Summary
Anemia is a common problem after renal transplantation. Therefore, the patients are treated with erythropoietin stimulating agents (ESAs). The varying response to treatment contributes to hemoglobin variability, which might be associated with mortality. We conducted a retrospective cohort study of first kidney allograft recipients between 1990 and 2008 represented in the Austrian Transplant Registry. We included 1441 patients of whom 683 received ESAs at any time after transplantation. Cox regression with cubic splines and linear estimates and the purposeful selection algorithm of covariables were used. The measure of variability was the moving standard deviation computed at three monthly intervals for the entire graft life. The hazard ratio (HR) of mortality and graft loss in the spline models increased with hemoglobin variability. The linear HR for mortality was 2.35 (95% confidence interval 1.75–3.17, P < 0.001) and functional graft loss 2.45 (1.76–3.40, P < 0.001). In an adjusted Cox model (ESA use, hemoglobin, age, diabetes, days on dialysis, eGFR, biopsy confirmed acute rejection and year of transplantation), hemoglobin variability was associated with mortality (HR: 2.11; 1.51–2.94; P < 0.001). No association with functional graft loss could be detected (HR: 1.34; 0.93-1.93; P = 0.121). These findings suggest that hemoglobin variability is associated with mortality of renal allograft recipients.
Keywords: Cox models, hemoglobin variability, kidney transplantation
Introduction
Anemia is a common problem in renal transplant patients. According to the Transplant European Survey on Anemia Management (TRESAM) the prevalence is 38.6%. Of these anemic patients 25% suffer from severe anemia, meaning their hemoglobin value is less than 11 or 10 g/dl for males or females, respectively [1]. Several factors are known to induce anemia in allograft recipients. These are sepsis, CMV, prophylactic co-trimoxazole, ganciclovir, immunosuppressive agents, ACE inhibitors, kidney injury during the transplantation procedure, poor graft function, and surgical problems in the recipient after transplantation. In a study performed by Chhabra and colleagues, it was shown that severe anemia is highly associated with reduced patient and graft survival as well as acute rejection [2]. It is also known that anemia increases the risk of cardiovascular disease and is related to left ventricular hypertrophy in renal transplant patients [3].
ESAs have been used over the last two decades to treat anemic renal transplant recipients. According to KDOQI guidelines, a target hemoglobin value of 12 g/dl was considered appropriate [4]. However, in most individuals there is a considerable amount of spread of hemoglobin levels over time. The consequence is a variability of hemoglobin levels, which have been thoroughly studied in dialysis patients, but not in transplant patients. In a recent retrospective study of subjects on hemodialysis, we showed that a higher hemoglobin variability was associated with increased mortality [5].
Furthermore, in transplanted patients the hemoglobin concentration is also a function of GFR and time after transplantation [6,7]. Usually, the hemoglobin concentration increases after the first months after transplantation. Chadban and colleagues showed that besides GFR other factors intrinsic to renal transplant recipients determine hemoglobin levels [8].
However, in transplant recipients the variability of hemoglobin over time was considered only in a few studies [9,10]. Therefore, we performed this cohort study to further elucidate the impact of hemoglobin variability on hard outcomes such as graft and patient survival in kidney transplanted patients.
Patients and methods
Patients
We analyzed the Austrian Registry of Transplanted Patients (OEDTR), which includes all transplants performed in Austria since 1970 [11]. This database holds 1808 first renal transplantations performed between the years 1990 and 2008 with known ESA or nonESA therapy. A list of variables reported in this repository may be found elsewhere [12]. Estimated GFR was computed by the abbreviated MDRD formula [13]. The recipients demographic data were stratified according to their ESA treatment (ever or never use). In the analysis, ESA users were defined as having received the drug at least for 10% of their graft life.
Outcomes
Mortality and graft loss were evaluated. Functional graft loss was defined as return to dialysis or retransplant.
