Summary
The increased use of older and/or marginal donor organs in liver transplantation over the last decade calls for strategies to minimize ischaemic reperfusion (I/R) injury to prevent early graft failure. Tacrolimus, a very potent and effective calcineurin inhibitor, was selected because of its ability to ameliorate I/R injury. A randomized, blinded, controlled single-centre trial of 26 liver transplant recipients was performed between February 2008 and December 2009. Donor organs were randomized to be perfused intraportally during liver transplantation with 1.5 l 5% albumin infusion containing either 20 ng/ml tacrolimus or placebo. The primary end point was liver function as assessed by aspartate transaminase (AST) or alanine transaminase (ALT) levels 6 days after transplantation. Treatment effectiveness was tested by transcriptome-wide analysis of biopsies. There was no difference in the primary end point, i.e. AST (IU/l) and ALT (IU/l) at day 6 after transplantation between groups. Furthermore, choleastatic parameters as well as parameters of liver synthesis were not different between groups. However, tacrolimus treatment suppressed inflammation and immune response in the transplanted liver on a genome-wide basis. Intrahepatic administration of tacrolimus did not result in a reduction of AST and ALT within the first week after transplantation. (ClinicalTrials.gov number: NCT00609388)
Keywords: early graft dysfunction, FK506, functional genomics, ischaemic reperfusion injury, microarrays, molecular mechanism
Introduction
The number of patients waiting for a liver transplant is continuously rising. The most important indications for liver transplantation in Europe are viral hepatitis (23%), alcoholic liver disease (19%), hepatocellular carcinoma (12%) and choleastatic liver disease (10%) [1]. In the Eurotransplant region, currently over 2600 patients are waiting for a liver graft, whereas only about 1600 liver donors become available per year. This discrepancy results in a median waiting time of 12–23 months in 2009 and a substantial mortality on the waiting list [2]. This situation has led to the acceptance of expanded criteria for donor livers. Currently, more than 20% of donors are above 50 years old [3] and livers with steatosis or grafts from donors with prolonged ICU stay, nonheart-beating donors and even such with HIV infection are being used with variable success rates [4]. At present, the 1-, 5- and 10-year patient survival rates are approximately 83%, 71% and 61%, respectively [3].
Transplantation using marginal grafts is unfortunately accompanied by increased rates of biliary strictures and early graft failure, leading to the need of retransplantation [5,6].
As early graft dysfunction dramatically influences graft and patient outcomes after liver transplantation, prevention of this event is mandatory. Ischaemia/reperfusion (I/R) injury is the underpinning mechanism of early graft dysfunction [4]. Potential ways to improve the outcome of transplantation and to revert or prevent I/R is the use of alternative preservation solutions or machine perfusion of the donor liver during cold ischaemia [7,8].
Intraportal infusions of immunosuppressants achieved relatively little attention in the literature as a potential therapeutic solution. St Peter et al. showed in a randomized pilot study that flushing the liver via portal vein and hepatic artery during transplantation surgery with tacrolimus resulted in superior early graft function as indicated by six liver markers in a multivariable rank test [9]. However, the unadjusted models in this randomized controlled trial (RCT) of these markers did not show differences at day 2 after engraftment. Therefore, it remains unclear whether tacrolimus in that dose and administration may exhibit suppression of the I/R injury on a molecular as well as clinical level as indicated by early liver transaminase levels.
We thus set out to elucidate the effect of intraportal tacrolimus flushing of the donor liver on genome-wide molecular signatures as well as early allograft function.
Materials and methods
This clinical trial is registered at http://clinicaltrials.gov/ct2/show/NCT00609388.
The study protocol was approved by the Institutional Review Board (Ethical Committee of the Medical University of Vienna # EK-597/2007, to be found at http://ohrp.cit.nih.gov/search) and conducted at the Medical University of Vienna according to IRB standards of the institution between February 2008 and December 2009.
CONSORT criteria
Twenty-six consecutive deceased but heart-beating organ donors above the age of 18 years who were reported to the organ procurement organizations (OPOs) were included in the randomized trial (see flowchart in Fig. 1). The donor and recipient demographics and follow-up data of the recipient were collected at the study website http://www.meduniwien.ac.at/nephrogene/trials/ by the local transplant coordinators.
Figure 1.
