992), characteristic of an estrogenic effect. Additionally, lipid accretion in ovarian theca and interstitial cells was observed in adult mice exposed orally to MXC (5.0 mg/d/4 wk) which also represent estrogenic effects (Martinez and Swartz, 1992). Conversely, MXC exhibits endocrine disruption through altered ovarian steroidogenesis. Cultured antral follicles from mice exposed to increasing concentrations of MXC (1–100 μg) over a time course of exposure (24–96 h) had dose-dependent decreased mRNA expression of the steroidogenic enzyme Hsd3b1 (10 μg MXC - 48 h). At a higher concentration and longer exposure to MXC (100 μg - 96 h), mRNA encoding Hsd17b1 was also decreased. CYP1B1 enzyme increases metabolism of E2 and was decreased by MXC exposure (100 μg - 96 h), along with a concomitant decrease in E2 level (Basavarajappa et al., 2011). Taken together, these data indicate that decreased E2 may contribute to MXC-induced inhibition of antral follicle growth in mice.
MXC is metabolized to 1,1,1-trichloro-2-(4-hrdroxyphenyl)-2-(4-methoxyphenyl)ethane (mono-OH) through the action of CYP2C9 and CYP1A2 enzymes (Hu and Kupfer, 2002; Hu et al., 2004; Hazai and Kupfer, 2005). Further conversion of mono-OH to 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) is mediated by CYP1A2, CYP2C8, CYP2C19, CYP2D6 and CYP3A4 (Hu and Kupfer, 2002). CYP enzymes (CYP1A1, CYP1B1, CYP1A2, CYP2C29) capable of metabolizing MXC are expressed in mouse ovarian tissues (Symonds et al., 2006). Also CYP2C29 mRNA expression level is increased through an E2 receptor (ER)-linked mechanism in mouse ovarian surface epithelium following MXC exposure (3 μM/14 d; Symonds et al., 2006).
The MXC metabolite mono-OH decreases E2 levels through impacts on mRNA encoding enzymes in the steroidogenic pathway (Craig et al., 2010). Cyp11a1, Cyp17a1, and Cyp19a1 mRNA were reduced in cultured mouse antral follicles exposed to mono-OH (10 μg - 96 h) with a decrease in E2 synthesis (Craig et al., 2010). In contrast, HPTE has not been observed to affect mRNA or protein expression of Cyp11a1 and while no impact of HPTE on steroidogenic gene mRNA has been demonstrated, HPTE (50 nM for 24 h) has been shown to inhibit progesterone (P4) formation in cultured rat theca-interstitial and granulosa cells and to decrease the catalytic activity of CYP11A1 (Akgul et al., 2008). Also, luteal cells exposed to HPTE have reduced P4 production (Akgul et al., 2011), however, in this cell type, an inhibitory effect of HPTE on the steroidogenic step catalyzed by CYP11A1 and CYP11A1 activity was observed (Akgul et al., 2011). No impact of HPTE on Cyp11a1 mRNA or protein level was observed however (akgul et al., 2011). Also, lack of any effect of HPTE on Gpx3, Gst, or Cyp17a1 was observed in vivo in mice (Waters et al., 2001). Moreover, mice dosed with HPTE (32 mg/kg/20 d; intraperitoneally (i.p.)) have increased antral follicle loss but, conversely, increased ovarian surface epithelium proliferation (Borgeest et al., 2002).
2,3,7,8-tetrachlorodibenzo-p-dioxin
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a halogenated aromatic hydrocarbon (HAH) and widespread environmental pollutant (Poland et al., 1982). TCDD is lipophilic and has a slow rate of metabolism and excretion, thus it readily concentrates in the food chain (Safe et al., 1991). TCDD is a by-product of the manufacture of herbicides, insecticides and disinfectants and human exposure comes in the form of waste incineration. Natural sources of TCDD also exist, including forest fire and volcanic eruptions (Ho et al., 2006).
TCDD is a reproductive toxicant (Peterson et al., 1993; Li et al., 1995). Female rats exposed to a single oral dose of TCDD (10 μg/kg) experienced prolonged diestrus stages with concomitant reduced times spent in proestrus and estrus. Also, a reduction in the number of oocytes ovulated due to TCDD has been reported (Li et al., 1995).
Developing ovaries are particularly sensitive to TCDD (Jablonska et al., 2010). Pregnant female Lewis rat dams were exposed to TCDD (50 ng/kg/wk) via oral gavage on both GD 14 and 21 and both PND 7 and 14. Following birth, pups were weaned at PND 21, followed by exposure to vehicle control or TCDD (50 ng/kg/wk; gavage). Ovaries from the TCDD exposed F1 females were then transplanted into control F1 females and vice versa, followed by TCDD exposure (50 ng/kg/wk; gavage) until sacrifice at 8 months. Both in utero and lactational TCDD exposure induced acyclicity as the females aged (Jablonska et al., 2010). These studies raise concerns over in utero TCDD exposure for female reproductive health.
Several studies have shown that TCDD acts as an EDC through inhibition of E2 production. Chronic TCDD exposure (200 ng/kg/wk) decreased E2 synthesis and caused irregular cyclicity in the absence of follicle depletion in rats (Franczak et al., 2006; Shi et al., 2007). TCDD exposure (10, 40, 100 ng/kg/14 d; orally) decreased E2 concentration, impaired follicular development (Heiden et al., 2006), and suppressed Cyp11a1, Cyp19a1a and Star mRNA expression (Heiden et al., 2008) in zebrafish. Additionally, E2 production was inhibited when human luteinized granulosa cells were exposed in vitro to TCDD (10 nM; alternate days; 8 d; Morán et al., 2000).
There is limited information on the impact of ovarian metabolism on TCDD ovotoxicity. A role for GSH conjugation to TCDD as a mechanism of detoxification has been indicated in female rats in which GSTM increased following an oral dose of TCDD (125 ng/Kg/d; Chen et al., 2009).