Hemoglobin variability
The time line of a transplant was divided in quarters of a year in which the median was calculated for the laboratory parameters. Hemoglobin variability was calculated by a moving standard deviation with a rolling window spanning four quarters, meaning that for every four quarters in a row the standard deviation was imputed. This implies that only patients who did not experience graft loss or died in the first year were included in the analysis. Therefore, data of 1441 patients were analyzed.
Statistical analyses
Cox proportional hazards model
The hazard ratio of mortality and functional graft loss was computed by a Cox proportional hazard model with restricted cubic splines using three knots to gain more flexibility in estimating nonlinear effects of continuous predictors such as hemoglobin variability [14,15]. For the crude analysis, we used a model including only moving average of hemoglobin level, moving standard deviation of hemoglobin and ESA therapy. In an adjusted clinical expertise model age at transplantation, diabetes, days on dialysis, GFR, biopsy confirmed acute rejection (BCAR) and year of transplantation, was added. To evaluate other covariables, we investigated a model with the purposeful selection algorithm in which all significant variables are included in the model and additionally all variables, which change the hazard ratio of others by more than 25% [16,17]. Missing values were imputed by linear regression. For all statistical tests a P-value less than 0.05 was considered significant. The statistical analysis was performed using sas for Windows 9.2TS1M0 (SAS Institute, Inc., Cary, NC, USA).
Results
Demographic variables as well as treatment and outcome relevant data are displayed in Table 1.
Table 1.
Demographic data of patients at time of transplantation. The P-value compares the two groups ESA and no-ESA therapy.
| Variable | No ESA therapy | ESA therapy | P-value |
|---|---|---|---|
| Number of patients | 758 | 683 | n. a. |
| Recipient sex (M/F) | 488/270 | 377/306 | <0.001 |
| Donor sex (M/F) | 444/287 | 387/276 | 0.368 |
| Renal Diagnosis (glomerulonephritis/vascular/diabetes/else) | 214/63/95/380 | 178/66/73/364 | 0.378 |
| Cause of death (cardvasc/infection/malign/else) | 70/33/25/28 | 69/51/14/41 | 0.039 |
| Diabetes mellitus (no/yes) | 644/114 | 570/113 | 0.434 |
| Number of Hypertensives (0/1/2/3/4/5/6/7) | 100/135/189/173/101/39/11/1 | 74/101/166/144/102/66/13/0 | 0.023 |
| BCAR (yes/no/unknown) | 342/307/43 | 369/213/19 | <0.001 |
| CMV (R-D−/R-D+/R+D-/R+D+/unknown) | 65/113/116/248/216 | 53/106/113/231/180 | |
| Insulin (yes/no/unknown) | 89/666/1 | 95/447/1 | 0.014* |
| Immunosuppression (steroid + AZA + CsA/steroid + MMF + CsA/no steroid/else and unknown) |
220/196/58/284 | 175/161/55/292 | 0.183 |
| CNI (no/yes) | 161/597 | 109/57 | 0.010 |
| CIT (h) | 17.8 (8.0) | 16.6 (8.5) | 0.009 |
| Donor age (years) | 40 (16) | 47 (15) | <0.001 |
| Weight (kg) | 71 (16) | 71 (16) | 0.493 |
| Sum of HLA mismatch | 2.5 (1.8) | 2.5 (1.4) | 0.648 |
| PRA | 4.5 (12.5) | 4.7 (12.6) | 0.774 |
| Recipient age (years) | 49 (15) | 49 (15) | 0.709 |
| GFR (ml/min/1.73 m2) | 46 (24) | 40 (24) | <0.001 |
| Creatinine (mg/dl) | 1.97 (1.36) | 2.27 (1.58) | <0.001 |
| Hemoglobin (g/l) | 11.4 (2.4) | 10.8 (2.6) | <0.001 |
Fisher exact test.
BCAR, biopsy confirmed acute rejection; CMV, cytomegalie virus; R, recipient; D, donor; CNI, calcineurin inhibitor; CIT, cold ischemic time; PRA, panel reactive antibody.