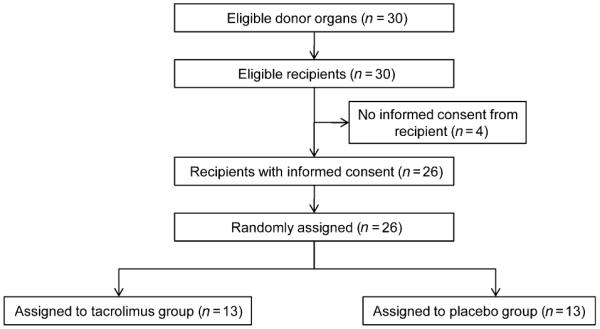
CONSORT flow chart of organ donor and liver graft recipients.
In the treatment group, the intraportal infusion contained tacrolimus (Prograf®, Astellas, Austria) at a concentration of 20 ng/ml. This was accomplished by adding 5 mg (daily dosage) of tacrolimus to a 250 ml 0.9% NaCl solution. Then 1.5 ml of this solution was added to 1.5 l 5% albumin (Octapharma, Vienna, Austria) solution. In the control group, 1.5 ml of normal saline was added to 1.5 l 5% albumin solution. Flush solutions of both groups were prepared by the hospital pharmacist at the beginning of the transplantation process, stored at 4 °C in a refrigerator, where maintenance of temperature was checked routinely every day. The infusion was administered passively according to the laws of gravitation using an i.v. pole at 2 m height. We applied our centre standard intraportal infusion of 1.5 l 5% albumin solution via portal vein to avoid any damage to the liver artery.
After recipient hepatectomy, the implantation of the donor allograft, which was preserved in HTK solution (Custodiol™; Köhler Chemie, Bensheim, Germany) and prepared on the back table, was started with the vena cava anastomosis. During the portal vein anastomosis time, the liver graft was flushed with 1.5 l of the previously mixed solution. The investigators were blinded to the contents of the solutions. After infusion, portal vein anastomosis was completed, and the liver was reperfused. Subsequently, the hepatic artery and biliary anastomosis completed the transplantation of the graft and the recipient was moved to a specialized transplantation intensive care unit for postoperative care. Biopsies were taken after the tacrolimus/placebo perfusion minutes before closure of the abdominal wall and skin of the transplant recipient.
Objectives
The primary study objective was to determine, on a genome-wide basis, whether or not intraoperative and intraportal treatment of the allograft with tacrolimus reduces the inflammatory signature in the liver. Inflammatory response was identified previously as a mediator of I/R injury. The secondary objective was the causality test, whether or not suppression of genes belonging to the ontologies of inflammation and immune response by tacrolimus will lead to a better initial function of the liver.
Study end point
The primary study end point was the serum concentration of the liver biomarkers aspartate transaminase (AST) (U/l) or alanine transaminase (ALT) (U/l) at day 6 after engraftment.
The secondary study end points included first week trajectories of AST and ALT, bilirubin (mg/dl), quick value (%), partial thromboplastin time (s) and international normalized ratio (INR) as well as rejection frequency [10], graft loss and death within the first 3 months after transplantation.
Sample size calculation
Thirteen recipients in each group were required to detect a 50% reduction in the mean value of AST or ALT with a standard deviation of 300 IU/l for an alpha value of 5% (two sides), statistical power of 80% and 20% drop-out rate. Inclusion criteria were, written consent of the recipient, age above 18 years and first transplantation. Exclusion criteria were fulminant liver failure, living donation, multi organ transplantation or re-transplantation, ABO incompatible donor organ and HIV-positive donors or recipients. Liver graft donation after cardiac death is not provided at our transplant centre.
Donors were randomized 1:1 to tacrolimus or placebo treatment. Randomization was based on a permuted block design with block sizes of four (https://www.meduniwien.ac.at/randomizer). Randomization was done centrally through our study website and concealed until data analysis. The randomization order did not have a repeating sequence and the randomization code was not revealed to recipients or investigators. Donors were enrolled by the local transplant coordinators in the centre. Recipients and investigators were blinded for the allograft treatment.
Statistical analysis
Continuous data were analysed by Wilcoxon’s rank sum test, categorical data by a chi-square test or Fisher’s exact test when appropriate. The analysis of the liver function parameters trajectories stratified by treatment was performed by a mixed linear model with time and therapy as the independent parameters.
A P-value less than 0.05 was considered statistically significant. For all analysis SAS for Windows 9.2 (The SAS Institute, Inc., Cary, NC, USA) was used.
