Abstract
Background
Interleukin-8 (IL-8) also referred to as CXCL8, a member of the CXC chemokine family that attracts neutrophils and other leukocytes, has been associated with cancer. Angiogenesis is a prime regulator of tumour expansion and data support that IL-8 is a potent angiogenic factor. Epigenomic instability has been postulated to play a role for the development of multiple neoplasias including colorectal cancer (CRC). DNA methylation of cytosine residues in CpG dinucleotides leads to transcriptional silencing of associated genes.
Method
In this study, we comparatively analysed the protein expression of IL-8 in plasma, tumour and paired normal tissue and methylation status of the IL-8 gene to evaluate its impact on CRC.
Results
Collectively, by using Luminex technology, we noted a significantly higher IL-8 level in cancer tissue compared to paired normal tissue and that CRC patients exhibit significantly higher plasma levels than healthy controls. Analysed by methylation-specific polymerase chain reaction, we detected IL-8 hypomethylation in 64% of the cancerous tissue cases but no hypomethylation was found in paired normal tissue. We noted that the CRC patients with IL-8 hypomethylation revealed a significant higher level of IL-8 protein in cancerous tissue, which tended to be associated with distant metastasis. We also observed that patients with distant metastasis showed a significantly higher plasma level of IL-8 in relation to patients without distant metastasis.
Conclusion
Our results suggest that the predominance of high plasma levels of IL-8 in patients with distant metastasis in combination with the hypomethylation of the IL-8 promoter region might be a useful marker of the disease advancement.
Keywords: Colorectal cancer, IL-8, DNA methylation, Protein expression
Introduction
Interleukin-8 (IL-8) also referred to as CXCL8, a member of the CXC chemokine family that attracts neutrophils and other leukocytes, has been associated with cancer [1–4]. IL-8 is produced by various cells including macrophages, neutrophils, endothelial cells and cancer cells [3, 5]. Several chemokines promote and regulate neoplastic progression including metastasis and angiogenesis [6]. IL-8 has been shown to be expressed higher in colon carcinoma compared with normal colon tissue [7, 8] and overexpression has also been noted in colon cancer cell lines [8–11]. Data showed that overexpression of IL-8 promotes tumour growth and metastasis and is associated with survival in colon cancer [8, 9]. Moreover, IL-8 acts as an autocrine growth factor for human colon carcinoma cells [12].
Angiogenesis is a prime regulator of tumour expansion and in the metastasis of tumour cells. The endogenous proangiogenic factor, vascular endothelial growth factor (VEGF), is a major regulator of both normal and pathological neovascularization and is upregulated in colorectal cancer (CRC) [13, 14]. Additionally, VEGF correlates with invasiveness, metastasis and prognosis [13, 14]. Besides VEGF, IL-8 is also a very potent angiogenic factor [3, 6] and data support the existence of a pathway by which IL-8 controls the expression of VEGF in endothelial cells [15].
Several investigations have indicated that matrix metalloproteinases (MMPs) have fundamental roles in pathological processes in cancer by degradation of the basal membranes and extracellular matrix [16]. Moreover, reduced MMP-9 expression is associated with a longer survival rate in CRC patients [17]. Interestingly, it has been observed that IL-8 can enhance the production of MMPs in ulcerative colitis and melanoma cells [18, 19].
Epigenetic modifications of DNA have been postulated to play a role in the development of multiple neoplasias including CRC [20, 21]. DNA methylation of cytosine residues in CpG dinucleotides leads to transcriptional silencing of associated genes. Promoters with methylated CpG units, which have their transcriptional activity lowered, may function as an alternative mechanism of repressing tumour suppressor genes. The aberrant methylation of gene promoter regions is widely studied and this epigenetic event in human malignancies may affect the cell cycle control and differentiation [20–22].
The human IL-8 gene is located on chromosome 4 and consists of four exons [23]. It has been reported that the expression of IL-8 can be modulated by the methylation of its promoter and that atypical methylation pattern correlates strongly with the metastatic potential of breast carcinoma cells [24]. Moreover, studies have proved hypomethylation of the IL-8 gene promoter in oral epithelial cells in aggressive and chronic periodontitis [25, 26].