Mortality and functional graft loss
Unadjusted analyses
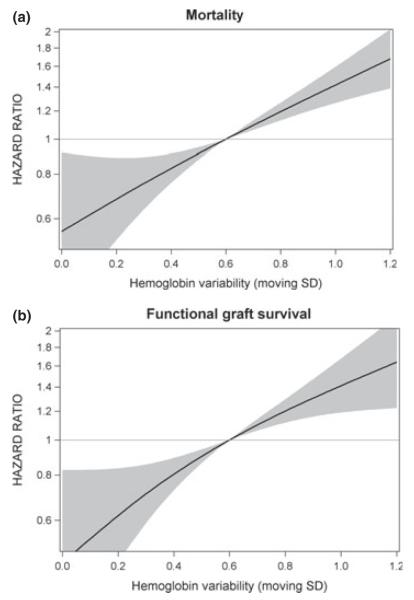
In all types of models, the hemoglobin variability was associated with mortality as well as functional graft loss (Fig. 1a and b). The estimated HRs over the full range of moving standard deviations estimated by linear models are indicated in Table 2.
Figure 1.
Hazard ratio for hemoglobin variability defined by moving standard deviation (SD) for (a) mortality and (b) functional graft survival. The Cox regression with restricted cubic splines was adjusted for hemoglobin and ESA therapy.
Table 2.
Hazard ratios and confidence intervals for mortality and functional graft loss in linear Cox models adjusted for Hb and ESA use.
| 95% Hazard Ratio | ||||
|---|---|---|---|---|
|
| ||||
| Parameter | Hazard ratio |
Confidence limits |
P-value | |
| Mortality | ||||
| Hb | 0.79 | 0.71 | 0.87 | <0.001 |
| Hb variability | 2.35 | 1.75 | 3.17 | <0.001 |
| ESA (yes versus no) | 1.00 | 0.73 | 1.3 | 0.998 |
| Functional graft loss | ||||
| Hb | 0.69 | 0.61 | 0.77 | <0.001 |
| Hb variability | 2.45 | 1.76 | 3.40 | <0.001 |
| ESA (yes versus no) | 1.73 | 1.19 | 2.52 | 0.004 |
Adjusted analyses
The clinical expertise models revealed no statistical association with functional graft loss (HR: 1.34, 95% CI 0.93–1.93, P = 0.121), whereas a statistically significant association with mortality (HR: 2.11, 95% CI 1.51–2.94, P < 0.001). The purposeful selection algorithm revealed similar results as the clinical experience model (Fig. 2).
Figure 2.
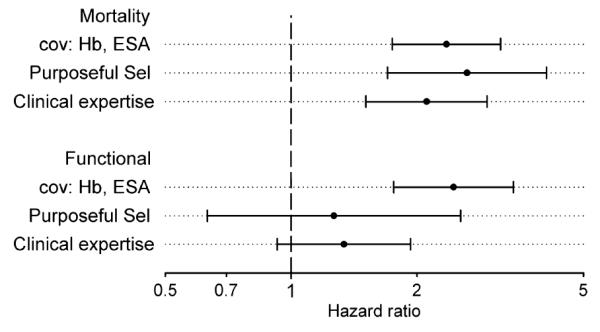
Hazard ratio and 95% confidence interval of hemoglobin variability computed for a clinical expertise model (covariates: age at transplantation, diabetes, vintage of dialysis, GFR, year of transplantation), a purposeful selection model and a model including only hemoglobin and ESA as covariates (cov).
Variables identified as significant counfounders by the purposeful selection algorithm were BCAR, eGFR, cold ischemic time, diabetes, comorbidities of lung, serum creatinine, phosphate, sodium, iron, and TRFS. In addition, the model for mortality included age at transplantation, donor age, PRA, sum of HLA mismatch, neoplasia, immunosuppression regimen, and iron. In the functional graft loss model, vintage of dialysis, cholesterin, and STRF turned out to be significant by using the purposeful selection method.
Discussion
In this study, we show that the risk of death was clearly associated with increased variability of hemoglobin levels. Depending on the statistical evaluation used, there may also be a small effect of variable hemoglobin levels with functional graft loss.
