Abstract
Deafferentation pain following nerve injury annoys patients, and its management is a challenge in clinical practice. Although the mechanisms underlying deafferentation pain remain poorly understood, progress in the development of multidimensional neuroimaging techniques is casting some light on these issues. Deafferentation pain likely results from reorganization of the nervous system after nerve injury via processes that interact with the substrates for pain perception (the pain matrix). Therapeutic effects of motor cortex stimulation on deafferentation pain suggest that the core mechanisms underlying deafferentation pain also interact with the motor system. Therefore, simultaneous neuroimaging and brain stimulation, an emerging neuroimaging technique, was developed to investigate complicated interactions among motor, somatosensory, and pain systems. In healthy participants, parts of the pain matrix (the anterior cingulate cortex, parietal operculum, and thalamus) show activity during both somatosensory stimulation and brain stimulation to the motor cortex. This finding indicates that motor, somatosensory, and pain systems communicate among each other via the neural network. A better understanding of the plastic mechanisms influencing such cross-talk among these systems will help develop therapeutic interventions using brain stimulation and neurofeedback.
Deafferentation pain
Deafferentation pain is an unfavorable outcome in orthopedic patients who have experienced injury to the nervous system. Such patients experience severe spontaneous pain in body parts distal to the injury despite reduced or no sensitivity to external noxious stimuli to that body part (hypoalgesia or analgesia). Deafferentation pain can follow spinal cord injuries, peripheral nerve injuries, brachial plexus avulsions, and limb amputations. Damage to the thalamus in the brain causes a similar symptom. It is widely accepted that the loss of pain-related afferent information to the brain (deafferentation) is responsible for this pain syndrome. In particular, deafferentation pain is considered to result from destruction of the spinothalamic tract, which transmits somatosensory information about pain, itch, and rough touch. Nonetheless, the mechanisms of how deafferentation-related changes produce spontaneous pain are not well understood.
Pain syndrome after limb amputation, known as phantom limb pain, is an extreme example of deafferentation pain. In patients with phantom limb pain, pain is perceived in body parts that no longer physically exist. The prevalence of phantom pain after amputation is quite high (60–80 %) [1–3]. Phantom limb pain may be described as burning or tingling at the time of onset and may eventually evolve into severe crushing, pinching, or shooting pain that can become extremely intense (e.g., as if a knife is twisting in the flesh). These descriptions suggest that phantom limb pain involves both superficial and deep pain sensations. The onset of phantom limb pain may be immediate, but it also may not become evident until years after the injury responsible for the pain. Hence, it is possible that plastic changes after deafferentation events play a role, at least in part, in the pathogenesis of phantom limb pain.
The pathophysiology of deafferentation pain remains to be elucidated. However, progress in neuroimaging and brain stimulation techniques has begun to cast light on the neural mechanisms underlying neuroplastic changes after limb amputation. Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation technique that can temporarily modulate brain functions. In brief, a coil placed onto the scalp surface produces rapidly changing magnetic fields, which transcranially induce eddy currents in the brain. The eddy currents activate a group of neurons beneath the coil. When applied to the primary motor cortex (M1), suprathreshold TMS stimulation can evoke muscle twitching that is measurable by electromyography (EMG); this activity is known as motor-evoked potentials (MEP). By changing the coil position over the M1 in the precentral gyrus, TMS can induce MEP in a somatotopical fashion. Thus, TMS has been widely used as a method for noninvasive motor mapping. Karl and colleagues [4] used TMS to map motor representations in the M1 in people with amputated forearms. The M1 contralateral to the amputation had expanded representations of the body parts (i.e., lip or upper arm) adjacent to the now missing forearm, as if the surrounding motor representations invaded the previous forearm motor representation. However, evidence indicates that the motor cortical representation of the missing limb is not completely gone in amputees. TMS applied over the M1 can elicit motor perception of the missing limb, providing an explanation for phantom limb perception [5].
