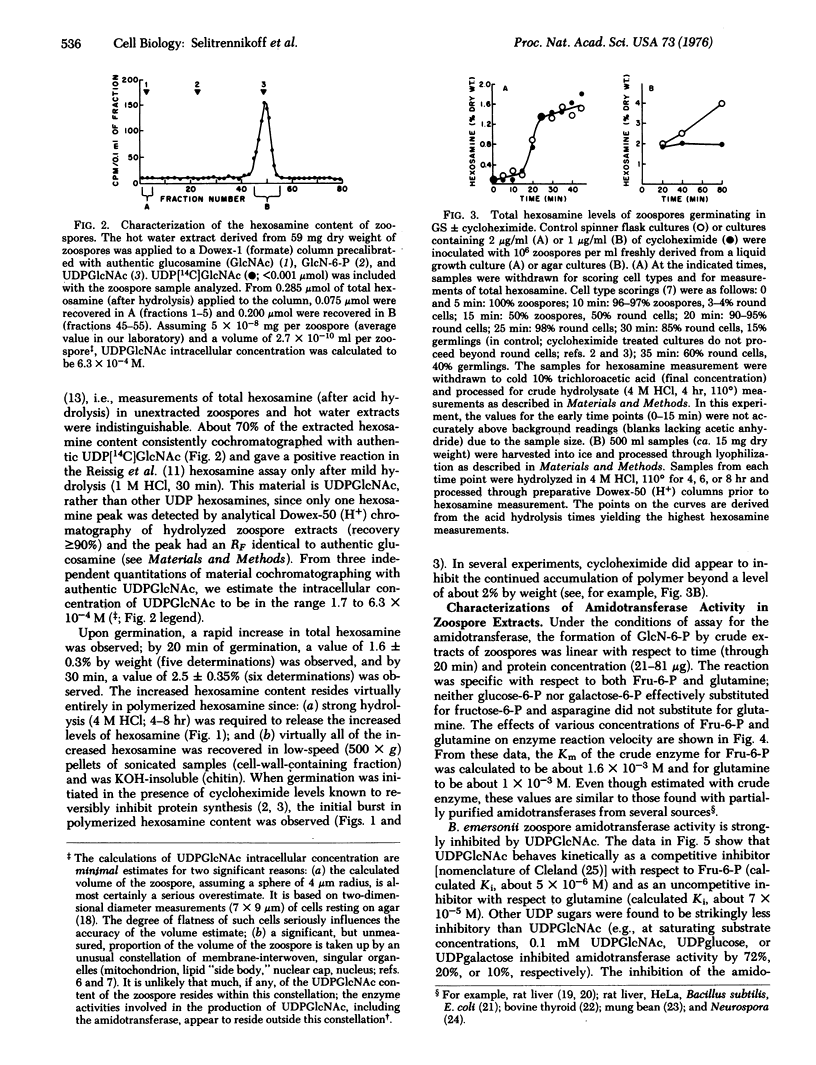
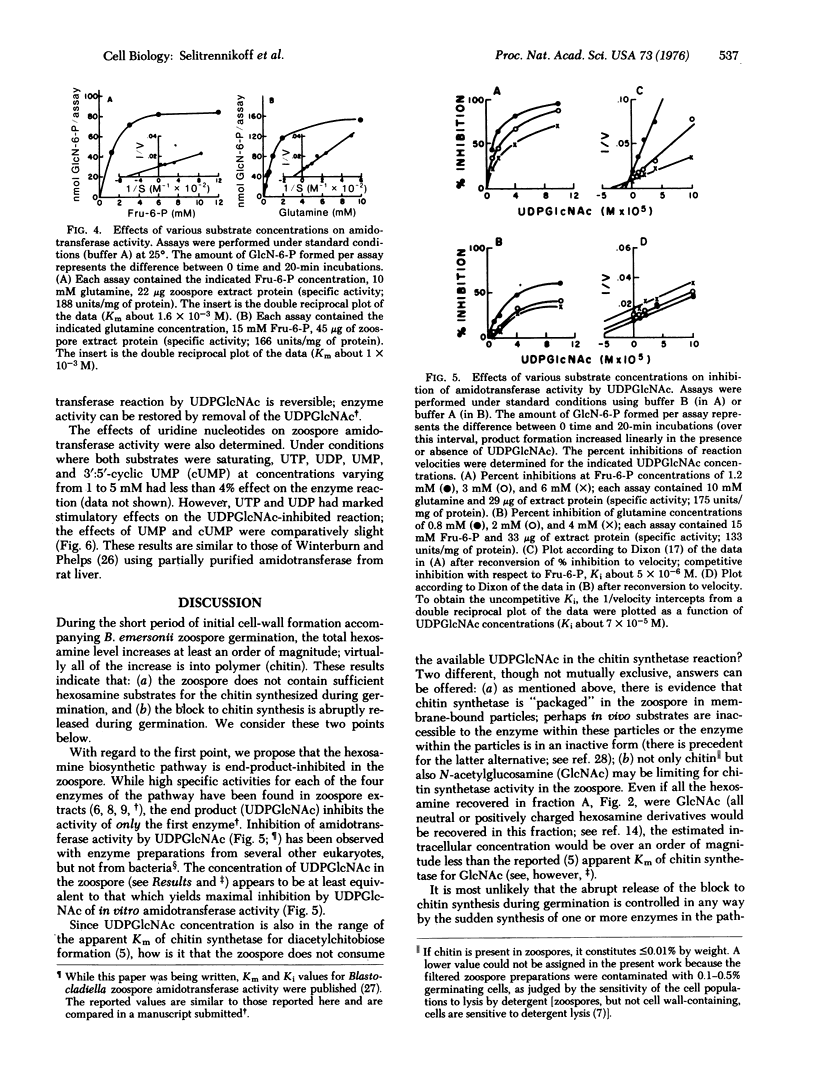
Abstract
De novo construction of a chitinous cell wall accompanies Blastocladiella emersonii zoospore germination. At least an order of magnitude increase in total hexosamine occurs during germination. This increase is into polymer (chitin) and occurs on schedule in the presence of cycloheximide. Uridine-5'-diphospho-N-acetylglucosamine (UDPGlcNAc), both the end product of hexosamine biosynthesis and a substrate for chitin biosynthesis, is a potent inhibitor of the activity of the first pathway-specific enzyme of hexosamine biosynthesis in zoospore extracts. Certain uridine nucleotides, not perceptibly influencing the activity of the first enzyme per se, counteract the inhibitory effects of UDPGlcNAc. The concentration of UDPGlcNAc in the zoospore is sufficient to act as an inhibitor of the enzyme, but the amount of UDPGlcNAc is sufficient, by over an order of magnitude, to account for the chitin synthesized during germination. Both the production of UDPGlcNAc and its utilization for chitin synthesis appear to be post-translationally regulated in zoospores and during zoospore germination.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANTINO E. C., HYATT M. T. Phenotypic sex determination in the life history of a new species of Blastocladiella, B. emersonii. Antonie Van Leeuwenhoek. 1953;19(1):25–70. doi: 10.1007/BF02594831. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Camargo E. P., Dietrich C. P., Sonneborn D., Strominger J. L. Biosynthesis of chitin in spores and growing cells of Blastocladiella emersonii. J Biol Chem. 1967 Jul 10;242(13):3121–3128. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A., Kakiki K., Misato T. Feedback inhibition of L-glutamine D-fructose 6-phosphate amidotransferase by uridine diphosphate N-acetylglucosamine in Neurospora crassa. J Bacteriol. 1970 Sep;103(3):588–594. doi: 10.1128/jb.103.3.588-594.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings T. H., Cantino E. C. Partial purification and properties of D-glucosamine 6'-phosphate N-acetyltransferase from zoospores of Blastocladiella emersonii. J Bacteriol. 1974 Nov;120(2):976–979. doi: 10.1128/jb.120.2.976-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R. Studies on L-glutamine D-fructose 6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem. 1967 Jul 10;242(13):3135–3141. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lodi W. R., Sonneborn D. R. Protein degradation and protease activity during the late cycle of Blastocladiella emersonii. J Bacteriol. 1974 Mar;117(3):1035–1042. doi: 10.1128/jb.117.3.1035-1042.