Data concerning the methylation status in the gene promoter of IL-8 in human CRC is limited. Therefore, we determined the methylation status in cancerous and paired normal tissue to evaluate its value in CRC and the association to the IL-8 protein expression in CRC patients and clinical factors.
Materials and methods
Patients and tissue sampling
The subjects of this study were 50 CRC patients from southeastern Sweden and informed consent was obtained from each subject. The study was approved by the Local Ethics Committee of Linköping University, Sweden.
Tissue samples were collected when the patients underwent surgical resections for primary colorectal adenocarcinomas at the Department of Surgery, Ryhov County Hospital, Jönköping, Sweden. Clinicopathological characteristics from the patients were received from surgical and pathological records. Tumour tissue and adjacent normal mucosa (about 5 cm from the tumour) from each patient were excised and immediately frozen at −70°C until analysis.
The patient group represented 28 males and 22 females with a mean age of 69 years (range, 36–90). The tumours were located in the colon (n = 29) and rectum (n = 21) and were classified according to the American Joint Committee on Cancer classification system: stage I (n = 3), stage II (n = 20), stage III (n = 14) and stage IV (n = 13). Histological grading was well differentiated in 7 patients, moderately differentiated in 29 patients and poorly differentiated in 14 patients.
Cell lines
Two established human colon cancer cell lines Caco-2 and HT-29 were purchased from American Type Culture Collection (Rockville, MD, USA). The cell lines were grown according to the supplier's instructions and the growth media were Essential Medium (Caco-2) and Mc Coy 5a (HT-29).
Lysates and plasma samples
Tumour tissue and paired normal mucosa as well as cell lines were homogenised in ice-cold lysis buffer containing PBS (9.1 mM dibasic sodium phosphate, 1.7 mM monobasic sodium phosphate, 150 mM NaCl, pH = 7.4) and 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 100 μg/ml phenylmethylsulphonyl fluoride, 2 μg/ml aprotinin, 1 mM sodium orthovanadate and 1 μg/ml leupeptin. The lysate was placed on ice for 30 min and then centrifuged at 13,000g for 10 min. Protein content of the supernatant fluid was determined for each sample using the Bradford protein assay (Bio-Rad Laboratories, CA, USA).
Forty-nine of the CRC patients were available for plasma samples collection before surgery. Blood donors (n = 51), from Ryhov County Hospital, with no known CRC history came from the same geographical region as the CRC patients and were selected as controls. This control group consisted of 28 males and 23 females with a mean age of 65 years (range, 46–71). All blood samples were centrifuged and the separated plasma was stored at −70°C.
Quantification of IL-8 in tissue, cell lines and plasma
IL-8 was measured in tissue, cell lines and plasma using a Luminex bead-based technology (Bio-Rad, Hercules, CA, USA). The tissue and cell line levels of IL-8 were expressed as picogrammes per milligramme of protein. IL-8 in the plasma was expressed as picogrammes per millilitre.
DNA extraction and bisulphite modification
DNA was isolated from tissue samples and cell lines using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Purified DNA (0.5 μg) was treated with bisulphite and purified using the EZ DNA methylation-gold kit (Zymo Research, Irvine, CA, USA) according to the manufacturer's instructions.
