Abstract
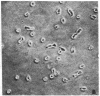
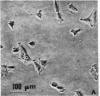
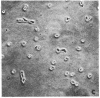
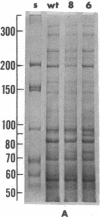
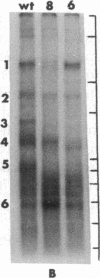
Two mutants of Balb/c 3T3 cells defective in adhesiveness to substratum have been isolated. After exposure to a mutagen a cell population was treated with prostaglandin E1 and methylisobutylxanthine to elevate the cells 3':5'-cyclic AMP levels and adhesion to the substratum; then, loosley adherent cells were harvested by gentle detachment. After four cycles of selection two clones were isolated. Both are characterized by a round morphology and a decreased adhesion to substratum. The defect in adhesion appears to be responsible for the change in cell shape, since the mutant cells become flattened and look similar to wild-type cells when treated with dibutyryl cyclic AMP even though their adhesiveness remains decreased. Further, the surface of both mutants is chemically altered. Lactoperoxidase-catalyzed iodination of the cell surface shows that two iodinated polypeptides (molecular weight, Mr, 90,000 and 135-140,000) present in wild-type cells are not detected on the surface of the mutants, and another iodinated band (110-120,000 Mr) is markedly decreased. In addition, one mutant is also missing the high-molecular-weight (230,000) cell surface protein that is sensitive to transformation. No changes have been found in the content of cyclic AMP or free or polymerized tubulin. The exponential growth rate and saturation density are not altered in these adhesion--eficient cells. This last result suggests that modulation of adhesion to substratum per se does not play an important role in the growth control of Balb/c 3T3 cells in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borisy G. G. A rapid method for quantitative determination of microtubule protein using DEAE-cellulose filters. Anal Biochem. 1972 Dec;50(2):373–385. doi: 10.1016/0003-2697(72)90046-2. [DOI] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Cyclic AMP and growth of fibroblasts: effect of environmental pH. Nat New Biol. 1973 Mar 21;242(116):78–80. doi: 10.1038/newbio242078a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Humphryes K. C. Characterization of the external proteins of hamster fibroblasts. J Cell Biol. 1974 Aug;62(2):438–448. doi: 10.1083/jcb.62.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Stanley P. Altered cell surface glycoproteins in phytohemagglutinin-resistant mutants of Chinese hamster ovary cells. Biochim Biophys Acta. 1975 May 6;389(2):401–406. doi: 10.1016/0005-2736(75)90332-6. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Rubin H. Effects of cell adhesion of the substratum on the growth of chick embryo fibroblasts. Exp Cell Res. 1974 Apr;85(2):319–333. doi: 10.1016/0014-4827(74)90133-5. [DOI] [PubMed] [Google Scholar]
- Pastan I., Johnson G. S. Cyclic AMP and the transformation of fibroblasts. Adv Cancer Res. 1974;19(0):303–329. doi: 10.1016/s0065-230x(08)60057-3. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Rubin R. W., Weiss G. D. Direct biochemical measurements of microtubule assembly and disassembly in Chinese hamster ovary cells. The effect of intercellular contact, cold, D2O, and N6,O2'-dibutyryl cyclic adenosine monophosphate. J Cell Biol. 1975 Jan;64(1):42–53. doi: 10.1083/jcb.64.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R., Pollock K. The adhesion of BHK and PyBHK cells to the substratum. Cell. 1974 Sep;3(1):31–38. doi: 10.1016/0092-8674(74)90034-8. [DOI] [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell. 1975 Oct;6(2):121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Carchman R. A., Pastan I. H. A mutant of 3T3 cells with cyclic AMP metabolism sensitive to temperature change. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2906–2910. doi: 10.1073/pnas.70.10.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]