Bisphenol A
BPA (4,4′-isopropylidenediphenol) is a diphenyl compound consisting of two hydroxyl groups in the “para” position making it very similar to diethylstilbestrol (DES) (Papaconstantinou et al., 2000; Markey et al., 2001). BPA is widely used in the manufacture of polycarbonated plastics, epoxy resins, dental sealants and as a stabilizing reagent in plastic production. Humans are exposed to BPA through the interior coating of food cansmilk containers, food storage vessels, wine storage vats, baby formula bottles, water pipes, automotive lenses, optical lenses, protective coatings, adhesives, protective window glazing, compact disks, thermal paper, paper coatings and developer in dyes (Markey et al., 2002). BPA derivates including tetrabromobisphenol A (TBBPA) and tetrachlorobisphenol A (TCBPA) are used as flame retardants for building materials and plastic products including epoxy resin electronic circuit boards (Markey et al., 2001).
BPA alters ovarian function through its action as an EDC. In vitro exposure of rat ovarian theca-interstitial and granulosa cells to BPA increased testosterone, pregnenolone and E2 production. Also there were increased mRNA levels of Cypc17, Cyp19, Cyp11a and StAR and decreased P4 levels with BPA exposure (Zhou et al., 2008). BPA can bind to both E2 receptors (ERα and ERβ) and induce E2-dependent gene expression including Cyp1b1 in rats (Naciff et al., 2002). PND1 female rats exposed to BPA (50 ug/kg/day; subcutaneous injection; s.c.) demonstrated premature puberty onset and anestrus (Adewale et al., 2009). Also, BPA increases initiation of primordial follicle recruitment from PND 1–7, subsequently reducing the primordial follicle number (Rodriguez et al., 2010). Neonatal BPA exposure from PND 1–10 (500 μg/kg/d) resulted in anovulation and infertility in female rats from 4 months of age onwards. These female rats also had an accelerated GnRH pulse frequency in hypothalamic explants (Fernandez et al., 2010), suggesting an effect of BPA on the hypothalamic-pituitary-gonadal (HPG) axis. BPA exposure also affects the ovary in adulthood following in utero exposure. Dams exposed to BPA (250 ng/kg/day) from GD 9 to 21 resulted in the appearance of blood-filled ovarian bursa in female offspring at 6 month of age (Markey et al., 2003).
Little is known about how BPA is metabolized in the ovary and whether such metabolism impacts BPA-induced ovotoxicity. An increase in GSTM expression in gonads of adult fish has been demonstrated during BPA exposure (600 μg/L; Yu et al., 2008), indicating that GSH conjugation catalyzed by GSTM may be a metabolism route for BPA.
Phthalates
Phthalates are diesters of phthalic acid mostly used as plasticizers in polyvinyl chloride products (DHHS., 2005). Phthalates are ubiquitous environmental toxicants to which million people are exposed daily and human phthalate exposure has been confirmed by detection of phthalate metabolites in urine (Blount et al., 2000a; Blount et al., 2000b). Around 18 billion pounds of phthalates are used in plastic product manufacture annually (Blount et al., 2000a), and the most commonly used phthalates are di-2-ethylhexyphthalate (DEHP), dibutyl phthalate (DBP) and diethyl phthalate (DEP).
Phthalates esters are E2 analogues and have estrogenic effects (Sonnenschein and Soto, 1998). DEHP causes ovarian toxicity in rodents (Reddy and Lalwai, 1983; Lake et al., 1987; Davis et al., 1994a). Female mice and rats exposed to DEHP demonstrate reduced implantations, increased fetus resorption, decreased fetal weights and malformations (Kaul et al., 1982; Lovekamp-Swan and Davis, 2003). DEHP (1–100 ml/kg; 1, 5, 10 d; s.c.) exposure in mice resulted in decreased pregnancy rates. Also, decreased growing follicle and corpora lutea numbers were observed (Agarwal et al., 1989). At higher doses, DEHP exposure (500–3000 mg/kg/day; gavage) in mice and rats delayed vaginal opening with prolonged estrous cycles (Grande et al., 2006), altered ovulation (Davis et al., 1994a), and decreased fertility (Lamb et al., 1987; Gray et al., 2006). The impact of DEHP on anovulation may due to defects in HPG axis signaling (Svechnikova et al., 2007) or may also be attributable to decreased E2 production. DEHP exposure (100 μM for 24 h) decreased production of E2 in cultured rat granulosa cells (Treinen et al., 1990).
The toxic effects of DEHP are mostly carried out by its active metabolite mono-(2-ethylhexyl) phthalate (MEHP). MEHP acts by activating peroxisome proliferator-activated receptors (PPARs), the main regulators for lipid metabolism and cell differentiation (Maloney and Waxman, 1999). All known isoforms of PPAR (α, β and γ) are expressed in the rat ovary (Braissant et al., 1996), where PPAR α and β are located in theca cells and stromal tissue and PPARγ is highly expressed in pre-ovulatory granulosa cells (Komar et al., 2001). MEHP (0–400 μM) decreases E2 production in a dose-dependent manner in cultured rat granulosa cells (Davis et al., 1994b), likely due to decreased Cyp19 transcription and increased mRNA expression of Hsd17b1 in cultured rat granulosa cells (200 μM MEHP; 48 h; Lovekamp and Davis, 2001). The observed decreased Cyp19 mRNA expression is mediated through the action of both PPAR α and γ receptors (Lovekamp-Swan and Davis, 2003).
Chemotherapeutics
Anti-neoplastic therapy destroys ovarian follicles and consequently predisposes women to infertility and premature menopause (Absolom et al., 2008). Increased survival rates (Blumenfeld et al., 1996; Maltaris et al., 2006) over the past few decades have raised the reproductive toxicity consequences of chemotherapeutic agents as a serious issue. Cyclophosphamide (CPA) is used to treat childhood leukemia, Hodgkin’s and non-Hodgkin’s lymphomas, and breast cancer, bone and tissue sarcomas in adults (Colvin, 1999; Hurley, 2002; Chemaitilly et al., 2006). It is also used as an immunosuppressant for multiple sclerosis and organ transplant rejection (Colvin, 1999).