The strength of our study lies in the way we defined variability in subjects with variable graft survival duration and the completeness of the OEDTR database. The moving standard deviation was used as solid measure for hemoglobin variability in the Cox models with splines for the covariable variability. Other common methods of variability estimates such as grouping of absolute hemoglobin values is disadvantageous in cases with longer follow up, because the changing of variability over time is neglected as it is more likely to have a higher variability in the first months after transplantation than later [6]. Therefore, ESA therapy is more frequently used in the extremes of follow up, i.e., early after engraftment and at the end of graft survival and ESA use will induce higher variability of hemoglobin.
In a recent study, Jason and colleagues examined 3 854 patients of a United Kingdom cohort [10]. The authors defined the variability by means of three hemoglobin values measured at 0, 3, and 6 months after including the patient into the study. Investigators could not find any association with mortality, which is in contrast to our study. However, we used another definition were the variability would change over time.
Furthermore, it remains unclear, whether Jason and coworkers included ESA use in the Cox model as covariable, although they collected data about ESA use. However, we found ESA use only significantly associated with functional graft loss. Therefore, we could have excluded that variable at least for mortality. Nevertheless, we found an association with hemoglobin variability.
Another reason for the difference might be that we assigned patients to ESA users when they used ESA in at least 10% of the follow-up time. However, as we used ESA as a covariable, in general this should not have an effect on the hazard ratio of variability. In contrast, a study performed by Kamar included patients into the nonESA group although they received ESA for 50% of the follow-up time [18].
The study performed by Jason also included only variables in the multivariate Cox model, which have been associated with a P-value below 0.15 in an univariate analysis. However, this approach has a higher likelihood of eliminating potential confounders with less-stringent associations as described by Hosmer and Lemeshow, who also developed the purposeful selection algorithm [16,19].
To find out predictors of immediate posttransplant anemia an observational study was performed by Poesen and colleagues [20]. The predictors for anemia after 3 months were donor age, gender, polycystic disease, pretransplant hemoglobin, ferritin level, GFR, and hospitalization. Another study dealing with the short term anemia showed similar predictive variables [18]. Many of these variables are also included in our model. Both studies did not investigate the hemoglobin variability and therefore no comparison is possible in that respect.
Fernandez Fresnedo et al. studied a patient population of 85 transplanted patients [9]. The investigators used the difference of hemoglobin values to baseline hemoglobin as a definition for variability. No difference in survival and graft loss in the univariable Kaplan–Meier analysis was observed. However, Kaplan–Meier product limit estimates should be reserved for randomized trials and no attempt has been made in this article to adjust for confounders and reversed causation. On the other hand, given the very few outcomes this was actually not feasible in that study.
A limitation of our study is the missing dose of ESA used for therapy for each patient. In a recent study with hemodialysis patients, we could show that there is a difference in the hazard ratio of mortality between ESA responders and such patients who respond less to ESA therapy [5].
On the other hand, a unique strength of our study is the long follow-up time of up to 19 years as well as the completeness of the database. Furthermore, the sophisticated analyses using Cox models with restricted splines for hemoglobin and its variability allowed the flexible calculation of risk over the whole range of the explanatory variable.
In conclusion, our data suggest that hemoglobin variability is strongly associated with mortality, but not with functional graft loss. As this is an observational study no treatment recommendation can be derived from our analyses.
Acknowledgements
We are indebted to the administrators and all contributors of the Austrian Dialysis and Transplant Registry who are listed in the Annual Data Report [11].
Funding
This study was supported by grants from the Austrian Science Fund (FWF P-21436) and the Austrian Academy of Science (OELZELT EST370/04) to R.O. and by an unrestricted educational grant from Amgen Austria.
Footnotes
Authorship
AK: participated in the writing of the paper and data analysis. JW: participated in data analysis. RF: participated in research design. RK: participated in research design. RO: participated in research design, writing of the paper and data analysis.
Conflict of interest There is no conflict of interest.