How, then, does the M1 retain representation of the phantom limb for a long period without linkage between motor efferents and somatosensory afferents? An emerging idea is that the stump muscles play a part. In amputees, an attempt to move phantom limbs induced EMG activity in stump muscles. Ischemic nerve block resulted in an inability to move phantom limbs [6]. This finding suggests that motor commands from the part of the M1, which previously controlled the now-missing limb, are retargeted to the stump muscles and that a new linkage between motor efferents and somatosensory afferents is formed. However, the brain possibly interprets stump muscle contraction and the resultant sensory information as phantom limb movement.
Functional magnetic resonance imaging (fMRI) noninvasively allows measurement of oxygenation/blood-flow-related (hemodynamic) changes in MRI signals as a surrogate marker of neural activity. Because of its high spatial localization, fMRI has remained the most reliable noninvasive brain mapping method for more than a decade. Using fMRI, brain activity during a sensory stimulation task was mapped in people with spinal cord injury (SCI) [7]. The study showed that activity during sensory stimulation to the little finger was expanded into the parts of the primary somatosensory cortex (S1) that would normally receive afferent information from the lower limbs. Namely, it appeared that the S1 was reorganized after SCI in such a way that representations of the remaining body parts invaded areas that had lost somatosensory afferents. This result parallels the findings of motor representations in the TMS study mentioned above [4]. Intriguingly, the degree of S1 reorganization was greater in SCI patients with deafferentation pain than in those without it [7].
To summarize, converging evidence suggests that motor and somatosensory representations undergo complex reorganization (Table 1) after deafferentation, including amputation, and that this reorganization may be associated with deafferentation pain.
Table 1.
Brain regions possibly involved in the pathogenesis of deafferentation pain
| Regions | Reorganization | Pain matrix | Somatosensory stimulation | M1 stimulation |
|---|---|---|---|---|
| Primary motor cortex (M1) | ++ | + (Nonthermal) | + | + |
| Primary somatosensory cortex (S1) | ++ | + (Nonthermal) | ++ | ++ |
| Supplementary motor/premotor areas | ? | + (Nonthermal) | ++ | ++ |
| Cingulate cortex | ? | ++ | ++ | ++ |
| Insula-parietal operculum (S2) | ? | ++ | ++ | ++ |
| Thalamus | + | ++ | ++ | ++ |
| Basal ganglia | ? | − | + | ++ |
++ frequently reported, + occasionally reported, ? not clear
Pain matrix
The International Association for the Study of Pain (http://www.iasp-pain.org) has defined pain as “an unpleasant sensory and emotional experience arising from actual or potential tissue damage or described in terms of such damage.” This implies that pain may be ascribed to the perception of tissue damage or to a perceptual experience interpreted as tissue damage. In any case, the above definition of pain signifies the importance of clarifying how the brain handles sensory afferents associated with tissue damage. Furthermore, it is important to understand how psychological and emotional factors influence the brain to interpret pain-related sensory signals. In fact, the neural substrates of pain perception have been intensively explored with neuroimaging techniques, such as fMRI and positron emission tomography (PET). Many imaging studies have consistently revealed a set of brain regions as substrates of pain perception [8]. These regions include the anterior cingulate cortex, the insula, the parietal operculum including the second somatosensory cortex (S2), and the thalamus, which are collectively called the pain matrix. Activity in the S1 is reported only during nonthermal stimulation [9] (Table 1; Fig. 1).
Fig. 1.