In hepatic tissue, CPA undergoes activation by CYP2B1 and CYP3A4 to 4-hydroxycyclophosphamide (4-HC) that is converted through non-enzymatic reactions to form aldophosphamide (AP), and subsequently phosphoramide mustard (PM) and acrolein (Ludeman, 1999). PM has been demonstrated to be the ultimate ovotoxic CPA metabolite (Plowchalk and Mattison, 1991; Desmeules and Devine, 2006). CPA can also be converted to carboxyphosphamide (CPM) or 4-ketocyclophosphamide (4-KTCP), which are nontoxic CPA metabolites due to their inability to form PM.
Ovarian follicles are the principal targets of CPA-induced ovotoxicity (Waxman, 1983). Sprague-Dawley female rats exposed to CPA (500 mg/kg; i.p.) demonstrate reduced ovarian follicle numbers in a time- and dose-dependent manner. Complete destruction of primordial follicles was observed within 3 d of CPA exposure (Shiromizu et al., 1984). Mouse primordial follicles are more susceptible than those of rats to CPA-induced depletion. Almost complete (98%) destruction of primordial follicles occurred when female mice were given a single CPA exposure (75 mg/kg; i.p; Plowchalk and Mattison, 1991).
Granulosa cells of large pre-antral and antral follicles are also substantially damaged by CPA treatment in mice and rats (Lopez and Luderer, 2004; Desmeules and Devine, 2006). Induction of the mitochondrial apoptotic pathway has been demonstrated in granulosa cells of secondary and antral follicles of rats following a single CPA exposure with a significant decrease in ovarian GSH level (300 mg/kg; i.p.; Lopez and Luderer, 2004). GSH has the capacity to detoxify reactive oxygen species (ROS; Dalton et al., 2004), however, no additional follicle loss was achieved however when GSH was suppressed using buthionine sulfoximine (BSO; 5 mmol/kg; i.p.) during CPA exposure (Lopez and Luderer, 2004). Additionally, human granulosa tumor cells, COV434, treated with a CPA metabolite, 4-hydroxycyclophosphamide (4-HC, 50 μM) had increased oxidative stress and reduction of GSH to oxidized GSH (GSSG; Tsai-Turton et al., 2007b). Similar to that of DMBA, use of TAM protects against CPA-induced primordial follicle loss (Ting and Petroff, 2010). These studies suggest that oxidative stress is involved in the apoptotic pathway induced by CPA and that GSH conjugation is a potential ovarian detoxification route.
Cultured PND4 mouse ovaries exposed to PM (3 μM) had rapid depletion of primordial oocytes and granulosa cells of larger follicles (Desmeules and Devine, 2006). Positive TUNEL staining of pyknotic granulosa cells was demonstrated, however, no increase in caspase-3 activation was observed (Desmeules and Devine, 2006). PM destroys tumor cells by binding covalently to DNA, inducing DNA-DNA and DNA-protein crosslinks and DNA double-strand breaks (DSB; (Colvin et al., 1999; Hurley et al., 2002; Helleday et al., 2008) and this mode of action has been confirmed in ovarian oocytes (Petrillo et al., 2011). Phosphorylation of H2AX (γH2AX) occurs in response to DSB and recruits DNA repair protein to the DSB sites (Rogakou et al., 1998; Paull et al., 2000; Modesti and Kanaar, 2001). γH2AX expression was observed within 9 h after PM (3–10 μM) exposure in cultured PND4 mouse and rat ovaries supporting that DSB were being induced by PM exposure (Petrillo et al., 2011). Interestingly, the DSB were detected at concentrations at which follicle loss was not observed, suggesting that germline DNA damage could occur prior to evidence of follicle depletion (Petrillo et al., 2011).
Aging
In mammals, ovarian aging is characterized by the reduction in ovarian follicle number and oocyte quality as well as dysfunction at the hypothalamic-pituitary-gonadal axis, culminating in reproductive senescence (Labhsetwar, 1967; Butcher and Page, 1981; Nozaki et al., 1995; Brann DW, 2005). Aging has also been associated with increased ROS generation and decreased antioxidant protection ultimately leading to a wide range of cellular damage (Dean et al., 1993). Ovarian cells are not an exception to this; there is increased ROS and decreased antioxidant levels in oocytes, cumulus cells as well as in follicular fluid of older women (45 years) undergoing assisted reproduction (Wiener-Megnazi et al., 2004; Tatone et al., 2006). GSH concentrations decreased in aged mouse oocytes compared to those from young mice (Hamatani et al., 2004; Brink et al., 2009), while superoxide dismutase (SOD) and glutathione peroxidase (GPX) enzymatic activities decreased in ovaries from postmenopausal compared to premenopausal women (Okatani et al., 1993). Furthermore, age-related increases in oxidative lipid, protein and DNA damage in ovarian interstitial cells and follicles have been reported with a decrease in glutaredoxin 1 (Glrx1) and Gstm2 and an increase in Gpx1 antioxidant gene expression (Lim and Luderer, 2010). Thus, due to the decrease in the ovarian protective response with aging, greater potential for damage caused by ovotoxicants is likely as females approach menopause.
In summary, ovarian metabolism plays an important role in determination of the ovotoxic impacts of a chemical exposure, and the studies on TCE, DMBA and VCD have begun to unravel mechanisms involved in xenobiotic metabolism. While insights on the impact of MXC, TCDD, BPA, Phthalates, and anti-neoplastic agents on normal ovarian processes are being delineated, there remains a dearth of information on whether and how these parent compounds are transformed to more active compound(s) in the ovary. Additionally, how these chemicals and their metabolites are detoxified and eliminated from the body remains poorly understood. Until greater insight is gained on ovarian xenobiotic biotransformation processes, attempts to develop therapeutic strategies to prevent follicle depletion and disruption to normal ovarian physiological processes will be limited.
Highlights.