Methylation-specific polymerase chain reaction
Methylation-specific polymerase chain reaction (MSP) was performed in principal as previously been described [25, 27]. The primer sequences were based on a previous report [25] and were forward: 5′-AAAATTTTCGTTATATTTCG-3′ and reverse: 5′-TCCGATAACTTTTTATATCAT-3′ for the methylated reaction and forward: 5′-AAAATTTTTGTTATATTTTG-3′ and reverse: 5′-TCCAATAACTTTTTATATCAT-3′ for the unmethylated reaction. The primers were synthesised commercially (TIB Molbiol, Berlin, Germany). The methylated and unmethylated MSP conditions were as follows: initial cycle at 95°C for 5 min followed by 30 cycles at 95°C for 45 s; 47°C for 45 s; 72°C for 45 s and final elongation at 72°C for 7 min. The amplification product (173 bp) was visualised by UV illumination on 3% agarose gel containing Gel Red (Biotium, CA, USA). The total volume of the PCR mixture was 25 μl and contained 60 ng bisulphite-modified DNA, 0.5 μM of each primer (TIB Molbiol), 1.5 mM MgCl2, 200 μM of each deoxynucleotide triphosphate, 2.5 U Taq DNA polymerase and reaction buffer 20 mM Tris–HCl (pH 8.3), 20 mM KCl and 5 mM (NH4)2SO4 (Fermentas, Burlington, Canada).
Statistical analysis
Chi-square test was used to investigate the difference in the methylation status in the groups. Differences of IL-8 levels between tumour and paired normal tissues were examined by the Wilcoxon signed rank test. Differences of IL-8 in plasma between CRC patients and controls were tested by the Mann–Whitney U test. Differences between IL-8 levels in cell lines were tested using Student's t test and the data were expressed as mean values±SEM. Statistical analyses were performed using SPSS for Windows computer package (Rel. 14.0, SPSS Inc., Chicago: 2005, USA). Results were considered significant at P < 0.05.
Results
IL-8 promoter methylation in CRC tissues and paired normal tissues
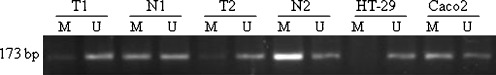
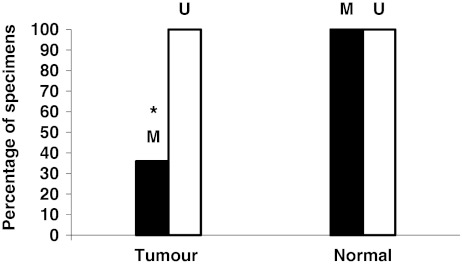
Representative band profiles of the MSP reactions of the IL-8 promoter region are illustrated in Fig. 1. The unmethylated (U) signals were detected in all colorectal samples and in both cell lines. In the paired normal tissue, methylated (M) signals were detected in all cases of the patients (50/50) and in Caco2 cells. In the cancer tissue, we observed that 36% (18/50) of the cases were methylated and that HT-29 cells showed hypomethylation status. The difference in methylation frequency between cancerous tissue and paired normal tissue was statistically significant (P < 0.05) (Fig. 2). When subdividing the patients in groups of no distant metastasis (stage I–III) (n = 37) and distant metastasis (stage IV), the stage IV patients (n = 13) revealed 15.3% (2/13) methylated signals in cancer tissue which tended to be a lower frequency (P = 0.07) compared to stage I–III 43.2% (16/37) (data not shown). There was no statistically significant association between MSP findings with other clinical parameters such as gender, location or histological grading (data not shown).
Fig. 1.
Representative results of MSP analysis of the IL-8 gene in two CRC patients and two cell lines. The PCR products (173 bp) in the lanes M and U indicate the presence of methylated and unmethylated fragments, respectively. T tumour and N normal paired tissue
Fig. 2.
Methylation (M) frequency of the IL-8 gene promoter between tumour and normal tissue was statistically significant, *P < 0.05. Unmethylated status (U) was detected in all specimens
Levels of IL-8 protein in colorectal tissue, plasma and cell lines
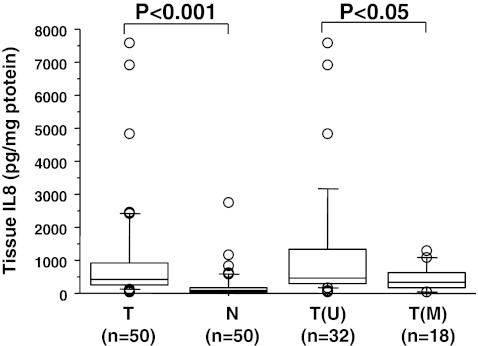
Using Luminex bead-based technology, we investigated the concentrations of IL-8 protein in lysates of CRC tissue and paired normal tissue and in plasma from 49 patients and 51 healthy controls. The CRC tissue levels of IL-8 (median, 419 pg/mg; range, 25–7,568) showed significant differences (P < 0.001) in comparison with normal tissue (median, 77 pg/mg; range, 3–2,760) (Fig. 3). Evaluation of the relative expression (tumour vs. normal tissue) showed 94% (47/50) upregulation for IL-8.