References
- 1.Vanrenterghem Y, Ponticelli C, Morales JM, et al. Prevalence and management of anemia in renal transplant recipients: a European survey. Am J Transplant. 2003;3:835. doi: 10.1034/j.1600-6143.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 2.Chhabra D, Grafals M, Skaro AI, Parker M, Gallon L. Impact of anemia after renal transplantation on patient and graft survival and on rate of acute rejection. Clin J Am Soc Nephrol. 2008;3:1168. doi: 10.2215/CJN.04641007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liefeldt L, Budde K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl Int. 2010;23:1191. doi: 10.1111/j.1432-2277.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 4.KDOQI KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Kainz A, Mayer B, Kramar R, Oberbauer R. Association of ESA hypo-responsiveness and haemoglobin variability with mortality in haemodialysis patients. Nephrol Dial Transplant. 2010;25:3701. doi: 10.1093/ndt/gfq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friend P, Russ G, Oberbauer R, et al. Incidence of anemia in sirolimus-treated renal transplant recipients: the importance of preserving renal function. Transpl Int. 2007;20:754. doi: 10.1111/j.1432-2277.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun CH, Ward HJ, Paul WL, Koyle MA, Yanagawa N, Lee DB. Serum erythropoietin levels after renal transplantation. N Engl J Med. 1989;321:151. doi: 10.1056/NEJM198907203210304. [DOI] [PubMed] [Google Scholar]
- 8.Chadban SJ, Baines L, Polkinghorne K, et al. Anemia after kidney transplantation is not completely explained by reduced kidney function. Am J Kidney Dis. 2007;49:301. doi: 10.1053/j.ajkd.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez Fresnedo G, de Francisco AL, Gomez Alamillo C, Ruiz JC, Rodrigo E, Arias M. The phenomenon of hemoglobin variability with erythropoiesis stimulating agents in renal transplant patients. Clin Nephrol. 2009;72:292. doi: 10.5414/cnp72292. [DOI] [PubMed] [Google Scholar]
- 10.Moore J, He X, Cockwell P, Little MA, Johnston A, Borrows R. The impact of hemoglobin levels on patient and graft survival in renal transplant recipients. Transplantation. 2008;86:564. doi: 10.1097/TP.0b013e318181e276. [DOI] [PubMed] [Google Scholar]
- 11.Kramar R, Oberbauer R. Austrian Dialysis and Transplantation Registry (OEDTR), Annual Report 2009. Austrian Society of Nephrology; Linz, Austria: [accessed January 20, 2012]. 2010. Available at: http://www.nephro.at/oedr2010/oedr2010.htm. [Google Scholar]
- 12.Heinze G, Mitterbauer C, Regele H, et al. Angiotensin-converting enzyme inhibitor or angiotensin II type 1 receptor antagonist therapy is associated with prolonged patient and graft survival after renal transplantation. J Am Soc Nephrol. 2006;17:889. doi: 10.1681/ASN.2005090955. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 14.Brown ER, Ibrahim JG, DeGruttola V. A flexible B-spline model for multiple longitudinal biomarkers and survival. Biometrics. 2005;61:64. doi: 10.1111/j.0006-341X.2005.030929.x. [DOI] [PubMed] [Google Scholar]
- 15.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S. Applied Survival Analysis. Regression Modeling of Time to Event Data. Wiley; New York, NY: 1999. p. 386. [Google Scholar]
- 17.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamar N, Reboux AH, Cointault O, et al. Impact of very early high doses of recombinant erythropoietin on anemia and allograft function in de novo kidney-transplant patients. Transpl Int. 2010;23:277. doi: 10.1111/j.1432-2277.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley; New York, NY: 2000. [Google Scholar]
- 20.Poesen R, Bammens B, Claes K, et al. Prevalence and determinants of anemia in the immediate postkidney transplant period. Transpl Int. 2011;24:1208. doi: 10.1111/j.1432-2277.2011.01340.x. [DOI] [PubMed] [Google Scholar]