Summary of ovotoxicant action during ovotoxicity
Discussion of impact of biotransformation on chemical toxicity
Identification of knowledge gaps in chemical metabolism
Acknowledgments
The project described was supported by award number R00ES016818 to AFK from the National Institutes of Environmental Health Sciences. The content is solely the responsibility of the authors and not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Conflicts of Interest Statement
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absolom K, Eiser C, Turner L, Ledger W, Ross R, Davies H, Coleman R, Hancock B, Snowden J, Greenfield D. Ovarian failure following cancer treatment: current management and quality of life. 2008;23:2506–2512. doi: 10.1093/humrep/den285. [DOI] [PubMed] [Google Scholar]
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81:690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal DK, Lawrence WH, Turner JE, Autian J. Effects of parenteral di-(2-ethylhexyl)phthalate (DEHP) on gonadal biochemistry, pathology, and reproductive performance of mice. J Toxicol Environ Health. 1989;26:39–59. doi: 10.1080/15287398909531232. [DOI] [PubMed] [Google Scholar]
- Akgul Y, Derk RC, Meighan T, Rao KM, Murono EP. The methoxychlor metabolite, HPTE, directly inhibits the catalytic activity of cholesterol side-chain cleavage (P450scc) in cultured rat ovarian cells. Reprod Toxicol. 2008;25:67–75. doi: 10.1016/j.reprotox.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Basavarajappa MS, Craig ZR, Hernandez-Ochoa I, Paulose T, Leslie TC, Flaws JA. Methoxychlor reduces estradiol levels by altering steroidogenesis and metabolism in mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2011;253:161–169. doi: 10.1016/j.taap.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Horner CM. In vivo exposure of female rats to toxicants may affect oocyte quality. Reprod Toxicol. 2003;17:273–281. doi: 10.1016/s0890-6238(03)00009-1. [DOI] [PubMed] [Google Scholar]
- Bevan C, Stadler JC, Elliott GS, Frame SR, Baldwin JK, Leung HW, Moran E, Panepinto AS. Subchronic toxicity of 4-vinylcyclohexene in rats and mice by inhalation exposure. Fundam Appl Toxicol. 1996;32:1–10. doi: 10.1006/faat.1996.0101. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Sen N, Hoyer PB, Keating AF. Ovarian expressed microsomal epoxide hydrolase: Role in detoxification of 4-vinylcyclohexene diepoxide and regulation by phosphatidylinositol-3 kinase signaling. Toxicol Appl Pharmacol. 2012;258:118–123. doi: 10.1016/j.taap.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Keating AF. Protective role for ovarian Glutathione S-transferase isoform pi during 7,12-dimethylbenz[a]anthracene-induced ovotoxicity. Toxicology and Applied Pharamacology. 2012 doi: 10.1016/j.taap.2012.02.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birner G, Vamvakas S, Dekant W, Henschler D. Nephrotoxic and genotoxic N-acetyl-S-dichlorovinyl-L-cysteine is a urinary metabolite after occupational 1,1,2-trichloroethene exposure in humans: implications for the risk of trichloroethene exposure. Environ Health Perspect. 1993;99:281–284. doi: 10.1289/ehp.9399281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000a;72:4127–4134. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000b;108:979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996;11:1620–1626. doi: 10.1093/oxfordjournals.humrep.a019457. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–478. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinol. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Brann DWMV. The aging reproductive neuroendocrine axis. Steroids. 2005;70:273–283. doi: 10.1016/j.steroids.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Brink TC, Demetrius L, Lehrach H, Adjaye J. Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontol. 2009;10:549–564. doi: 10.1007/s10522-008-9197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Page RD. Introductory remarks: environmental and endogenous hazards to the female reproductive system. Environ Health Perspect. 1981;38:35–37. doi: 10.1289/ehp.813835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady EA, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of microsomal epoxide hydrolase in follicles isolated from mouse ovaries. Toxicol Sci. 2002;68:24–31. doi: 10.1093/toxsci/68.1.24. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, Robison LL, Sklar CA. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- Chen X, Ma XM, Ma SW, Coenraads PJ, Zhang CM, Liu J, Zhao LJ, Sun M, Tang NJ. Proteomic analysis of the rat ovary following chronic low-dose exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) J Toxicol Environ Health A. 2009;72:717–726. doi: 10.1080/15287390902841136. [DOI] [PubMed] [Google Scholar]
- Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des. 1999;5:555–560. [PubMed] [Google Scholar]
- Commandeur JN, Vermeulen NP. Identification of N-acetyl(2,2-dichlorovinyl)- and N-acetyl(1,2-dichlorovinyl)-L-cysteine as two regioisomeric mercapturic acids of trichloroethylene in the rat. Chem Res Toxicol. 1990;3:212–218. doi: 10.1021/tx00015a005. [DOI] [PubMed] [Google Scholar]
- Cosby NC, Dukelow WR. Toxicology of maternally ingested trichloroethylene (TCE) on embryonal and fetal development in mice and of TCE metabolites on in vitro fertilization. Fundam Appl Toxicol. 1992;19:268–274. doi: 10.1016/0272-0590(92)90160-j. [DOI] [PubMed] [Google Scholar]
- Craig ZR, Leslie TC, Hatfield KP, Gupta RK, Flaws JA. Mono-hydroxy methoxychlor alters levels of key sex steroids and steroidogenic enzymes in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2010;249:107–113. doi: 10.1016/j.taap.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings AM, Gray LE., Jr Antifertility effect of methoxychlor in female rats: dose- and time-dependent blockade of pregnancy. Toxicol Appl Pharmacol. 1989;97:454–462. doi: 10.1016/0041-008x(89)90250-0. [DOI] [PubMed] [Google Scholar]