Functional genomics
The detailed workflow can be found in the supplementary data file. Briefly, wedge biopsies of each liver were taken under sterile conditions minutes before closure of the abdominal wall and skin of the transplant recipient and liver gene expression analysis from total RNA was performed according to the NuGEN-recommended protocol using the Affymetrix GeneChip Human Gene 1.0 ST Array.
Raw data files as well as the MIAME checklist are available at the GEO Omnibus Database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=jzerxmockuuwqdk&acc=GSE25338)
Results
Baseline characteristics of organ donor and recipients
Demographic data of organ donors and recipients are displayed in Table 1. There were no significant differences between the treatment groups. The CONSORT flow chart in Fig. 1 indicates that 30 donors needed to be enrolled into the study until the present recipients’ number of 13 was achieved in both study arms (see CONSORT chart in Fig. 1).
Table 1.
Demographic data of donors and recipients stratified by treatment assignment. Continuous data are provided as median (first, third quartile), categorical data are shown as counts.
| Tacrolimus | Placebo | P-value | |
|---|---|---|---|
| Donor | 13 | 13 | |
| Gender (f/m) | 8/5 | 7/6 0 | 691* |
| Pretreatment steroids (no/yes) | 5/8 | 4/9 | 1.000† |
| Cause of death (else/intracranial haemorrhage/trauma) |
2/9/4 | 3/9/2 | 0.762† |
| Comorbidities (DM/hypertension/MCI/stroke/none/unknown) |
1/6/1/4/3/3 | 0/3/2/0/6/3 | 0.231† |
| Age [years] | 53 (38, 64) | 50 (33, 57) | 0.396 |
| Steatosis in donor liver [%] | 1 (0, 5) | 5 (0, 10) | 0.554 |
| ALT [IU/l] | 20 (18, 50) | 42.5 (16, 61) | 0.608 |
| AST [IU/l] | 34 (29, 61) | 35 (23, 76) | 0.959 |
| Bilirubin [mg/dl] | 0.60 (0.43, 0.83) | 0.54 (0.30, 0.80) | 0.758 |
| Creatinine [mg/dl] | 0.75 (0.62, 1.20) | 0.99 (0.70, 1.78) | 0.238 |
| INR | 0.0(0.0, 1.2) | 0.0 (0.0, 1.1) | 0.887 |
| Quick value [%] | 71 (65, 89) | 82 (73, 99) | 0.397 |
| PTT [s] | 35 (22.4, 38.2) | 36.2 (24, 47) | 0.699 |
| Recipient | 13 | 13 | |
| Gender (f/m) | 4/9 | 3/10 | 1.000† |
| Age [years] | 55.5 (50, 59) | 55 (53, 60) | 0.959 |
| CIT [h] | 8.0 (6.3, 9.0) | 7.8 (5.8, 9.8) | 0.878 |
| MELD | 20 (16; 23) | 16 (14; 20) | 0.137 |
| Indication for OLT | |||
| PHCC | 2 | 3 | 1.000† |
| HCCA | 1 | 3 | 0.593† |
| ALCI | 4 | 6 | 0.688† |
| PBCI | 2 | 1 | 1.000† |
| OTCI | 3 | 0 | 0.220† |
| AUCI | 1 | 0 | 1.000† |
| Operation data | |||
| Operation time [min] | 330 (260; 445) | 340 (285; 420) | 0.719 |
| WIT [min] | 71 (65; 78) | 77 (70; 90) | 0.157 |
| Blood products | |||
| Packed cells | 0 (0; 4) | 2 (0; 8) | 0.572 |
| FFP | 6 (2; 10) | 8 (6; 12) | 0.457 |
| Platelets | 0 (0; 0) | 0 (0; 0) | 0.939 |
AST, aspartate transaminase; ALT, alanine transaminase; PTT, partial thromboplastin time; INR, international normalized ratio; MELD, model for end-stage liver disease; OLT, orthotopic liver transplantation; PHCC, post-hepatitis C cirrhosis; HCCA, hepatocellular carcinoma; ALCI, alcoholic cirrhosis; PBCI, primary biliary cirrhosis; OTCI, other cirrhosis: unknown causes; AUCI, autoimmune cirrhosis; WIT, warm ischaemic time; FFP, fresh frozen plasma
Chi-square test
Fisher's exact test
The immunosuppression protocol of all subjects included 40 mg dexamethasone intraoperatively with subsequent step-wise tapering and 3 days ATG (Thymoglobulin 2.5 mg/kg/day) induction therapy postoperatively, followed by tacrolimus maintenance therapy started on postoperative day 3. First tacrolimus serum levels were measured at day 4. No difference in serum tacrolimus levels could be detected between the study groups (P = 0.23).