Fig. 3.
Box plot illustration of the protein levels of IL-8 in colorectal tissue from 50 CRC patients. The levels of IL-8 in tumour tissue (T) were higher compared to normal (N) paired tissue. The IL-8 levels are higher in tumour tissue with only unmethylated (U) signals analysed by MSP in relation to tumour tissue with methylated (M) signals. Medians are shown by horizontal bars
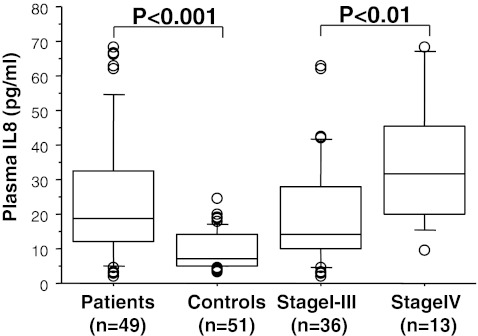
When assessing the cancer tissue levels of IL-8 in detail, we observed that the patients with a hypomethylated status (n = 32) (median, 473 pg/mg; range, 60–7,568) showed significantly (P < 0.05) higher levels compared to the patients (n = 18) (median, 320 pg/mg; range, 25–1,290) which displayed promoter methylation (Fig. 3). Furthermore, we found that the IL-8 concentration in plasma from CRC patients (median, 18.7 pg/ml; range, 1.9–68.2 pg/ml) differed significantly from the healthy controls (median, 7.12 pg/ml; range, 3.4–24.6 pg/ml), P < 0.001 (Fig. 4).
Fig. 4.
Box plot illustration of the protein levels of IL-8 in plasma from 49 CRC patients and 51 healthy controls. The plasma concentrations of IL-8 in CRC patients were higher compared to controls and revealed higher levels in patients with distant metastasis (stage IV) than in those without distant metastasis (stage I–III). Medians are shown by horizontal bars
The levels of IL-8 in all the analysed tissue and plasma samples from the CRC patients did not correlate with any clinical characteristics such as age, gender, histological grading, location and stage apart from the fact that the patients with distant metastasis showed a significantly (P < 0.01) higher plasma level (median, 31.7 pg/ml; range, 9.6–68.2 pg/ml) in comparison with patients without distant metastasis (median, 14.4 pg/ml; range, 1.9–62.7 pg/ml) (Fig. 4). In the human colon adenocarcinoma cell lines, we noted in three experiments that the protein level of IL-8 was 17.7 ± 3.6 and 3.3 ± 1.4 pg/mg in HT-29 and Caco2, respectively. The difference of IL-8 concentrations between HT-29 and Caco2 was statistically significant at the 0.05 level.
Discussion
IL-8 is a proinflammatory cytokine and chemoattractant factor for leukocytes and is involved in tumour growth, metastasis and survival in colon cancer [6, 8, 9]. Moreover, IL-8 is a very potent angiogenic factor [3, 6].
Epigenomic instability has been postulated to play a role for the development of multiple neoplasias including CRC [20, 21]. Methylation of gene promoter regions is widely studied, and this epigenetic event affect the cell cycle control and differentiation in human malignancies. Studies have described that aberrant hypermethylation of promoter CpG islands is linked to gene silencing and loss of tumour suppressor function [20–22].