- Cummings BS, Lash LH. Metabolism and toxicity of trichloroethylene and S-(1,2-dichlorovinyl)-L-cysteine in freshly isolated human proximal tubular cells. Toxicol Sci. 2000;53:458–466. doi: 10.1093/toxsci/53.2.458. [DOI] [PubMed] [Google Scholar]
- Dalton T, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Davidson IW, Beliles RP. Consideration of the target organ toxicity of trichloroethylene in terms of metabolite toxicity and pharmacokinetics. Drug Metab Rev. 1991;23:493–599. doi: 10.3109/03602539109029772. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994a;128:216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2-ethylhexyl) phthalate suppresses estradiol production independent of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994b;128:224–228. doi: 10.1006/taap.1994.1201. [DOI] [PubMed] [Google Scholar]
- Dean RT, Gieseg S, Davies MJ. Reactive species and their accumulation on radical-damaged proteins. Trends Biochem Sci. 1993;18:437–441. doi: 10.1016/0968-0004(93)90145-d. [DOI] [PubMed] [Google Scholar]
- Desmeules P, Devine PJ. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicol Sci. 2006;90:500–509. doi: 10.1093/toxsci/kfj086. [DOI] [PubMed] [Google Scholar]
- Diagaradjane P, Yaseen MA, Yu J, Wong MS, Anvari B. Synchronous fluorescence spectroscopic characterization of DMBA-TPA-induced squamous cell carcinoma in mice. J Biomed Opt. 2006;11:014012. doi: 10.1117/1.2167933. [DOI] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nature rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Eroschenko VP, Swartz WJ, Ford LC. Decreased superovulation in adult mice following neonatal exposures to technical methoxychlor. Reprod Toxicol. 1997;11:807–814. doi: 10.1016/s0890-6238(97)00064-6. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118:1217–1222. doi: 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Keating AF, Christian PJ, Sen N, Hoying JB, Brooks HL, Hoyer PB. Involvement of the KIT/KITL signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol Reprod. 2008;79:318–327. doi: 10.1095/biolreprod.108.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franczak A, Nynca A, Valdez KE, Mizinga KM, Petroff BK. Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats. Biol Reprod. 2006;74:125–130. doi: 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- Grande SW, Andrade AJ, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol Sci. 2006;91:247–254. doi: 10.1093/toxsci/kfj128. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Laskey J, Ostby J. Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol Sci. 2006;93:189–195. doi: 10.1093/toxsci/kfl035. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991a;4:168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Turvy CG. Comparison of levels of several human microsomal cytochrome P-450 enzymes and epoxide hydrolase in normal and disease states using immunochemical analysis of surgical liver samples. J Pharmacol Exp Ther. 1991b;256:1189–1194. [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13:2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- Hazai E, Kupfer D. Interactions between CYP2C9 and CYP2C19 in reconstituted binary systems influence their catalytic activity: possible rationale for the inability of CYP2C19 to catalyze methoxychlor demethylation in human liver microsomes. Drug Metab Dispos. 2005;33:157–164. doi: 10.1124/dmd.104.001578. [DOI] [PubMed] [Google Scholar]
- Heiden TC, Struble CA, Rise ML, Hessner MJ, Hutz RJ, Carvan MJ., 3rd Molecular targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) within the zebrafish ovary: insights into TCDD-induced endocrine disruption and reproductive toxicity. Reprod Toxicol. 2008;25:47–57. doi: 10.1016/j.reprotox.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiden TK, Carvan MJ, 3rd, Hutz RJ. Inhibition of follicular development, vitellogenesis, and serum 17beta-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2006;90:490–499. doi: 10.1093/toxsci/kfj085. [DOI] [PubMed] [Google Scholar]
- Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. PNAS. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Ho HM, Ohshima K, Watanabe G, Taya K, Strawn EY, Hutz RJ. TCDD increases inhibin A production by human luteinized granulosa cells in vitro. J Reprod Dev. 2006;52:523–528. doi: 10.1262/jrd.18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooser SB, Douds DP, DeMerell DG, Hoyer PB, Sipes IG. Long-term ovarian and gonadotropin changes in mice exposed to 4-vinylcyclohexene. Reprod Toxicol. 1994;8:315–323. doi: 10.1016/0890-6238(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Taii S, Narimoto K, Noda Y, Nishikawa S, Nakayama H, Fujita J, Mori T. The expression of c-kit protein during oogenesis and early embryonic development. Biol Reprod. 1991;45:547–552. doi: 10.1095/biolreprod45.4.547. [DOI] [PubMed] [Google Scholar]
- Hoyer PB. Reproductive toxicology: current and future directions. Biochem Pharmacol. 2001;41:1557–1564. doi: 10.1016/s0006-2952(01)00814-0. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Sipes IG. Assessment of follicle destruction in chemical-induced ovarian toxicity. Annu Rev Pharmacol Toxicol. 1996;36:307–331. doi: 10.1146/annurev.pa.36.040196.001515. [DOI] [PubMed] [Google Scholar]
- Hu X, Christian P, Sipes IG, Hoyer PB. Expression and redistribution of cellular Bad, Bax, and Bcl-X(L) protein is associated with VCD-induced ovotoxicity in rats. Biol Reprod. 2001;65:1489–1495. doi: 10.1095/biolreprod65.5.1489. [DOI] [PubMed] [Google Scholar]
- Hu Y, Krausz K, Gelboin HV, Kupfer D. CYP2C subfamily, primarily CYP2C9, catalyses the enantioselective demethylation of the endocrine disruptor pesticide methoxychlor in human liver microsomes: use of inhibitory monoclonal antibodies in P450 identification. Xenobiotic. 2004;34:117–132. doi: 10.1080/00498250310001644535. [DOI] [PubMed] [Google Scholar]
- Hu Y, Kupfer D. Metabolism of the endocrine disruptor pesticide-methoxychlor by human P450s: pathways involving a novel catechol metabolite. Drug Metab Dispos. 2002;30:1035–1042. doi: 10.1124/dmd.30.9.1035. [DOI] [PubMed] [Google Scholar]