Efficacy of treatment
The dendrogram of the gene expression profiles in Fig. 2 shows excellent discrimination between the blinded tacrolimus and placebo treatment. The intraoperative, intraportal treatment of the allograft with 20 ng/ml of tacrolimus resulted in alterations of genes belonging to the significant enriched processes immune system and cellular amino acid, derivative metabolism (Table 2). The 128 significant DEGs are provided in the supplemental data, Table S1.
Figure 2.
Dendrogram derived by hierarchical clustering of gene expression profiles characterizing the tacrolimus group (black bar) and the placebo group (blue bar).Pearson correlation was used as distance metric and complete linkage as linkage method. Red spots indicate up-regulated transcripts, whereas green spots indicate down-regulated transcripts relative to the mean overall samples.
Table 2.
Significant enriched biological processes according to PANTHER classification separating tacrolimus and placebo treatment as derived on the level of liver differential gene expression. Categories are ranked by the P-value (comparison of expected number of genes and observed number of genes in each biological process) indicating the relevance of a particular process.
| Enriched biological processes | Symbol | Number of genes | P-value |
|---|---|---|---|
| Immune system process | ABCG8, ARSA, BMPER, CRTAM, DUSP26, ELK3, GPR52, HLA-DRB3, HRG, HSP90AA1, IGHD, IL1A, IL34, IRAK3, LGALS9, LRRC40, NLRP1, PGLYRP3, POLM, PTPN22, SAA2, SDC1, SIGIRR, STK33, UTS2R |
25 | 0.008 |
| Cellular amino acid and derivative metabolic process |
BST1, SDC1, ARHGEF2, AANAT, TKTL2, AZIN1 | 6 | 0.020 |
In the interactome analysis, 21 genes (10 DEGs down-regulated and 11 DEGs up-regulated in the tacrolimus group) built together with their interacting partner according to Online Predicted Human Interaction Database, a network with 35 nodes, 105 edges and 20 self loops (Fig. 3). This network shows the molecular interaction of tacrolimus with the NF-κB complex on the transcriptional level and subsequently the efficacy of immunosuppression.
Figure 3.
Protein–protein interaction network of significant DEGs with a fold change over 1.2, respectively. Blue nodes (10 DEGs) indicate down-regulated genes and red nodes (11 DEGs) indicate up-regulated genes with tacrolimus use. Grey nodes represent proteins/complexes identified by the nearest neighbour expansion method.
Primary and secondary study end points
The complete data set for all measured parameters is listed in Table 3. AST and ALT show no significant difference between the two groups at day 6 after engraftment. The median values of AST and ALT in the tacrolimus group were 32 IU/l and 79 IU/l. The corresponding values in the placebo group were 35 IU/l and 101 IU/l with P-values 0.98 or 0.88, respectively.
Table 3.
Liver function parameters after transplantation at day 0, 1 and 6. Data are provided as median (first, third quartile). P-values are derived by Wilcoxon rank sum test.
| Day | Tacrolimus (n = 13) | Placebo (n = 13) | P-value |
|---|---|---|---|
| AST [IU/l] | |||
| 0 | 560 (397, 785) | 391 (308, 730) | 0.383 |
| 1 | 242 (173, 389) | 210 (150, 353) | 0.538 |
| 6 | 32 (29, 66) | 35 (25, 50) | 0.980 |
| ALT [IU/l] | |||
| 0 | 450 (211, 689) | 427 (195, 550) | 0.644 |
| 1 | 281 (194, 650) | 360 (166, 425) | 0.538 |
| 6 | 79 (61, 128) | 101 (53, 160) | 0.878 |
| Bilirubin [mg/dl] | |||
| 0 | 5.28 (3.57, 6.38) | 4.96 (2.45, 9.82) | 0.918 |
| 1 | 2.51 (1.87, 4.12) | 3.3 (1.72, 5.99) | 0.505 |
| 6 | 1.71 (1.55. 2.77) | 2.21 (1.38, 3.62) | 0.644 |
| Quick value [%] | |||
| 0 | 44 (39, 55) | 51 (38, 62) | 0.505 |
| 1 | 61 (48, 68) | 63 (52, 83) | 0.305 |
| 6 | 81 (71, 105) | 82 (71, 91) | 0.758 |
| INR | |||
| 0 | 0.0 (0.0, 1.2) | 0.0 (0.0, 1.3) | 0.864 |
| 1 | 0.0 (0.0, 1.2) | 0.0 (0.0, 0.0) | 0.423 |
| 6 | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 1.000 |
| PTT [s] | |||
| 0 | 47.1 (44.7, 51.5) | 45.4 (43.7, 51.5) | 0.798 |
| 1 | 38.9 (36.3, 44.2) | 38.0 (35.4, 40.3) | 0.573 |
| 6 | 35.0 (30.3, 37.7) | 34.6 (31.6. 37.2) | 0.918 |
AST, aspartate transaminase; ALT, alanine transaminase; PTT, partial thromboplastin time; INR, international normalized ratio
Further, no difference between the other liver function parameters was observed (Table 3).