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett J. S. Reactivation of ribonucleic acid and protein synthesis during germination of Blastocladiella zoospores and the role of the ribosomal nuclear cap. J Bacteriol. 1968 Oct;96(4):962–969. doi: 10.1128/jb.96.4.962-969.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi T., Tsuiki S. Effect of phosphoglucose isomerase and glucose 6-phosphate on UDP-N-acetylglucosamine inhibition of L-glutamine-D-fructose 6-phosphate aminotransferase. Biochim Biophys Acta. 1971 Oct;250(1):51–62. doi: 10.1016/0005-2744(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Molnar J., Chao H., Ikehara Y. Phosphoryl-N-acetylglucosamine transfer to a lipid acceptor of liver microsomal preparations. Biochim Biophys Acta. 1971 Sep 1;239(3):401–410. doi: 10.1016/0005-2760(71)90033-6. [DOI] [PubMed] [Google Scholar]
- Norrman J., Myers R. B., Giddings T. H., Cantino E. C. Partial purification of L-glutamine:D-fructose 6-phosphate aminotransferase from zoospores of Blastocladiella emersonii. Biochim Biophys Acta. 1973 Mar 15;302(1):173–177. doi: 10.1016/0005-2744(73)90020-x. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Rothman L. B., Cabib E. Regulation of glycogen synthesis in the intact yeast cell. Biochemistry. 1969 Aug;8(8):3332–3341. doi: 10.1021/bi00836a030. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Huh M. M., Sun L. Protein synthesis during zoospore germination in the aquatic phycomycete Blastocladiella emersonii. Dev Biol. 1974 Sep;40(1):59–70. doi: 10.1016/0012-1606(74)90107-9. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Bromberg R., Sonneborn D. R. Zoospore germination in the water mold. Blastocladiella emersonii. I. Measurement of germination and sequence of subcellular morphological changes. Dev Biol. 1969 Sep;20(3):183–217. doi: 10.1016/0012-1606(69)90012-8. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Sonneborn D. R. Zoospore germination in Blastocladiella emersonii: cell differentiation without protein synthesis? Proc Natl Acad Sci U S A. 1971 Feb;68(2):459–463. doi: 10.1073/pnas.68.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessal M., Hassid W. Z. Partial Purification and Properties of l-Glutamine d-Fructose 6-Phosphate Amidotransferase from Phaseolus aureus. Plant Physiol. 1972 Jun;49(6):977–981. doi: 10.1104/pp.49.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. Binding of substrates and modifiers to glucosamine synthetase. Biochem J. 1971 Feb;121(4):721–730. doi: 10.1042/bj1210721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. Studies on the control of hexosamine biosynthesis by glucosamine synthetase. Biochem J. 1971 Feb;121(4):711–720. doi: 10.1042/bj1210711. [DOI] [PMC free article] [PubMed] [Google Scholar]