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- Igawa Y, Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB. Evaluation of ovotoxicity induced by 7, 12-dimethylbenz[a]anthracene and its 3,4-diol metabolite utilizing a rat in vitro ovarian culture system. Toxicol Appl Pharmacol. 2009;234:361–369. doi: 10.1016/j.taap.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska O, Shi Z, Valdez KE, Ting AY, Petroff BK. Temporal and anatomical sensitivities to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin leading to premature acyclicity with age in rats. Int J Androl. 2010;33:405–412. doi: 10.1111/j.1365-2605.2009.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter EM, Walker RM, Marion SL, Brewer M, Hoyer PB, Barton JK. Dual modality imaging of a novel rat model of ovarian carcinogenesis. J Biomed Opt. 2006;11:041123. doi: 10.1117/1.2236298. [DOI] [PubMed] [Google Scholar]
- Kaul AF, Souney PF, Osathanondh R. A review of possible toxicity of di-2-ethylhexylphthalate (DEHP) in plastic intravenous containers: effects on reproduction. Drug Intell Clin Pharm. 1982;16:689–692. doi: 10.1177/106002808201600908. [DOI] [PubMed] [Google Scholar]
- Keating AF, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol Appl Pharmacol. 2009;241:127–134. doi: 10.1016/j.taap.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Fernandez SM, Mark-Kappeler CJ, Sen N, Sipes IG, Hoyer PB. Inhibition of PIK3 signaling pathway members by the ovotoxicant 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2011;84:743–751. doi: 10.1095/biolreprod.110.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sen N, Sipes IG, Hoyer PB. Dual protective role for glutathione S-transferase class pi against VCD-induced ovotoxicity in the rat ovary. Toxicol Appl Pharmacol. 2010;247:71–75. doi: 10.1016/j.taap.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sipes IG, Hoyer PB. Expression of ovarian microsomal epoxide hydrolase and glutathione S-transferase during onset of VCD-induced ovotoxicity in B6C3F(1) mice. Toxicol Appl Pharmacol. 2008;230:109–116. doi: 10.1016/j.taap.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Kim SG. Phase II enzyme induction by alpha-lipoic acid through phosphatidylinositol 3-kinase-dependent C/EBPs activation. Xenobiotica. 2008;38:587–604. doi: 10.1080/00498250802126920. [DOI] [PubMed] [Google Scholar]
- Komar CM, Braissant O, Wahli W, Curry TE., Jr Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinol. 2001;142:4831–4838. doi: 10.1210/endo.142.11.8429. [DOI] [PubMed] [Google Scholar]
- Labhsetwar A. Age-dependent changes in the pituitary-gonadal relationship: a study of ovarian compensatory hypertrophy. J Endocrinol. 1967;39:387–393. doi: 10.1677/joe.0.0390387. [DOI] [PubMed] [Google Scholar]
- Lake BG, Gray TJ, Lewis DF, Beamand JA, Hodder KD, Purchase R, Gangolli SD. Structure-activity relationships for induction of peroxisomal enzyme activities by phthalate monoesters in primary rat hepatocyte cultures. 1987;3:165–183. doi: 10.1177/074823378700300212. [DOI] [PubMed] [Google Scholar]
- Lamb JC, 4th, Chapin RE, Teague J, Lawton AD, Reel JR. Reproductive effects of four phthalic acid esters in the mouse. Toxicol Appl Pharmacol. 1987;88:255–269. doi: 10.1016/0041-008x(87)90011-1. [DOI] [PubMed] [Google Scholar]
- Lash LH, Qian W, Putt DA, Jacobs K, Elfarra AA, Krause RJ, Parker JC. Glutathione conjugation of trichloroethylene in rats and mice: sex-, species-, and tissue-dependent differences. Drug Metab Dispos. 1998;26:12–19. [PubMed] [Google Scholar]
- Lash LH, Xu Y, Elfarra AA, Duescher RJ, Parker JC. Glutathione-dependent metabolism of trichloroethylene in isolated liver and kidney cells of rats and its role in mitochondrial and cellular toxicity. Drug Metab Dispos. 1995;23:846–853. [PubMed] [Google Scholar]
- Lawther PJ, Waller RE. Coal fires, industrial emissions and motor vehicles as sources of environmental carcinogens. IARC. 1976:27–40. [PubMed] [Google Scholar]
- Li X, Johnson DC, Rozman KK. Reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female rats: ovulation, hormonal regulation, and possible mechanism(s) Toxicol Appl Pharmacol. 1995;133:321–327. doi: 10.1006/taap.1995.1157. [DOI] [PubMed] [Google Scholar]
- Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2010;84:775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Lopez SG, Luderer U. Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic Biol Med. 2004;36:1366–1377. doi: 10.1016/j.freeradbiomed.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172:217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Ludeman SM. The chemistry of the metabolites of cyclophosphamide. Curr Pharm Des. 1999;5:627–643. [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ. Trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- Maltaris T, Boehm D, Dittrich R, Seufert R, Koelbl H. Reproduction beyond cancer: a message of hope for young women. Gynecol Oncol. 2006;103:1109–1121. doi: 10.1016/j.ygyno.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova RF. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Mark-Kappeler CJ, Sen N, Lukefahr A, McKee L, Sipes IG, Konhilas J, Hoyer PB. Inhibition of ovarian KIT phosphorylation by the ovotoxicant 4-vinylcyclohexene diepoxide in rats. Biol Reprod. 2011;85:755–762. doi: 10.1095/biolreprod.111.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev. 2003;5:67–75. doi: 10.1046/j.1525-142x.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- Markey CM, Rubin BS, Soto AM, Sonnenschein C. Endocrine disruptors: from Wingspread to environmental developmental biology. J Steroid Biochem Mol Biol. 2002;83:235–244. doi: 10.1016/s0960-0760(02)00272-8. [DOI] [PubMed] [Google Scholar]
- Martinez EM, Swartz WJ. Effects of methoxychlor on the reproductive system of the adult female mouse. 1 Gross and histologic observations. Reprod Toxicol. 1991;5:139–147. doi: 10.1016/0890-6238(91)90042-e. [DOI] [PubMed] [Google Scholar]