Recently, a marked hypomethylated status of the IL-8 gene promoter in aggressive periodontitis, which may have relevance for the inflammatory reactions, has been shown [25]. The protein expression profile of IL-8 in relation to methylation of the IL-8 gene promoter in human CRC has not been studied.
In agreement with previous observations, we found that CRC patients have a significantly higher level of IL-8 protein in cancer tissue in comparison with paired normal tissue [7, 8]. Analysed by MSP, we detected IL-8 hypomethylation in 64% of the cancer tissue cases but no hypomethylation was found in paired normal tissues. Furthermore, we noted that the CRC patients with IL-8 hypomethylation revealed significantly higher levels of IL-8 protein in cancer tissue. Associations between tissue IL-8 level or methylation status and a series of clinical characteristics were not found. However, our results indicate that patients with distant metastasis tend to show lower methylation frequency (P = 0.07) in cancer tissue compared with patients without distant metastasis. According to previous reports, we observed a higher constitutive level of IL-8 protein in the colon cancer cell line HT-29 compared to Caco2 [8–11]. This observation can be explained by the hypomethylation status we found in HT-29 and is in line with the higher levels of IL-8 protein in cancer tissue with hypomethylation status.
A possible explanation for the observed higher expression of IL-8 in cancerous compared with normal tissue is that the expression may be controlled by specific transcription factors. The evaluated CpG dinucleotides in this study, which represent potential targets for methylation and subsequent transcriptional repression, are located within nucleotides −136 and +43 in the IL-8 gene promoter [25]. This region has been shown to contain the binding sites for the transcription factors activator protein-1 and nuclear factor-ĸB which are responsible for the constitutive expression of the IL-8 gene [3]. These factors are enhanced in CRC and have a role in the colorectal carcinogenesis [28].
Our study showed significantly higher IL-8 levels in cancer tissue compared with normal tissue, both overall and particularly in patients with IL-8-hypomethylated gene in cancer tissue. Methylation of the IL-8 promoter region may play a pivotal role to modulate the transcriptional activity and is related to overtranscription.
The functional consequence of the IL-8 gene promoter methylation in human CRC is not established. Our results suggest that the hypomethylation of the IL-8 promoter region could have a complementary driving force for the development of CRC by modulating the cancer tissue IL-8 level. In this study we noted that CRC patients exhibit significantly higher plasma levels of IL-8 in relation to healthy controls, which is in agreement with previous reports [10, 29]. Moreover, the patients with distant metastasis showed significantly higher plasma levels than in patients with no distant metastasis. These findings are in line with the study by Ueda et al. [29] showing that circulating levels of IL-8 were significantly higher in patients with metastasis than in patients without metastasis.
Taken together, our study suggest that hypomethylation of the IL-8 promoter region may play a part as a prognostic factor in CRC. The predominance of high plasma levels of IL-8 in patients with distant metastasis in combination with the hypomethylation of the IL-8 promoter region might be a useful indicator of advanced CRC. We are aware that our finding needs to be confirmed by extended studies before drawing a final conclusion regarding these suggestions.
The data presented in this report are prerequisite to a forthcoming study of CRC patients to evaluate the influence of IL-8 protein expression and IL-8 gene methylation status in cancer and normal tissue on a 5-year survival and recurrence rate. Moreover, the clinical significance of free-circulating tumour-associated DNA in CRC patients will be evaluated regarding DNA methylation status of the IL-8 gene promoter.