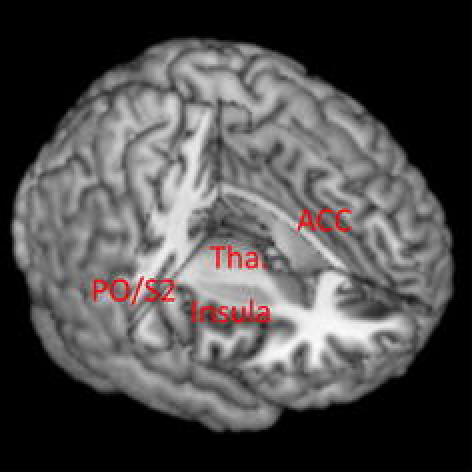
Pain matrix. The anterior cingulate cortex (ACC), posterior insula, parietal operculum (PO) including the second somatosensory cortex (S2) and thalamus (Tha.) are considered to be the key structures for pain perception. The primary motor and somatosensory areas may be involved in the perception of nonthermal (deep) pain
Activity in parts of the pain matrix is modulated by psychological and/or emotional factors. Such modulation may explain how these factors influence the perception of pain. Sawamoto and colleagues [10] showed that the anterior cingulate cortex and parietal operculum/insula area are activated during both painful (stimulus intensity above the pain threshold) and nonpainful (below the threshold) thermal stimuli. Moreover, uncertainty about the forthcoming stimulus enhances the unpleasantness of the stimulus and brain responses in the anterior cingulate cortex and parietal operculum/insula. By contrast, in a yoga master who claimed not to feel pain during meditation, brain activity in the pain matrix in response to painful thermal stimuli was reduced during meditation [11]. These lines of evidence suggest that activity in the pain matrix is influenced by psychological factors and that the degree of pain matrix activity could reflect the subjective experience of pain.
Deafferentation pain appears to involve both superficial and deep pain sensations. Although most previous neuroimaging studies of pain used stimuli affecting superficial pain sensations, a few studies investigated brain responses during the experience of muscular pain. Such studies used injection of hypertonic saline into muscles [12, 13] or electric stimulation of a myofascial trigger point [14] to evoke deep pain sensations. The results of these studies suggest that deep pain perception induces brain activity in motor-related areas, including the M1, as well as in the pain matrix. Therefore, it is possible that motor-related brain areas are involved in addition to the pain matrix in the development of deafferentation pain involving both superficial and deep pain sensations.
Motor cortex stimulation to relieve deafferentation pain
Therapeutic interventions to deafferentation pain also indicate a link between the pathophysiology of deafferentation pain and functions of the motor-related brain areas. Invasive and noninvasive stimulation to the M1 seem effective for ameliorating deafferentation pain. Motor cortex stimulation using surgically implanted electrodes can control poststroke pain after thalamic infarction [15], although its effects on phantom limb pain require further investigation [16]. Moreover, noninvasive stimulation to the M1 with high-frequency repetitive TMS relieves deafferentation pain, whereas low-frequency stimulation or stimulation to other brain areas does not [17, 18].
The mechanisms by which M1 stimulation reduces deafferentation pain are poorly understood. It has been suggested that “abnormal processing of nociceptive information develops at the level of deafferentation and spreads to higher levels” [15]. Together with evidence discussed in the “Deafferentation pain” section above, it seems plausible to hypothesize that reorganization of the nervous system upstream to the deafferentation level is responsible for deafferentation pain. Reorganization appears to occur in the motor and somatosensory areas after deafferentation, but the connection between those areas and the pain matrix has not been well documented. To clarify the pathophysiology of deafferentation pain, we need to know how the motor/somatosensory areas and the pain matrix interact through neural networks.
Multidimensional neuroimaging approach
Advances in multidimensional neuroimaging techniques allow us to conduct TMS with EMG monitoring in the fMRI environment [19, 20]. This experimental setup may provide a novel approach with which to explore the neural mechanisms underlying deafferentation pain. When single-pulse TMS was delivered to the hand representation of the left M1 at various intensities, it evoked MEPs in the right-hand muscles in a dose-dependent manner [19]. Suprathreshold TMS induced brain activity not only in the stimulated M1 but also in remote motor and somatosensory areas, such as the anterior cingulate cortex, supplementary motor areas, premotor cortex, S1, insula/parietal operculum (S2), thalamus, basal ganglia, and cerebellum (Table 1; Fig. 2). Counterintuitively, a detailed analysis of the relationship between TMS intensities and fMRI responses showed that activity in the remote motor/somatosensory areas was induced at lower intensities than those that were required to evoke M1 activity. These findings indicate that brain activity is evoked through the network comprising the M1 and other motor/somatosensory areas by means of combined TMS and fMRI. It should be noted that because brain stimulation to the M1 can relieve deafferentation pain, the neural substrates responsible for deafferentation pain are likely included in the brain network activated by M1 stimulation.
Fig. 2.