- Martinez EM, Swartz WJ. Effects of methoxychlor on the reproductive system of the adult female mouse: 2. Ultrastructural observations Reprod Toxicol. 1992;6:93–98. doi: 10.1016/0890-6238(92)90026-p. [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nature Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Schulman JD. How xenobiotic chemicals can destroy oocytes. Am J Ind Med. 1980:4. [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod Toxicol. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Miyata M, Judo G, Lee Y, Yang TJ, Gelboni HV, Fernandez-Salguero P, Kimura S, Gonzalez FJ. Targeted disruption of the microsomal epoxide hydrolase gene. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12-dimethylbenz[a]anthracene. J Biol Chem. 1999;274:23963–23968. doi: 10.1074/jbc.274.34.23963. [DOI] [PubMed] [Google Scholar]
- Modesti M, Kanaar R. DNA repair: spot(light)s on chromatin. Curr Biol. 2001;11:R229–232. doi: 10.1016/s0960-9822(01)00112-9. [DOI] [PubMed] [Google Scholar]
- Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, Daston GP. Gene expression profile induced by 17alpha-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci. 2002;68:184–199. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Okino T, Okuyama S, Kaneko T, Yonekura I, Sato A. Ethanol-induced enhancement of trichloroethylene metabolism and hepatotoxicity: difference from the effect of phenobarbital. Toxicol Appl Pharmacol. 1988;94:227–237. doi: 10.1016/0041-008x(88)90264-5. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Wang RS, Elovaara E, Park SS, Gelboin HV, Vainio H. A comparative study on the contribution of cytochrome P450 isozymes to metabolism of benzene, toluene and trichloroethylene in rat liver. Biochem Pharmacol. 1992a;43:251–257. doi: 10.1016/0006-2952(92)90285-q. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Wang RS, Elovaara E, Park SS, Gelboin HV, Vainio H. Cytochrome P450-related differences between rats and mice in the metabolism of benzene, toluene and trichloroethylene in liver microsomes. Biochem Pharmacol. 1993;45:1079–1085. doi: 10.1016/0006-2952(93)90252-r. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Wang RS, Katakura Y, Kishi R, Elovaara E, Park SS, Gelboin HV, Vainio H. Sex-, age- and pregnancy-induced changes in the metabolism of toluene and trichloroethylene in rat liver in relation to the regulation of cytochrome P450IIE1 and P450IIC11 content. J Pharmacol Exp Ther. 1992b;261:869–874. [PubMed] [Google Scholar]
- Nakajima T, Wang RS, Murayama N, Sato A. Three forms of trichloroethylene-metabolizing enzymes in rat liver induced by ethanol, phenobarbital, and 3-methylcholanthrene. Toxicol Appl Pharmacol. 1990;102:546–552. doi: 10.1016/0041-008x(90)90049-z. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Mitsunaga F, Shimizu K. Reproductive senescence in female Japanese monkeys (Macaca fuscata): age- and season-related changes in hypothalamic-pituitary-ovarian functions and fecundity rates. Biol Reprod. 1995;52:1250–1257. doi: 10.1095/biolreprod52.6.1250. [DOI] [PubMed] [Google Scholar]
- Okatani Y, Morioka N, Wakatsuki A, Nakano Y, Sagara Y. Role of the free radical-scavenger system in aromatase activity of the human ovary. Horm Res. 1993;39(Suppl 1):22–27. doi: 10.1159/000182753. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: role of the estrogen receptor. Toxicol Sci. 2000;56:332–339. doi: 10.1093/toxsci/56.2.332. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Ogilvie BW. Casarett and Doull’s Toxicology:the basic science of poisons. In: Klassen CD, editor. Biotransformation of xenobiotics. Chapter 6. The McGraw-Hill Companies publishing; 2008. pp. 131–160. [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Petrillo SK, Desmeules P, Truong TQ, Devine PJ. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol Appl Pharmacol. 2011;253:94–102. doi: 10.1016/j.taap.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Plowchalk DR, Mattison DR. Phosphoramide mustard is responsible for the ovarian toxicity of cyclophosphamide. Toxicol Appl Pharmacol. 1991;107:472–481. doi: 10.1016/0041-008x(91)90310-b. [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Rajapaksa KS. Dissertation. Department of Physiology, University of Arizona; 2007. The role of ovarian metabolism in 4-vinylcyclohexene metabolites and 7, 12-dimethylbenz[a]anthracene-induced ovotoxicity in mice. [Google Scholar]
- Rajapaksa KS, Cannady EA, Sipes IG, Hoyer PB. Involvement of CYP 2E1 enzyme in ovotoxicity caused by 4-vinylcyclohexene and its metabolites. Toxicol Appl Pharmacol. 2007a;221:215–221. doi: 10.1016/j.taap.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksa KS, Sipes IG, Hoyer PB. involvement of microsomal epoxide hydrolase enzyme in ovotoxicity caused by 7,12-dimethylbenz[a]anthracene. Toxicol Sci. 2007b;96:327–334. doi: 10.1093/toxsci/kfl202. [DOI] [PubMed] [Google Scholar]
- Rappaport SM, Fraser DA. Air sampling and analysis in a rubber vulcanization area. Am Ind Hyg Assoc J. 1977;38:205–210. doi: 10.1080/0002889778507601. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Lalwai ND. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12:1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol. 2005;281:160–170. doi: 10.1016/j.ydbio.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30:550–557. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- Safe S, Astroff B, Harris M, Zacharewski T, Dickerson R, Romkes M, Biegel L. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and related compounds as antioestrogens: characterization and mechanism of action. Pharmacol Toxicol. 1991;69:400–409. doi: 10.1111/j.1600-0773.1991.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Serve H, Hsu YC, Besmer P. Tyrosine residue 719 of the c-kit receptor is essential for binding of the P85 subunit of phosphatidylinositol (PI) 3-kinase and for c-kit-associated PI 3-kinase activity in COS-1 cells. J Biol Chem. 1994;269:6026–6030. [PubMed] [Google Scholar]
- Shi Z, Valdez KE, Ting AY, Franczak A, Gum SL, Petroff BK. Ovarian endocrine disruption underlies premature reproductive senescence following environmentally relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol Reprod. 2007;76:198–202. doi: 10.1095/biolreprod.106.053991. [DOI] [PubMed] [Google Scholar]