One recipient in the placebo group died 3 months after transplantation of haemorrhage of a duodenal ulcer. Acute rejection occurred in three recipients in the placebo group at day 7 (rejection activity index (RAI) 6 from 9, moderate rejection), day 42 (RAI 3 from 9, borderline) and day 89 (RAI 3 from 9, borderline) whereas no acute rejection was observed in the treatment group (P = 0.22).
Time course of post-transplant graft function
Post-transplant liver function measured by the continuous variable AST and ALT is shown in Fig. 4. No differences between the two treatment groups were detectable. The repeated measures analysis using an unstructured covariance matrix of the longitudinal enzyme values revealed no difference in the trajectories of AST and ALT (P = 0.390 and 0.387, respectively). No effect modification was observed between treatment and time after transplantation.
Figure 4.
Trajectories of aspartate transaminase (AST) (a) and alanine transaminase (ALT) (b) concentrations in the first 6 days after transplantation stratified by therapy. Solid line: placebo, dashed line: tacrolimus. Vertical bars represent standard deviation; the P-value was derived from the mixed linear model for longitudinal data (treatment effect).
Discussion
In this RCT, we found that intraportal administration with tacrolimus during liver transplantation did not enhance early graft function, despite suppression of inflammation in the donor graft.
In 2003, St Peter et al. published a similar RCT study revealing that intraportal tacrolimus administration during liver transplantation surgery has a beneficial effect on early graft function [9]. This conclusion is based on the multivariate comparison of all six liver function parameters. However, the study was not powered for such a comparison and one or more prespecified primary study end(s) point was/were not provided in the study. Furthermore, the authors investigated the delta of continuous liver parameters between baseline and day 1 and 2 after engraftment as main outcome. This, however, is not statistically appropriate and in addition, no adjustment for the multiple testing was performed. Moreover, it remained unclear from this trial whether the administered dose and timing of tacrolimus flushes were appropriately chosen to suppress inflammation in the donor organ and also subsequently donor liver necrosis.
Thus, we showed on the molecular level that dose and timing of this intervention suppressed inflammation in the donor liver.
Three highly connected, differentially regulated features in the protein–protein interaction network are reported as tacrolimus targets in the literature [11-13]. One of these down-regulated targets, fibroblast growth factor 2, plays a central role in the network and interacts with a multitude of proteins like proinflammatory cytokines, immunoglobulins and the NF-κB complex. Especially NF-κB, a ubiquitous transcription factor, mediates early gene expression of cytokines, chemokines, growth factors, immunoreceptors and cell adhesion molecules during I/R injury [14]. Tacrolimus blockades NF-κB activity and may positive modulate I/R injury in allografts [15-19]. Despite the reported benefits of tacrolimus treatment after I/R injury, we found no positive modification of early graft function in our study cohort. However, we found clearly the molecular interaction with NF-κB and suppression of the immune response.
Our study strengths include the random assignment of organ donors to receive tacrolimus or placebo, the blinding of investigators to treatment allocation and the fact that no patient was lost to follow up. Furthermore, our study is the first to show that tacrolimus used to flush the donor liver during surgery was sufficient to exhibit immunosuppressive action in the donor organ. A precisely followed prespecified study design with objective end points supports the strengths of this trial. However, our study was designed to detect only a large, clinically meaningful difference in liver transaminase at 6 days after surgery of 50%. In addition, all limitations of a single-centre RCT apply.
In conclusion, this trial shows intraoperative, intraportal treatment of the liver allograft with tacrolimus has no beneficial effects on the early graft function within the first week after transplantation. However, tacrolimus treatment suppressed inflammation and immune response in the transplanted liver on a genome-wide basis.