Brain activities induced by deep somatosensory stimulation (blue) and transcranial magnetic stimulation to the primary motor cortex (red) in healthy volunteers. The overlap of the two is shown in green. The overlapping activity partially corresponds to the pain matrix, which is composed of the cingulate cortex, the insula/parietal operculum including the second somatosensory cortex, and the thalamus. Overlap is also observed in nonpain matrix areas, such as the primary motor cortex, the primary somatosensory cortex, the supplementary motor cortex, and the basal ganglia. The figure shows a new illustration based on data from Shitara et al. [20]
Another possibility is that the regions responsible for deafferentation pain receive somatosensory afferent information from the body. Shitara and colleagues [20] investigated brain activity evoked by superficial and deep somatosensory stimulation of a limb, along with brain activity evoked by M1 stimulation. As superficial and deep somatosensory stimuli, electrical stimulation was delivered to the median nerve at the right wrist above the sensory threshold (below the motor threshold) and above the motor threshold, respectively. Median nerve stimulation above the motor threshold induced brain activity in the M1, S1, S2, anterior cingulate cortex, insula, basal ganglia, and thalamus. These regions partially overlapped with the zones activated by M1 stimulation (Figs. 2, 3; Table 1).
Fig. 3.
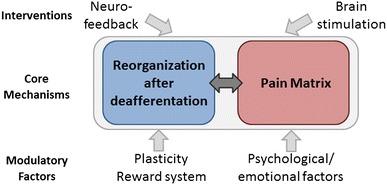
Hypothetical mechanisms of deafferentation pain. Deafferentation pain may result from an interaction between reorganization processes after deafferentation and their influences on the pain matrix (core mechanisms). These processes and perception of deafferentation pain are likely to be influenced by many factors, including plasticity, the reward system, and psychological/emotional factors. We need to look for therapeutic interventions (e.g., brain stimulation, neurofeedback) that can effectively modulate the activity of these core mechanisms, most likely represented in the form of a neural network
Deafferentation pain could be represented in parts of the pain matrix. Considering the intersection of the pain matrix, somatosensory-stimulation-induced brain activity, and M1-stimulation-induced brain activity, the candidate regions responsible for deafferentation pain are S2, the cingulate cortex, and the thalamus (Table 1). It is appealing to examine brain responses during M1 stimulation in patients with deafferentation pain to prove the essential roles of these areas in the pathophysiology of deafferentation pain.
Neuroplasticity: future perspective for imaging studies and therapeutic interventions
As discussed, accumulating evidence suggests that the complex interaction between reorganization of the nervous system after deafferentation and its influences on the pain perception system (pain matrix) underlies the pathophysiology of deafferentation pain. Moreover, it is critical to examine the mechanisms of neuroplasticity operating after nerve injury and their relationship to deafferentation pain. Recent anatomical imaging studies show brain reorganization in the motor/somatosensory areas and thalamus at the structural level (i.e., reduced gray matter volume) after SCI [21] and amputation [22]. The meaning of these structural changes should be clarified in relation to functional changes in those regions.
To understand the plasticity associated with deafferentation pain, we also need to consider the roles of basal ganglia. Geha and colleagues [23] found that activity in the basal ganglia was related to tactile allodynia after nerve injury, although the basal ganglia are not regarded as a part of the pain matrix. Intriguingly, the basal ganglia are activated during both deep somatosensory stimulation and M1 stimulation [20]. Because the basal ganglia are implicated in plastic brain changes that occur in association with reward and punishment, it is possible that the basal ganglia exert modulation effects on S2, anterior cingulate cortex, and thalamus in the course of the development of deafferentation pain. This hypothesis should be investigated in future studies.
Finally, the effects of M1 stimulation on the relief of deafferentation pain suggest that the abnormal activity responsible for such pain can be functionally modulated. A recent study shows that training with fMRI-based neurofeedback of pain-induced activity can relieve pain [24]. These exciting findings will direct researchers toward the development of a variety of neurofeedback techniques to relive deafferentation pain. In conclusion, neuroimaging studies are providing, and will continue to provide, indispensible knowledge that will aid our understanding of the pathophysiology of deafferentation pain and lead to the development of new treatment strategies for such pain.