- Shimada T, Sugie A, Shindo M, Nakajima T, Azuma E, Hashimoto M, Inoue K. Tissue-specific induction of cytochromes P450 1A1 and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in engineered C57BL/6J mice of arylhydrocarbon receptor gene. Toxicol Appl Pharmacol. 2003;187:1–10. doi: 10.1016/s0041-008x(02)00035-2. [DOI] [PubMed] [Google Scholar]
- Shiromizu K, Mattison DR. Murine oocyte destruction following intraovarian treatment with 3-methylcholanthrene or 7,12-dimethylbenz(a)anthracene: protection by alpha-naphthoflavone. Teratog Carcinog Mutagen. 1985;5:463–472. doi: 10.1002/tcm.1770050609. [DOI] [PubMed] [Google Scholar]
- Shiromizu K, Thorgeirsson SS, Mattison DR. Effect of cyclophosphamide on oocyte and follicle number in Sprague-Dawley rats, C57BL/6N and DBA/2N mice. Pediatr Pharmacol. 1984;4:213–221. [PubMed] [Google Scholar]
- Smith BJ, Mattison DR, Sipes IG. The role of epoxidation in 4-vinylcyclohexene-induced ovarian toxicity. Toxicol Appl Pharmacol. 1990;105:372–381. doi: 10.1016/0041-008x(90)90141-g. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the Villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci. 2011;123:563–575. doi: 10.1093/toxsci/kfr195. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. 1998;65:143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996a;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer LN, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol Appl Pharmacol. 1996b;139:402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Stresser DM, Kupfer D. Human cytochrome P450-catalyzed conversion of the proestrogenic pesticide methoxychlor into an estrogen. Role of CYP2C19 and CYP1A2 in O-demethylation. Drug Metab Dispos. 1998;26:868–874. [PubMed] [Google Scholar]
- Svechnikova I, Svechnikov K, Soder O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J Endocrinol. 2007;194:603–609. doi: 10.1677/JOE-07-0238. [DOI] [PubMed] [Google Scholar]
- Swartz WJ, Corkern M. Effects of methoxychlor treatment of pregnant mice on female offspring of the treated and subsequent pregnancies. Reprod Toxicol. 1992;6:431–437. doi: 10.1016/0890-6238(92)90006-f. [DOI] [PubMed] [Google Scholar]
- Symonds DA, Miller KP, Tomic D, Flaws JA. Effect of methoxychlor and estradiol on cytochrome p450 enzymes in the mouse ovarian surface epithelium. Toxicol Sci. 2006;89:510–514. doi: 10.1093/toxsci/kfj044. [DOI] [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod. 2006;12:655–660. doi: 10.1093/molehr/gal080. [DOI] [PubMed] [Google Scholar]
- Ting AY, Petroff BK. Tamoxifen decreases ovarian follicular loss from experimental toxicant DMBA and chemotherapy agents cyclophosphamide and doxorubicin in the rat. J Asst Repro Genet. 2010;27:591–597. doi: 10.1007/s10815-010-9463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinen KA, Dodson WC, Heindel JJ. Inhibition of FSH-stimulated cAMP accumulation and progesterone production by mono(2-ethylhexyl) phthalate in rat granulosa cell cultures. Toxicol Appl Pharmacol. 1990;106:334–340. doi: 10.1016/0041-008x(90)90252-p. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Nakamura BN, Luderer U. Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol Reprod. 2007a;77:442–451. doi: 10.1095/biolreprod.107.060368. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luong BT, Tan Y, Luderer U. Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol Sci. 2007b;98:216–230. doi: 10.1093/toxsci/kfm087. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Service. Toxicological profile for methoxychlor (update) Atlanta, GA: Public Health Service; 2002. [Google Scholar]
- United States Department of Health and Human Service. NCEH Pub No 05-0570. Center for Disease Control and Prevention (CDC); 2005. [Google Scholar]
- United States Environmental Protection Agency. Final Report EPA/600/8-82/006F. Washington, DC: Environmental Protection Agency, Office of Health and Environmental Assessment; 1985. Health Assessment Document for Tricholoroethylene. [Google Scholar]
- United States National Toxicology Program. NTP Tech Rep No 362. 1989. [Google Scholar]
- Waters KM, Safe S, Gaido KW. Differential gene expression in response to methoxychlor and estradiol through ERα, ERβ, and AR in reproductive tissues of female mice. Toxicol Sci. 2001;63:47–56. doi: 10.1093/toxsci/63.1.47. [DOI] [PubMed] [Google Scholar]
- Waxman J. Chemotherapy and the adult gonad: a review. J R Soc Med. 1983;76:144–148. doi: 10.1177/014107688307600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NS. Cancer in relation to occupational exposure to trichloroethylene. Occup Environ Med. 1996;53:1–5. doi: 10.1136/oem.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Pediatrics. 1992;90:342–349. [PubMed] [Google Scholar]
- Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M, Dirnfeld M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82:1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Wu C, Schaum J. Exposure assessment of trichloroethylene. Environ Health Perspect. 2000;108(Suppl 2):359–363. doi: 10.1289/ehp.00108s2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Berger T. Trichloroethylene metabolism in the rat ovary reduces oocyte fertilizability. Chem Biol Interact. 2007;170:20–30. doi: 10.1016/j.cbi.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Berger T. Ovarian gene expression is stable after exposure to trichloroethylene. Toxicol Lett. 2008;177:59–65. doi: 10.1016/j.toxlet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IT, Rhee JS, Raisuddin S, Lee JS. Characterization of the glutathione S-transferase-Mu (GSTM) gene sequence and its expression in the hermaphroditic fish, Kryptolebias marmoratus as a function of development, gender type and chemical exposure. Chem Biol Interact. 2008;174:118–125. doi: 10.1016/j.cbi.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283:12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]