Acknowledgments
This work was supported by grants from Futurum—the Academy of Healthcare, County Council of Jönköping, Sweden, the Foundation of Clinical Cancer Research, Jönköping, Sweden and the University College of Health Sciences, Jönköping Sweden.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 2.Strieter RM, Kasahara K, Allen RM, Standiford TJ, Rolfe MW, et al. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol. 1992;14:397–407. [PMC free article] [PubMed] [Google Scholar]
- 3.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/S1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 4.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 5.Rossi D, Zliotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Strieter RM, Belperio JA, Phillis RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Baier PK, Eggstein S, Wolff-Vorbeck G, Baumgartner U, Hopt UT. Chemokines in human colorectal cancer. Anticancer Res. 2005;25:3581–3584. [PubMed] [Google Scholar]
- 8.Doll D, Keller L, Maak M, Boulesteix A-L, Siewert JR, et al. Differential expression of the chemokines GRO-2, GRO-3 and interleukin-8 in colon cancer and their impact on metastasis disease and survival. Int J Colorectal Dis. 2010;25:573–581. doi: 10.1007/s00384-010-0901-1. [DOI] [PubMed] [Google Scholar]
- 9.Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malicki S, Winiarski M, Matlok M, Kostarczyk W, Guzdek A, et al. IL-6 and IL-8 responses of colorectal cancer in vivo and in vitro cancer cells subjected to simvastatin. J Physiol Pharmacol. 2009;60:141–146. [PubMed] [Google Scholar]
- 11.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- 12.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, et al. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Abe Y, Tokunaga T. Pathological significance of vascular endothelial growth factor A isoform expression in human cancer. Pathol Int. 2002;52:331–339. doi: 10.1046/j.1440-1827.2002.01367.x. [DOI] [PubMed] [Google Scholar]
- 14.George ML, Tutton MG, Janssen F, Arnaout A, Abulafi AM, et al. VEGF-A, VEGF-C and VEGF-D in colorectal cancer progression. Neoplasia. 2001;3:420–427. doi: 10.1038/sj.neo.7900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial grown factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stetler-Stevenson WG. The role of matrix metalloproteinases in tumor invasion, metastasis and angiogenesis. Surg Oncol Clin N Am. 2001;10:383–392. [PubMed] [Google Scholar]
- 17.Bendardaf R, Buhmeida A, Hilska M, Laato M, Syrjänen S, et al. MMP-9 (gelatinase B) expression is associated with disease-free survival and disease-specific survival in colorectal cancer patients. Cancer Invest. 2010;28:38–43. doi: 10.3109/07357900802672761. [DOI] [PubMed] [Google Scholar]
- 18.Pallone F, Monteleone G. Mechanisms of tissue damage in inflammatory bowel disease. Curr Opin Gastroenterol. 2001;17:307–312. doi: 10.1097/00001574-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, et al. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol. 1997;151:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo Y, Issa JP. Epigenetic changes in colorectal cancer. Cancer Metastasis Rev. 2004;23:29–39. doi: 10.1023/A:1025806911782. [DOI] [PubMed] [Google Scholar]
- 21.Venkatachalam R, Ligtenberg MJL, Hoogerbrugge N, de Bruijn DRH, Kuiper RP, et al. The epigenetics of (hereditary) colorectal cancer. Cancer Genet Cytogenet. 2010;203:1–6. doi: 10.1016/j.cancergencyto.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukaida N, Shiroo MK. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 24.De Larco JE, Wuertz BRK, Yee D, Rickert BL, Furcht LT. Atypical methylation of the interleukin-8 gene correlates strongly with the metastatic potential of breast carcinoma cells. PNAS. 2003;100:13988–13993. doi: 10.1073/pnas.2335921100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andia DC, de Oliveira NFP, Casarin RCV, Casati MZ, Line SRP, et al. DNA methylation status of the IL8 gene promoter in aggressive periodontitis. J Periodontol. 2010;81:1336–1341. doi: 10.1902/jop.2010.100082. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira NFP, Damm GR, Andia DC, Salmon C, Nociti FH, Jr, et al. DNA methylation status of the IL8 gene promoter in oral cells of smokers and non-smokers with chronic periodontitis. J Clin Periodontol. 2009;36:719–725. doi: 10.1111/j.1600-051X.2009.01446.x. [DOI] [PubMed] [Google Scholar]
- 27.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaiopoulos AG, Papachroni KK, Papavassiliou AG. Colon carcinogenesis: learning from NF-kappaB and AP-1. Int J Biochem Cell Biol. 2010;42:1061–1065. doi: 10.1016/j.biocel.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–429. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]