Acknowledgments
The author thanks Dr. Hitoshi Shitara for his help with making a figure and Drs. Kenji Takagshi and Manabu Honda for their general support. This study was supported in part by KAKENHI (23500485).
References
- 1.Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87:33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- 2.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983;17:243–256. doi: 10.1016/0304-3959(83)90097-0. [DOI] [PubMed] [Google Scholar]
- 3.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985;21:267–278. doi: 10.1016/0304-3959(85)90090-9. [DOI] [PubMed] [Google Scholar]
- 4.Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 2001;15(21):3609–3618. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercier C, Reilly KT, Vargas CD, Aballea A, Sirigu A. Mapping phantom movement representations in the motor cortex of amputees. Brain. 2006;129(Pt 8):2202–2210. doi: 10.1093/brain/awl180. [DOI] [PubMed] [Google Scholar]
- 6.Reilly KT, Mercier C, Schieber MH, Sirigu A. Persistent hand motor commands in the amputees’ brain. Brain. 2006;129(Pt 8):2211–2223. doi: 10.1093/brain/awl154. [DOI] [PubMed] [Google Scholar]
- 7.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37(Suppl 1):S80–S88. doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 9.Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage. 2011;15(58):1070–1080. doi: 10.1016/j.neuroimage.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;1(20):7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakigi R, Nakata H, Inui K, Hiroe N, Nagata O, Honda M, Tanaka S, Sadato N, Kawakami M. Intracerebral pain processing in a Yoga Master who claims not to feel pain during meditation. Eur J Pain. 2005;9:581–589. doi: 10.1016/j.ejpain.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Henderson LA, Bandler R, Gandevia SC, Macefield VG. Distinct forebrain activity patterns during deep versus superficial pain. Pain. 2006;120:286–296. doi: 10.1016/j.pain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007;128:20–30. doi: 10.1016/j.pain.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Niddam DM, Chan RC, Lee SH, Yeh TC, Hsieh JC. Central representation of hyperalgesia from myofascial trigger point. Neuroimage. 2008;1(39):1299–1306. doi: 10.1016/j.neuroimage.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 15.Katayama Y, Yamamoto T, Kobayashi K, Kasai M, Oshima H, Fukaya C. Motor cortex stimulation for post-stroke pain: comparison of spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77:183–186. doi: 10.1159/000064618. [DOI] [PubMed] [Google Scholar]
- 16.Katayama Y, Yamamoto T, Kobayashi K, Kasai M, Oshima H, Fukaya C. Motor cortex stimulation for phantom limb pain: comprehensive therapy with spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77:159–162. doi: 10.1159/000064593. [DOI] [PubMed] [Google Scholar]
- 17.Saitoh Y, Hirayama A, Kishima H, Shimokawa T, Oshino S, Hirata M, Tani N, Kato A, Yoshimine T. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high-frequency repetitive transcranial magnetic stimulation of the primary motor cortex. J Neurosurg. 2007;107:555–559. doi: 10.3171/JNS-07/09/0555. [DOI] [PubMed] [Google Scholar]
- 18.Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, Kato A, Yoshimine T. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006;122:22–27. doi: 10.1016/j.pain.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Hanakawa T, Mima T, Matsumoto R, Abe M, Inouchi M, Urayama S, Anami K, Honda M, Fukuyama H. Stimulus-response profile during single-pulse transcranial magnetic stimulation to the primary motor cortex. Cereb Cortex. 2009;19:2605–2615. doi: 10.1093/cercor/bhp013. [DOI] [PubMed] [Google Scholar]
- 20.Shitara H, Shinozaki T, Takagishi K, Honda M, Hanakawa T. Time course and spatial distribution of fMRI signal changes during single-pulse transcranial magnetic stimulation to the primary motor cortex. Neuroimage. 2011;1(56):1469–1479. doi: 10.1016/j.neuroimage.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- 22.Draganski B, Moser T, Lummel N, Gänssbauer S, Bogdahn U, Haas F, May A. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006;1(31):951–957. doi: 10.1016/j.neuroimage.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008;15(138):641–656. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]