Supplementary Material
Acknowledgements
We acknowledge the valuable contribution of the OPO coordinators.
Funding
This study was supported from an unrestricted grant from Astellas and the FP7 European Union grant ‘SysKid’, grant No. HEALTH-F2-2009-241544.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Significant differentially expressed genes between tacrolimus and placebo treatment listed by fold change.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.ELITA . European Liver Transplant Registry. Birmingham; UK: 2010. [Google Scholar]
- 2.Eurotransplant Annual Report 2009. 2010 [Google Scholar]
- 3.Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29:3. doi: 10.1055/s-0029-1192052. [DOI] [PubMed] [Google Scholar]
- 4.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 5.Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled nonheart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659. doi: 10.1097/01.TP.0000062574.18648.7C. [DOI] [PubMed] [Google Scholar]
- 6.Foley DP, Fernandez LA, Leverson G, et al. Donation after cardiac death: the University of Wisconsin experience with liver transplantation. Ann Surg. 2005;242:724. doi: 10.1097/01.sla.0000186178.07110.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luer B, Koetting M, Efferz P, Minor T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl Int. 2010;23:944. doi: 10.1111/j.1432-2277.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 8.Yuan X, Theruvath AJ, Ge X, et al. Machine perfusion or cold storage in organ transplantation: indication, mechanisms, and future perspectives. Transpl Int. 2010;23:561. doi: 10.1111/j.1432-2277.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 9.St Peter SD, Post DJ, Rodriguez-Davalos MI, Douglas DD, Moss AA, Mulligan DC. Tacrolimus as a liver flushsolution to ameliorate the effects of ischemia/reperfusion injury following liver transplantation. Liver Transpl. 2003;9:144. doi: 10.1053/jlts.2003.50018. [DOI] [PubMed] [Google Scholar]
- 10.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 11.Fong S, Mounkes L, Liu Y, et al. Functional identification of distinct sets of antitumor activities mediated by the FKBP gene family. Proc Natl Acad Sci USA. 2003;100:14253. doi: 10.1073/pnas.2332307100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogina K, Shoda H, Yamaguchi Y, et al. Tacrolimus differentially regulates the proliferation of conventional and regulatory CD4(+) T cells. Mol Cells. 2009;28:125. doi: 10.1007/s10059-009-0114-z. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi J, Takai S, Matsushima-Nishiwaki R, et al. Tacrolimus but not cyclosporine A enhances FGF-2-induced VEGF release in osteoblasts. Int J Mol Med. 2009;23:267. [PubMed] [Google Scholar]
- 14.Squadrito F, Altavilla D, Squadrito G, et al. Tacrolimus limits polymorphonuclear leucocyte accumulation and protects against myocardial ischaemia-reperfusion injury. J Mol Cell Cardiol. 2000;32:429. doi: 10.1006/jmcc.1999.1089. [DOI] [PubMed] [Google Scholar]
- 15.Krishnadasan B, Naidu B, Rosengart M, et al. Decreased lung ischemia-reperfusion injury in rats after preoperative administration of cyclosporine and tacrolimus. J Thorac Cardiovasc Surg. 2002;123:756. doi: 10.1067/mtc.2002.120351. [DOI] [PubMed] [Google Scholar]
- 16.Oltean M, Olofsson R, Zhu C, Mera S, Blomgren K, Olausson M. FK506 donor pretreatment improves intestinal graft microcirculation and morphology by concurrent inhibition of early NF-kappaB activation and augmented HSP72 synthesis. Transplant Proc. 2005;37:1931. doi: 10.1016/j.transproceed.2005.02.069. [DOI] [PubMed] [Google Scholar]
- 17.Oltean M, Pullerits R, Zhu C, Blomgren K, Hallberg EC, Olausson M. Donor pretreatment with FK506 reduces reperfusion injury and accelerates intestinal graft recovery in rats. Surgery. 2007;141:667. doi: 10.1016/j.surg.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Oltean M, Zhu C, Mera S, et al. Reduced liver injury and cytokine release after transplantation of preconditioned intestines. J Surg Res. 2009;154:30. doi: 10.1016/j.jss.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Tsoulfas G, Geller DA. NF-kappaB in transplantation: friend or foe? Transpl Infect Dis. 2001;3:212. doi: 10.1034/j.1399-3062.2001.30405.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





